Background. Chronic renal failure (CRF) is a significant cause of morbidity and mortality in post-liver transplantation (LT) recipients. The risk factors associated with the development of renal dysfunction are not clearly elucidated. Objectives. To examine the risk factors in the development of CRF in these patients.
Material and methods. Retrospective case-cohort of liver transplant patients without baseline kidney dysfunction who developed chronic renal failure during their follow-up.
Results. Of 370 patients, 254 met the inclusion criteria. 30% (76) of these patients had CRF of which 57% (43) were male. Age, estimated glomerular filtration rate (eGFR) at discharge, and HCV infection were found to be risk factors for CRF post-LT. The odds ratio of developing CRF was 1.4 (0.6-3.3) in males with HCV, 1.6 (0.7-3.9) in females without HCV and 4.4 (1.5-13.2) among females with HCV when compared to men without HCV.
Conclusions. In this cohort of LT receipients of a major Canadian city, age, eGFR, and HCV infection were risk factors for CRF. Female gender and HCV increased this odds by a factor of more than 4.
Chronic renal failure (CRF) is a significant cause of morbidity and mortality in post-liver transplantation (LT) recipients, affecting graft function and survival. In one study, the 13-year survival rate in patients with end-stage renal disease after liver transplantation was 28.2% vs. 54.6% in those without kidney disease.1 In another study, the risk of death increased by 4.55 in patients with occurrence of CRF.2 The mechanisms causing this CRF may relate to the development of diabetes mellitus (DM) post-transplantation3,4 and calcineurin-induced nephro-toxicity.5,6 Previous studies have identified hepatitis C (HCV) infection,2,7,8 pre-LT serum creatinine (CR),7,9 pre-LT diabetes mellitus (DM),2,7,8 pre-LT hypertension (HTN),2 female gender,2,8,9 and age2,9 as risk factors. The relationships of these factors are not clearly defined. Furthermore, Canadian data is lacking. The main objective of this study was to examine the risk factors for the development of CRF in post-LT recipients of a major Canadian city.
Material and MethodsThis study was approved by the Clinical Research Ethics Board of the University of British Columbia. Cases were identified from a cohort of all LT recipients at a single tertiary center (Vancouver General Hospital) from January 2005 to December 2013. Those who were over the age of 18, with a first liver transplant, who survived 90 days post-LT and had an estimated glomerular filtration rate (eGFR) equal to or above 60 mL/min at the time of discharge were included in the study. The MDRD formula was used to calculate the eGFR. We excluded patients who had a prior liver transplant or more than one organ transplanted, intrinsic renal disease (e.g. diabetic nephropathy or acute tubular necrosis), hepatorenal syndrome prior to liver transplant and acute kidney injury. The presence of intrinsic renal disease was based on the clinical history identified at the time of transplant assessment. CRF was defined as a sustained decrease in eGFR below 50 mL/min over 3 months. This eGFR value was selected as this is the value that is used in our center to substitute CNI based immunosuppression to sirolimus and it represents the stage 3 of chronic kidney disease according to the K/ DOQI guidelines.10 All our patients with progressive CKD after transplant were assessed for possibility of pre-renal disease and proteinuria. They were also referred to a nephrologist for evaluation and it was based on their individual assessment that a diagnosis of CNI induced kidney injury was concluded.
Clinical information including age, gender, reason for LT, donor type, comorbidities (diabetes mellitus and hypertension), type of immunosuppression (CNI, myco-phenolate mofetil, azathioprine) as well as average CNI levels during follow-up were recorded. Diabetes melli-tus was defined as use of insulin or any other hypoglyc-emic agent during follow-up. Hypertension was defined as use of an anti-hypertensive agent during follow-up.
Patients in the cohort were followed until they reached the pre-defined outcome of chronic renal failure or the end of follow-up period which was set at May 1, 2014.
Cases were patients who developed CRF and controls were all the other patients in the cohort who have not developed CRF at the end of the study. Potential pre-defined exposures that would increase the risk of CRF were analyzed and included age, gender, presence of diabetes, presence of hypertension, hepatitis C infection as an underlying disease, mean CNI levels during follow-up and the eGFR at the time of discharge. Only a minority of patients with hepatitis C in this cohort were treated after their transplant.
Statistical analysisContinuous variables were presented as means with standard deviations or medians with IQR when appropriate. Categorical variables were presented as proportions. Student t-test for was used for comparison of normally distributed variables and the Mann-Whitney U test for non-parametric data. Categorical variables were compared using Pearson’s χ2 test or Fisher’s exact test when appropriate.
A logistic regression model was performed to identify independent predictors of CRF adjusting for pre-set variables that included patient age, history of hypertension, history of diabetes, nephrotoxic medications, maintenance immunosuppression and average trough CNI levels. Results are presented as odds-ratios with their 95% confidence interval. All statistical analyses were performed using Stata 11.2 (StataCorp, College Station, Texas).
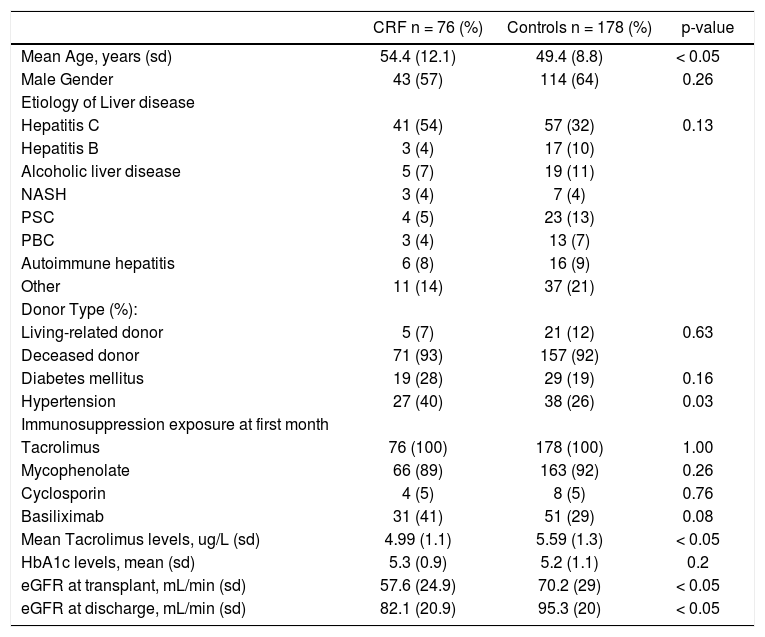
ResultsOf 370 patients identified that were transplanted during the study period, 254 met the inclusion criteria. Seventy-six (30%) patients developed post-LT CRF during their follow-up. Table 1 shows the demographic characteristics of the post-LT CRF patients as well as the control population. The median age at transplant was 54.4 years in the CRF group compared to 49.4 years in the control group. Gender was similar to in both groups. HCV was the most common reason for LT in both groups, but HCV was more common in the CRF group when compared to the control group (54% vs. 32%, p < 0.01). No differences were found when the type of donor was compared (deceased vs. living donor). Prevalence of diabetes mellitus was similar in both groups as well, but hypertension was more prevalent in the CRF group (40% vs. 26%, p = 0.03). During the first month after transplant all patients were exposed to tacrolimus, 5.2% of CRF patients vs 4.5% were exposed to cyclosporine; 86.8% of CRF patients vs. 92.1% were exposed to mycophenolate, all patients except for one in the CRF group received steroids. There was a non-statistical significant difference in the exposure to basilixi-mab (40.1% in CRF group vs. 28.8% in controls). Finally, both groups had similar exposure to antithymocyte-globu-lin (3.4% in CRF vs. 5.2% in controls) in the first month after transplant.
Demographic characteristics of post-liver transplantation chronic renal failure (CRF) and control populations.
| CRF n = 76 (%) | Controls n = 178 (%) | p-value | |
|---|---|---|---|
| Mean Age, years (sd) | 54.4 (12.1) | 49.4 (8.8) | < 0.05 |
| Male Gender | 43 (57) | 114 (64) | 0.26 |
| Etiology of Liver disease | |||
| Hepatitis C | 41 (54) | 57 (32) | 0.13 |
| Hepatitis B | 3 (4) | 17 (10) | |
| Alcoholic liver disease | 5 (7) | 19 (11) | |
| NASH | 3 (4) | 7 (4) | |
| PSC | 4 (5) | 23 (13) | |
| PBC | 3 (4) | 13 (7) | |
| Autoimmune hepatitis | 6 (8) | 16 (9) | |
| Other | 11 (14) | 37 (21) | |
| Donor Type (%): | |||
| Living-related donor | 5 (7) | 21 (12) | 0.63 |
| Deceased donor | 71 (93) | 157 (92) | |
| Diabetes mellitus | 19 (28) | 29 (19) | 0.16 |
| Hypertension | 27 (40) | 38 (26) | 0.03 |
| Immunosuppression exposure at first month | |||
| Tacrolimus | 76 (100) | 178 (100) | 1.00 |
| Mycophenolate | 66 (89) | 163 (92) | 0.26 |
| Cyclosporin | 4 (5) | 8 (5) | 0.76 |
| Basiliximab | 31 (41) | 51 (29) | 0.08 |
| Mean Tacrolimus levels, ug/L (sd) | 4.99 (1.1) | 5.59 (1.3) | < 0.05 |
| HbA1c levels, mean (sd) | 5.3 (0.9) | 5.2 (1.1) | 0.2 |
| eGFR at transplant, mL/min (sd) | 57.6 (24.9) | 70.2 (29) | < 0.05 |
| eGFR at discharge, mL/min (sd) | 82.1 (20.9) | 95.3 (20) | < 0.05 |
Maintenance immunosuppression consisted mainly of a combination of tacrolimus and mycophenolate mofetil. Patients in the CRF group had lower mean through levels of tacrolimus (5 ng/mL vs. 5.59 ng/mL, mean difference 0.6, 95% CI 0.26-0.93). No differences in the mean hemoglobin A1c between two groups was identified (5.3% in CRF vs. 5.2% in controls).
On the day of transplant, the eGFR was lower in the CRF cases (57.6 mL/min) than in the controls (70.2 mL/ min) with a mean difference of 12.4 mL/min (95% CI 5.04-20.03). Despite an improvement at the day of discharge, CRF patients continued to have a lower eGFR (82.1 mL/ min) than controls (95.3 mL/min) with a mean difference of 13.2 mL/min (95% CI 7.5-18.9).
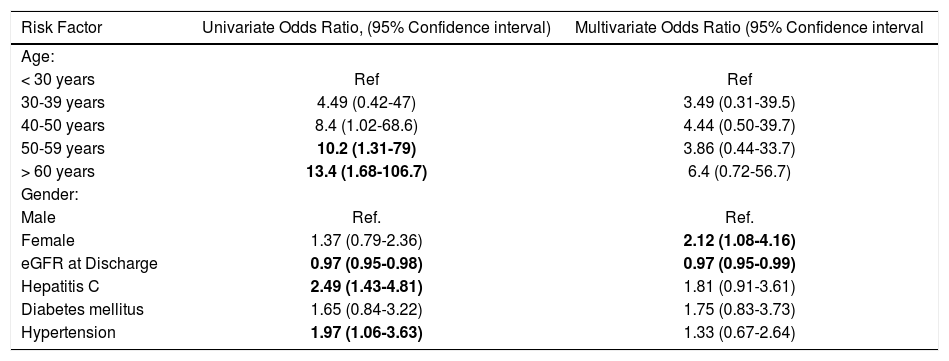
Predictors of CRFWe first performed a univariate analysis to determine predictors of developing CRF from pre-defined potential risks including age, gender, diabetes status, hypertension status, diagnosis of HCV, mean tacrolimus levels an eGFR at the time of discharge. Age was divided in groups (less than 30 years old, 30-39 years old, 40-49 years old, 50-59 years old and more than 60 years old at the time of transplant) and each group was compared to the younger group as a reference. In this univariate analysis, higher tacrolimus levels (OR 0.68, 95% CI 0.54-0.85) and eGFR at discharge (OR 0.97, 95% CI 0.95-0.98) were associated with lower odds for CRF. On the other hand, HCV (OR 2.48, 95% CI 1.43-4.31), hypertension (OR 1.91, 95% CI 1.07-3.63) were associated with an increased odds of developing CRF. The odds of CRF also increased for each age group when compared to the younger group. Gender and diabetes were not associated with increased odds of CRF. The results are summarized in table 2.
Univariate logistic regression analysis of risk factors for post-liver transplantation chronic renal failure.
| Risk Factor | Univariate Odds Ratio, (95% Confidence interval) | Multivariate Odds Ratio (95% Confidence interval |
|---|---|---|
| Age: | ||
| < 30 years | Ref | Ref |
| 30-39 years | 4.49 (0.42-47) | 3.49 (0.31-39.5) |
| 40-50 years | 8.4 (1.02-68.6) | 4.44 (0.50-39.7) |
| 50-59 years | 10.2 (1.31-79) | 3.86 (0.44-33.7) |
| > 60 years | 13.4 (1.68-106.7) | 6.4 (0.72-56.7) |
| Gender: | ||
| Male | Ref. | Ref. |
| Female | 1.37 (0.79-2.36) | 2.12 (1.08-4.16) |
| eGFR at Discharge | 0.97 (0.95-0.98) | 0.97 (0.95-0.99) |
| Hepatitis C | 2.49 (1.43-4.81) | 1.81 (0.91-3.61) |
| Diabetes mellitus | 1.65 (0.84-3.22) | 1.75 (0.83-3.73) |
| Hypertension | 1.97 (1.06-3.63) | 1.33 (0.67-2.64) |
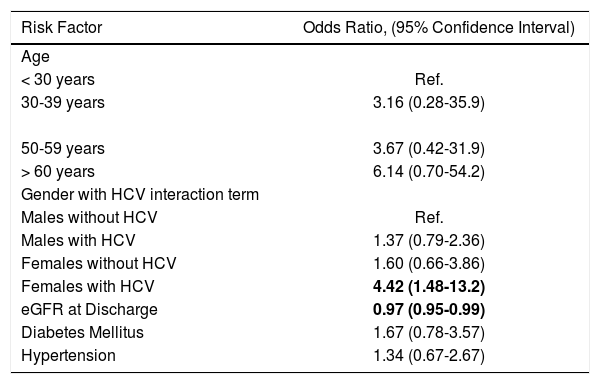
We ran a multivariate analysis using the same variables, except for mean tacrolimus levels and including an interaction term between gender and diagnosis of HCV. In this model, the higher eGFR at discharge was still associated with a decreased odds of CRF (0.97, 95% CI 0.95-0.98). Interestingly, while the effect remained stable on most variables, we noted a change in the strength and direction of the effect for HCV and gender suggesting an interaction between the two variables or effect modification (Table 2). There was no difference in the eGFR at discharge between men and women (91.6 vs. 91.0 mL/min respectively) or HCV infected an non-infected patients (88.1 vs. 93.4 mL/min, difference 5.3; 95% CI -0.04-10.71). Therefore, we proceeded to a repeat analysis introducing an interaction term between gender and HCV infection. When compared to males without HCV infection, males with HCV infection (OR 1.4, 95% CI 0.6-3.31) and females without HCV infection (OR 1.6, 95% CI 0.66-3.86) had an increased, but statistically not-significant odds of developing CRF. However, females with HCV infection, compared to males without HCV infection had a higher odds of CRF (OR 4.42, 95% CI-1.48-13.2). The expected joint effect of HCV and gender in a multiplicative scale was 2.25 (1.6 multiplied by 1.4), therefore at 4.42 the interaction between gender and HCV was supermultiplicative. In this model, increasing age groups, diabetes and hypertension were not statistically significant. The results are summarized in table 3.
Multivariate logistic regression of factors associated with CRF, including an interaction term between gender and hepatitis C infection.
| Risk Factor | Odds Ratio, (95% Confidence Interval) |
|---|---|
| Age | |
| < 30 years | Ref. |
| 30-39 years | 3.16 (0.28-35.9) |
| 50-59 years | 3.67 (0.42-31.9) |
| > 60 years | 6.14 (0.70-54.2) |
| Gender with HCV interaction term | |
| Males without HCV | Ref. |
| Males with HCV | 1.37 (0.79-2.36) |
| Females without HCV | 1.60 (0.66-3.86) |
| Females with HCV | 4.42 (1.48-13.2) |
| eGFR at Discharge | 0.97 (0.95-0.99) |
| Diabetes Mellitus | 1.67 (0.78-3.57) |
| Hypertension | 1.34 (0.67-2.67) |
In this cohort of LT recipients in a major Canadian city, we found that gender, lower eGFR at discharge, and HCV infection were risk factors for developing post-LT CRF. To our knowledge, this is the first study to report a multiplicative effect of female gender and HCV for post-LT CRF.
The risk factors we found in our study were comparable to previous studies. Ojo and colleagues studied nearly 70,000 patients with nonrenal transplantation of which 36,489 received a LT. These investigators found that age, female gender, HCV, pre-LT HTN, and pre-LT DM were risk factors for the development of CRF.2 Similarly, a Swiss study of 406 patients with LT found that HCV and pre-LT CR were independent risk factors for CRF7while an Australian study of 130 patients with LT showed that HCV, female gender, and pre-LT DM were risk factors.8 A recent German study by Weismuller and colleagues proposed a “pocket guide” to identify patients at risk of CRF post-LT.11 They demonstrated that pre-LT eGFR, age, pre-LT DM, HCV, and PSC as risk factors.
In our study, we did not find that presence of hypertension or diabetes were risk factors as other studies when adjusted for age, gender, liver disease and eGFR at the time of discharge. Diabetes and hypertension were defined in this study as the use of any medication to treat these conditions. It is possible that this definition may not have been enough sensitive leading to misclassification of exposure. We did look at average HbA1c levels during follow-up and we did not find a difference in average levels between.
In the univariate analysis, mean tacrolimus levels were associated with risk of CRF. Patients with CRF had lower average tacrolimus levels during the length of follow-up. This likely reflects that patients in which a declining kidney function was observed were given lower doses of tac-rolimus to preserve their kidney function and not due to a protective effect of tacrolimus. For this reason, we did not include tacrolimus levels in our final multivariate model.
Interestingly, we found a multiplicative effect of female gender and HCV on post-LT CRF. Female patients with HCV had higher odds of developing CRF than males without HCV, females without HCV and males with HCV. The observed combined effect was greater than the individual effects of gender and hepatitis C. This was not explained by a lower eGFR in women with HCV or by differences in kidney function or MELD score in women within our cohort at the time of transplant. The exact mechanism for this is unclear. It has been suggested that renal injury due to HCV is related to deposition of immune complexes containing HCV proteins, anti-HCV antibodies, and rheumatoid factor immunoglobulin M with anti-immunoglobulin G activity.12 Perhaps, gender plays a role in the interaction of these factors. Further studies would be necessary to examine this relationship.
The relevance of our study may be challenged in the era of highly effective therapy for HCV. However, in our study we did not assess the association of fibrosis stage on the progression of CKD. It is possible, however, that the observed findings are reflective of the presence of HCV, irrespectively of the severity of the fibrosis especially given that few, if any, of our patients would have had decom-pensated cirrhosis that is often associated with renal dysfunction (ie. acute kidney injury, hepatorenal syndrome etc.). The significance of renal dysfunction secondary to HCV post transplant is potentially clinically important as the traditional surrogate markers for the initiation of treatment are liver fibrosis and not renal function. Access to DAA remains challenging and many programs (and third party payers), including ours, will not reimburse these treatments unless some degree of fibrosis is present given the high acquisition costs of the current antiviral drugs. Our findings, suggest that HCV post-transplant affects renal function, especially in women, and therefore may be a clinical indication for anti-HCV therapy with the expensive new agents. Indeed, a recent national Canadian post-liver transplant study has reported that renal function improved in the majority of patients who successfully eradicated HCV with the new direct acting antiviral agents.13 Therefore, hypothetically, women with HCV and F1 fibrosis may not be treated and still have the increased odds of progression of CKD, an unfortunate clinical circumstance that is potentially avoidable. It is therefore important to recognize this possibility and adapt target levels of CNI to minimize risk and consider early antiviral therapy.
A strength of this study was the relatively large sample study of Canadian patients. Cases and controls were taken from the same study base and selection bias was limited. There were no patients excluded from the study because of missing data. We used a pre-defined set of variables in our analysis to minimize the risk of spurious findings from over-testing. We were able to demonstrate a multiplicative effect of gender and HCV that, to our knowledge, has not been previously reported.
Our study has several limitations that should be noted. It is a retrospective case-cohort study and as such we can only show association and not causation. Our study does not provide an a priori hypothesis for a biological mechanism to explain our findings. However, like all clinical studies that investigate associations between disease entities and clinical variables, our study was not designed to provide a definitive pathophysiologic answer and further future studies, based on our hypothesis generation, will be needed. We do note that other database studies have hinted at similar associations also without providing a biological mechanism. This suggests to us that our findings are not spurious. The majority of our patients were on a cal-cineurin inhibitor, thus it is unknown if the same effect will be observed in patients under a different imunosu-pression regimen. We did not have data on eradication of HCV after transplant and it is therefore possible that patients continued to be labeled as HCV when in fact they were no longer infected, introducing information bias from misclassification of exposure. However, the study period is set at an era where only a small minority of patients were able to achieve post-transplant sustained viral response because effective medications were lacking (ie. interferon-free direct acting antiviral agents). Finally, it is possible that the observed effect is a spurious finding although given the strength of the association, we feel that this is less likely.
The results of this study should be validated with larger cohorts and if confirmed a search for a biological explanation should follow. Minimization of CNI doses in at-risk individuals would preserve renal function, improve graft survival, and decrease mortality. Our findings do suggest that patients with HCV infection post-liver transplant, especially females, should be given consideration for antiviral treatment, regardless of liver fibrosis score, that is the traditional determinant of the need for therapy.
ConclusionsIn this cohort of LT recipients of a major Canadian city, age, eGFR, and HCV infection were risk factors for cal-cineurin inhibitor related CRF. Female gender and HCV increased these odds by a factor of more than 4.