The natural history of intrapulmonary vascular dilations (IPVD) and their impact on patient outcomes in the setting of portal hypertension has only been described in small series.
AimsTo assess the development of hepatopulmonary syndrome (HPS) in patients with isolated IPVD and to evaluate outcomes of IPVD and HPS among patients evaluated for liver transplantation (LT).
Material and methodsData from a prospective cohort of patients evaluated for LT with standardized screening for HPS were analyzed. IPVDs were defined as the presence of microbubbles in the left atrium > 3 cycles following right atrial opacification. HPS was defined as the presence of IPVD and hypoxemia (Alveolar-arterial gradient ≥ 15 mmHg) in the absence of concomitant cardiopulmonary disease.
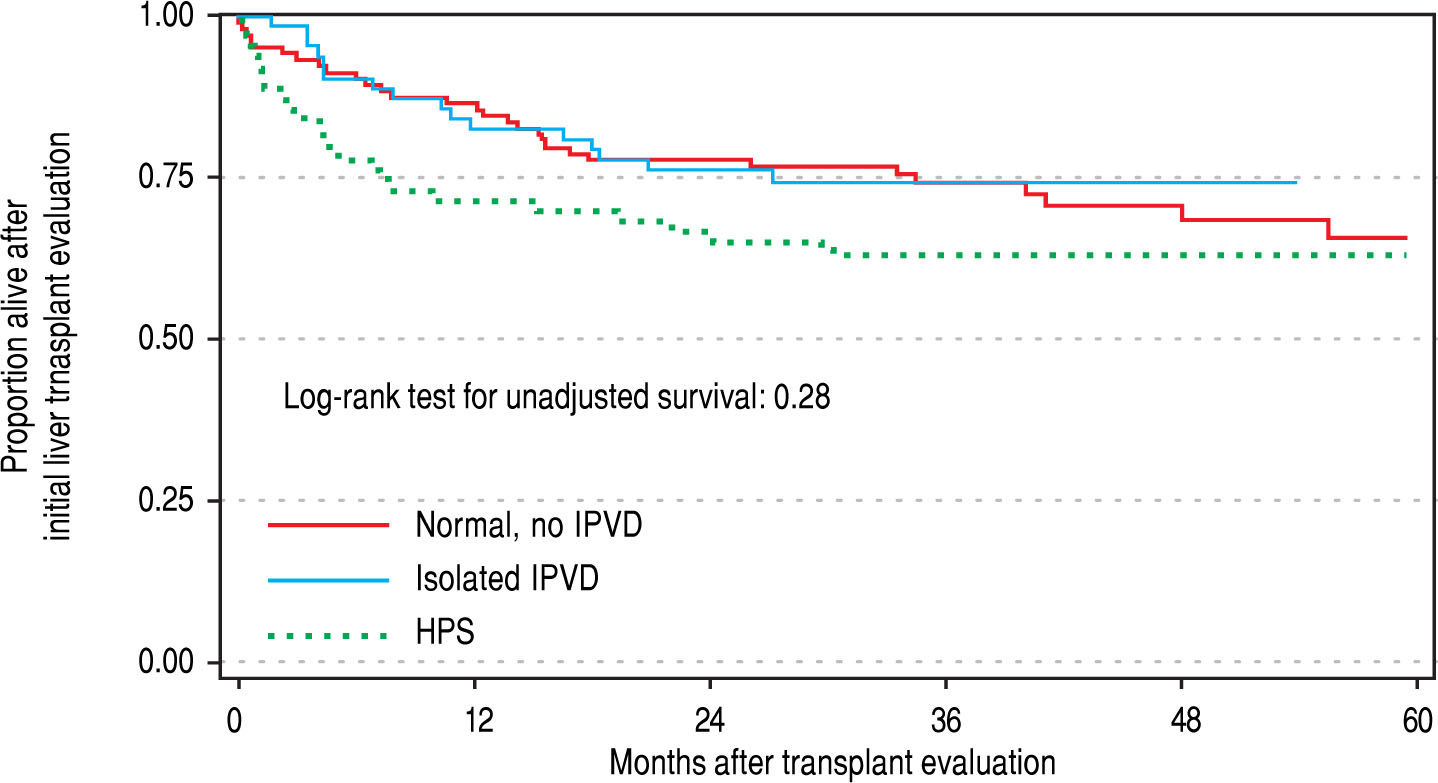
ResultsA total of 104 patients with negative contrast-enhanced echocardiogram (CE) were compared to 63 patients with IPVD and 63 patients with HPS. Only four patients were categorized as ‘severe’ HPS based on degree of hypoxemia (defined as PaO2 < 60 mmHg). Twenty IPVD patients were followed with ABG over a mean duration of 21 months (range 9-43), of whom 7 (35%) subsequently met HPS criteria. Overall unadjusted survival from the time of LT evaluation using multistate survival models that accounted for pre- and post-LT time was not statistically different among the three groups (negative CE, IPVD, and HPS; p > 0.5).
ConclusionsPatients with IPVD appear to have a substantial risk of developing oxygenation impairment over time and progress to HPS. In our cohort, survival in patients with HPS and isolated IPVD is not different when compared to those without IPVDs.
Hepatopulmonary syndrome (HPS) is the triad of abnormal systemic oxygenation due to intrapulmonary vascular dilatations (IPVD) in the setting of liver disease or portal hypertension.1 The reported prevalence of HPS in liver transplant candidates is approximately 10% to 32%.2-4 HPS is associated with poor quality of life and increased mortality.3,5 Currently, liver transplantation (LT) is the only established treatment for HPS.
The presence of IPVD in liver transplant candidates, defined by positive contrast-enhanced transthoracic echocardiography (CE) and normal oxygenation, is common (40-50%).6 However, the natural history and impact on outcome of IPVD is poorly characterized, and it is unknown whether hypoxemia can develop and progress over time to severe HPS. Pulse oximetry (SpO2) is one tool to estimate changes in oxygenation over time in patients with IPVD, but few studies have quantified changes using serial arterial blood gases (ABG).7
The objective of our study was to assess oxygenation over time in patients with IPVD. Furthermore, because most of the data on HPS is based on North American and European cohorts, we sought to evaluate survival in patients with IPVD and HPS in a well-characterized Argentinian cohort of patients with portal hypertension or cirrhosis referred to our clinic for liver transplant evaluation.
Material and MethodsStudy designWe conducted a prospective cohort study of consecutive patients with cirrhosis or severe portal hypertension who were referred for further management and LT evaluation at the outpatient clinic of the Hepatology and Liver Transplant Unit at the Hospital Universitario Austral in Buenos Aires, between January 1, 2009 and December 31, 2012 were included. All procedures followed were in accordance with STROBE guidelines for cohort studies and complied with the ethical standards of the responsible committee on human experimentation and with the Helsinski Declaration 1975, as revised in 2008.8 The Institutional Review Board at the Hospital Universitario Austral approved the study.
ParticipantsEligibility criteria consisted of patients ≥ 18 years with cirrhotic or non-cirrhotic portal hypertension referred to our clinic for liver transplant consideration. Cirrhosis and portal hypertension were defined histologically or by a combination of clinical, laboratory, radiologic and upper endoscopy findings. Patients with acute liver failure, intracardiac shunting (appearance of microbubbles in the left heart < 3 cardiac cycles after venous injection), intrinsic cardiopulmonary disease and/or with pleural effusions > 20% of the pleural space were excluded from the analysis. Cardiopulmonary disease as a rule-out diagnosis for HPS was up to the discretion of the investigators, which is aligned with expert consensus opinion for diagnosing HPS.9
Population DefinitionHPS was Defined by (1) CE with late appearance of microbubbles after venous injection of agitated saline, (2) an alveolar-arterial oxygen gradient (A-a) value ≥ 15 mmHg (or ≥ 20 mmHg if age older than 64 years), as previously recommended.1,10 Severity of HPS was classified according to the following classes: mild (PaO2 ≥ 80 mmHg), moderate (PaO2 < 80 and ≥ 60 mmHg) and severe (PaO2 < 60 mmHg). Patients who presented positive CE, an A-a value < 15 mmHg and PaO2 > 80 mmHg were considered to be in the IPVD group. Subjects with negative CE and normal oxygenation were included as control group.
Clinical variable assessment and definitionsClinical, demographic and laboratory data were prospectively recorded. Severity of liver disease was measured based on the Child-Pugh class and Model for End-Stage Liver Disease (MELD) score. Basal and follow up ABG measurements were performed while the subject was breathing ambient room air in the seated position. The samples were analyzed immediately using a gasometer (Cobas B 221, Hoffman-La Roche), and the A-a gradient was calculated using the standard formula A-a,
PO2 = [(BP-47) FIO2 - PaCO2/0.8] - PaO2
Where BP is the barometric pressure, FIO2 is the fraction of inspired oxygen, PaCO2 is the partial pressure of arterial carbon dioxide and PaO2 is the partial pressure of arterial oxygen.11 Starting 9 months after the initial clinic evaluation, patients with IPVD underwent serial ABG measurements. We considered this an adequate interval given that yearly interval ABG evaluation of HPS patients has been recommended to assess worsening of oxygenation.12 Oximetry was not evaluated during periods of acute decompensation, including those requiring hospitalization. Clinic staff blinded to the study obtained SpO2 measurements during the initial clinic evaluation using a pulse oximeter. Survival and LT status were prospectively recorded in our database. Time-to-event was measured from the date of transplant evaluation, and patients were followed until death, end of follow-up, or date a patient was lost to follow-up. Death was the primary outcome, and all other patients were censored at the last date of follow-up.
Contrast echocardiographyAll subjects underwent CE which was performed by two experienced cardiologists at our hospital who were blinded from the ABG results and clinical data. A 10-mL agitated saline solution was injected via a peripheral vein during CE. The solution was injected via an 18G catheter into a peripheral vein in an upper limb. An apical four-chamber view with a 3.5 MHz transducer in a Vivid 7 echocardiograph (General Electric, Connecticut, United States) was used to detect the microbubbles. Appearance of microbubbles in the left heart ≥ 3 cardiac cycles after saline injection was considered consistent with intrapulmonary shunting.
Pulmonary function testsSpirometry was performed in all the patients, and forced vital capacity (FVC), forced expiratory volume in 1s (FEV1) and the FEV1/FVC ratio were recorded. The spirometric studies were performed using a pneumotachograph spirometer (SensorMedics; Vmax series, USA). The patient was considered to have obstructive lung disease when the FEV1/FVC ratio was < 70% or when the FEV1 value was < 80% of the reference value. Restrictive lung disease was considered when the FVC value was < 80% of the reference value.
Statistical analysisCategorical data are presented in frequencies and percentages and were compared using Fisher's exact test (2-tailed) or Chi-Square (χ2) test, as appropriate. Continuous variables are presented in mean ± standard deviation or median and interquartile ranges (IQR) according to their distribution and were compared with Student's T test or Mann-Whitney U test, respectively. Correlations were obtained by using either the Pearson or Spearman tests as appropriate. We fit multivariable Cox regression models to compare overall patient survival based on their HPS status (control vs. IPVD vs. HPS) and hazard ratios with the corresponding 95% confidence intervals (95% CI) were calculated. The multivariate models included adjustments for age, gender, MELD and Child-Pugh scores at first evaluation, blood type and liver disease etiology, as well as waitlisting status and transplant status in multistate models. To account for the impact of transplantation on survival, transplantation (binary yes/no) was considered a time-varying covariate in survival models. For all comparisons, 2-tailed statistical significance was defined as a P value < 0.05. Statistical analysis was carried out using Stata 14.0.
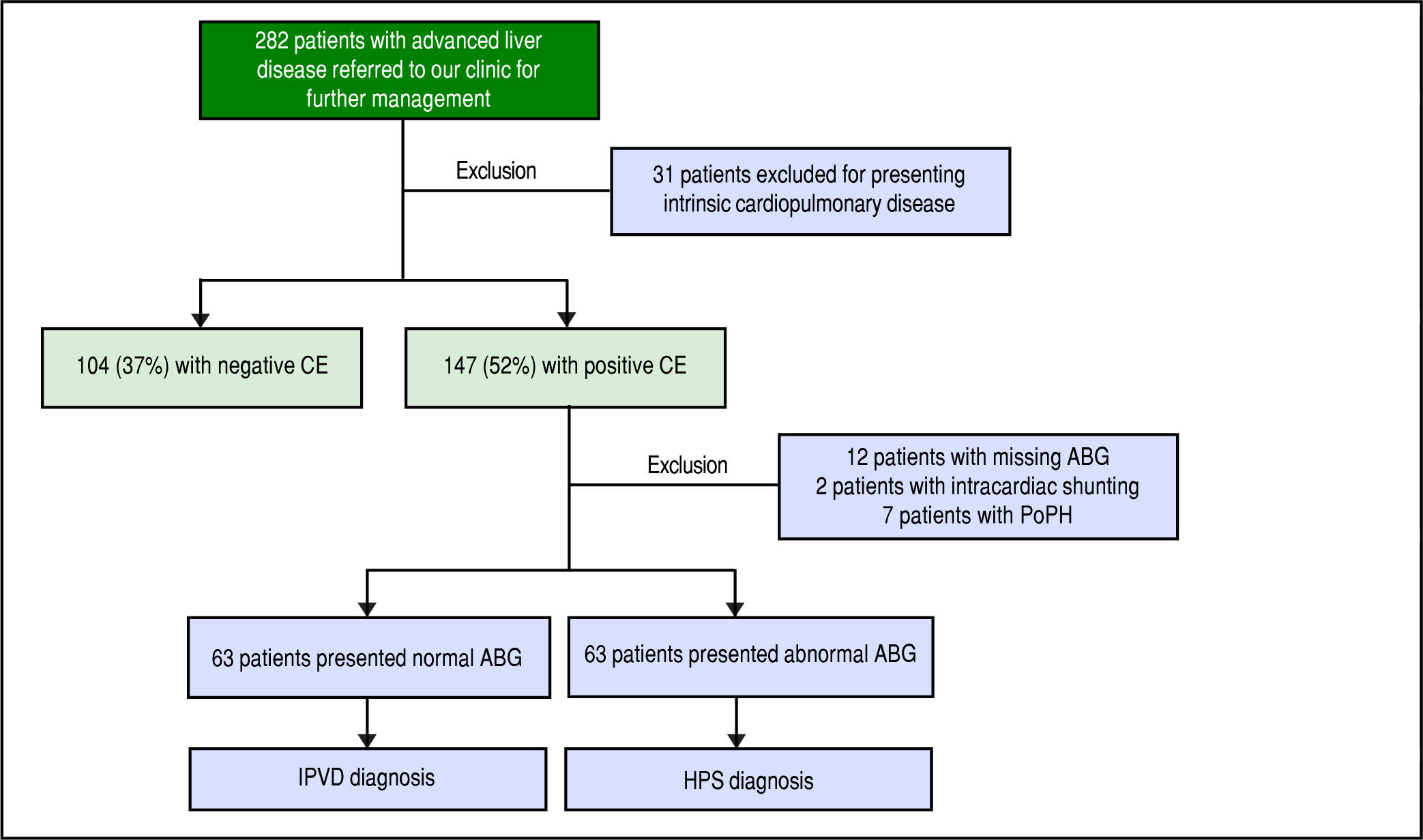
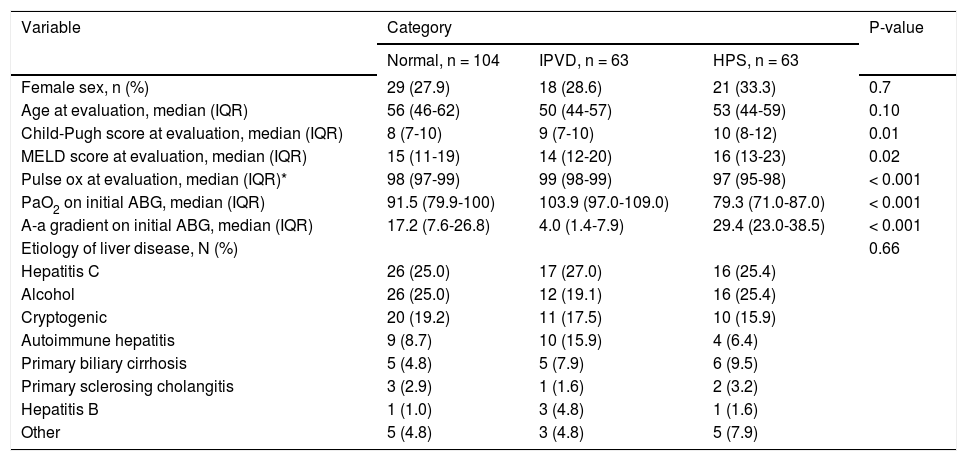
ResultsBaseline characteristicsA total of 282 patients were referred to our clinic for management of advanced liver disease during the study period. Thirty-one patients were excluded for intrinsic cardiopulmonary disease. After performing a CE, 2 patients with intracardiac shunting and 7 patients with portopulmonary hypertension were excluded. Twelve patients with positive CE were excluded because the results of the ABG were missing (Figure 1Figure 1). There were 63 (23%) patients with HPS, 63 (23%) patients with IPVD and 104 (37%) patients with negative CE (normals; Table 1Table 1). There were no clinically significant differences in the demographics or etiology of liver disease between the three groups, but patients with HPS presented more advanced liver disease at baseline (Table 1). Consistent with the diagnoses, patients with HPS had the lowest PaO2 values and highest A-a gradients, but unexpectedly patients without IPVDs presented abnormal A-a gradient as well (Table 1).
Baseline demographics and clinical characteristics.
| Variable | Category | P-value | ||
|---|---|---|---|---|
| Normal, n = 104 | IPVD, n = 63 | HPS, n = 63 | ||
| Female sex, n (%) | 29 (27.9) | 18 (28.6) | 21 (33.3) | 0.7 |
| Age at evaluation, median (IQR) | 56 (46-62) | 50 (44-57) | 53 (44-59) | 0.10 |
| Child-Pugh score at evaluation, median (IQR) | 8 (7-10) | 9 (7-10) | 10 (8-12) | 0.01 |
| MELD score at evaluation, median (IQR) | 15 (11-19) | 14 (12-20) | 16 (13-23) | 0.02 |
| Pulse ox at evaluation, median (IQR)* | 98 (97-99) | 99 (98-99) | 97 (95-98) | < 0.001 |
| PaO2 on initial ABG, median (IQR) | 91.5 (79.9-100) | 103.9 (97.0-109.0) | 79.3 (71.0-87.0) | < 0.001 |
| A-a gradient on initial ABG, median (IQR) | 17.2 (7.6-26.8) | 4.0 (1.4-7.9) | 29.4 (23.0-38.5) | < 0.001 |
| Etiology of liver disease, N (%) | 0.66 | |||
| Hepatitis C | 26 (25.0) | 17 (27.0) | 16 (25.4) | |
| Alcohol | 26 (25.0) | 12 (19.1) | 16 (25.4) | |
| Cryptogenic | 20 (19.2) | 11 (17.5) | 10 (15.9) | |
| Autoimmune hepatitis | 9 (8.7) | 10 (15.9) | 4 (6.4) | |
| Primary biliary cirrhosis | 5 (4.8) | 5 (7.9) | 6 (9.5) | |
| Primary sclerosing cholangitis | 3 (2.9) | 1 (1.6) | 2 (3.2) | |
| Hepatitis B | 1 (1.0) | 3 (4.8) | 1 (1.6) | |
| Other | 5 (4.8) | 3 (4.8) | 5 (7.9) | |
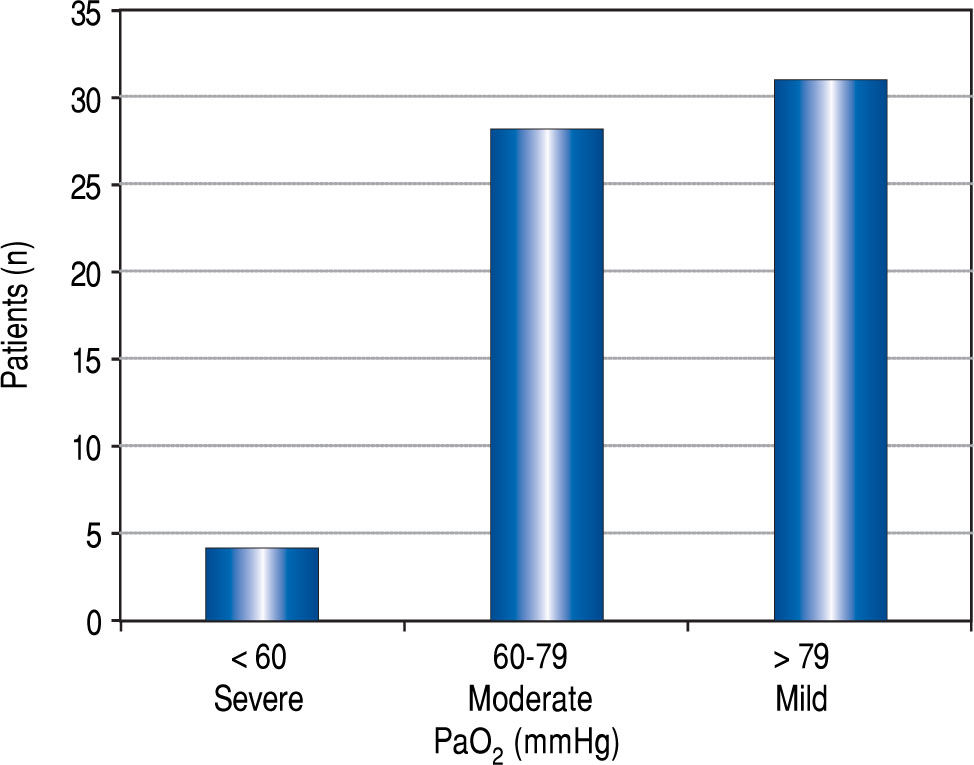
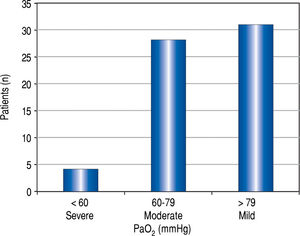
Figure 2Figure 2 demonstrates the distribution of PaO2 values in patients with HPS. Four of 63 (6%) patients with HPS had a PaO2 <60 mmHg, and three were granted HPS MELD exception points. The fourth patient with a PaO2 died before concluding transplant evaluation. Almost half of the HPS patients (n = 31, 49%) were categorized as having mild HPS.
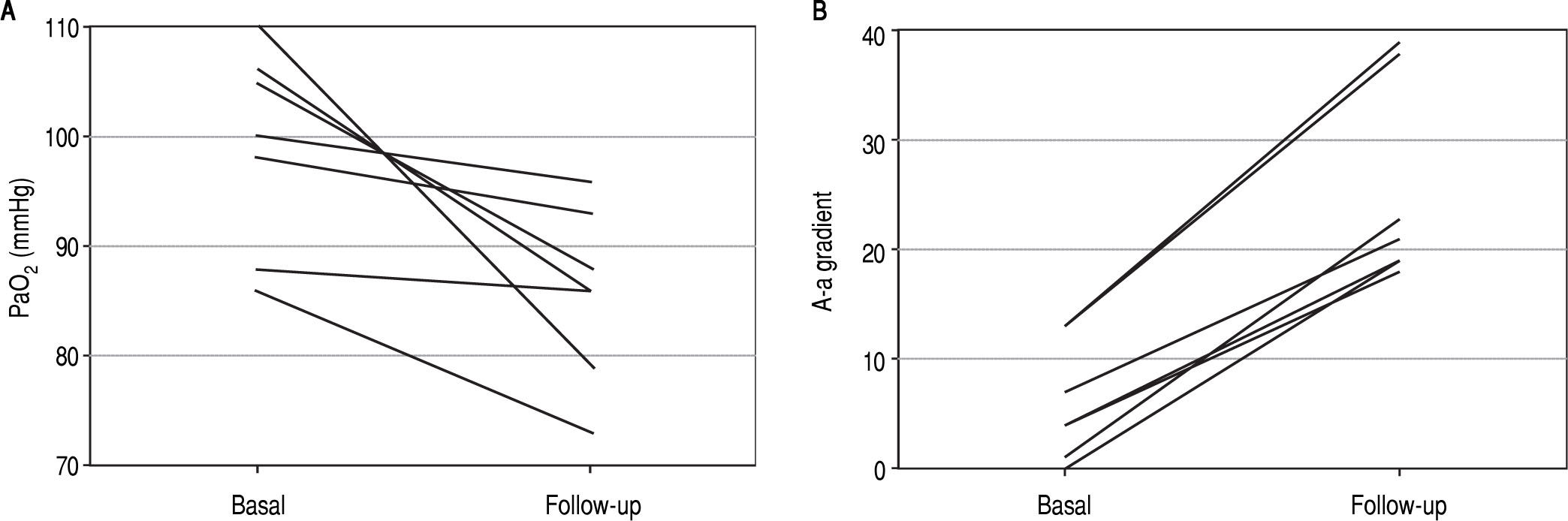
Serial oxygenation values over time in patients with intrapulmonary vascular dilatationsA total of 20 out of 63 patients with IPVD had at least two ABG measurements with a median follow up of 18 months (range 9-50 months). The rest of the IPVD patients were not followed over time with ABG measurements because they died on the waiting list or underwent LT before having their second ABG assessment. Seven patients developed hypoxemia (35%) and fulfilled criteria for HPS, five were categorized as mild, two as moderate (Figure 3Figure 3).
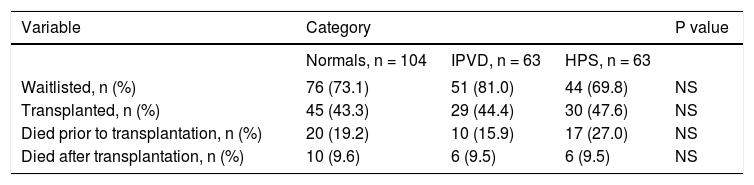
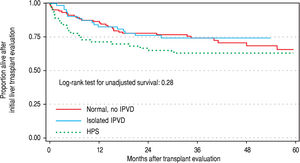
Patient outcomesThere were no significant differences in the three groups with respect to the probability of being waitlisted for LT or receiving a transplant. Wait listing times among the three groups were 287, 226 and 127 days for normals, IPVD and HPS patients, respectively. In multivariable Cox regression models that adjusted for age, gender, etiology and MELD score, the pre- and post-LT mortality was not significantly different between HPS, IPVD and normal patient groups (Table 2Table 2; Figure 4Figure 4).
Outcomes of patients with advanced liver disease included in our study.
| Variable | Category | P value | ||
|---|---|---|---|---|
| Normals, n = 104 | IPVD, n = 63 | HPS, n = 63 | ||
| Waitlisted, n (%) | 76 (73.1) | 51 (81.0) | 44 (69.8) | NS |
| Transplanted, n (%) | 45 (43.3) | 29 (44.4) | 30 (47.6) | NS |
| Died prior to transplantation, n (%) | 20 (19.2) | 10 (15.9) | 17 (27.0) | NS |
| Died after transplantation, n (%) | 10 (9.6) | 6 (9.5) | 6 (9.5) | NS |
To our knowledge, this is the first study to assess oxygenation over time solely in patients with IPVD. We found no significant differences in overall survival from the time of initial transplant evaluation between patients with IPVD, HPS and control, which stand in contrast to previously published reports from the Pulmonary Vascular Complications of Liver Disease (PVCLD) study group.3 The differences in survival may simply be attributable to a low prevalence of severe HPS in our region, but could also be due to other factors due to differences in the timing of transplant evaluation in our cohort, differences in transplant rates, or other factors related to the care of cirrhotic patients, all of which require further exploration. More importantly however, we were able to evaluate changes in oxygenation over time in patients with IPVD, and in the subset with serial ABGs, found that 35% (7/20) developed hypoxemia and progressed to mild or moderate HPS. These findings suggest that natural history of IPVD may involve a decline in oxygenation over time and further development of HPS.
The HPS prevalence data in our cohort is consistent with prior studies, with 23% of our patients meeting formal diagnostic criteria for HPS.2,3,10 As aforementioned, we did not see worse overall survival in patients with HPS, which may be explained by the low proportion of patients with severe hypoxemia included in our series, specifically with only 4 of 63 patients developing PaO2 < 60 mmHg during the study period. These findings are similar to a prior study whereby only 2 out of 22 patients with HPS presented with severe hypoxemia, and did not have worse survival compared with non-HPS patients.6 Our findings may reflect different genetics of our patient population, as Roberts, et al. has identified genes involved in the regulation of angiogenesis associated with increased risk of developing HPS.14 MELD score was implemented as the organ allocation system in Argentina in 2005, since then, only 42 patients over 4,857 listed were granted MELD exception points for HPS (unpublished data: Instituto Nacional Central Único Coordinador de Ablación e Implante [INCUCAI]). This data can suggest a very low prevalence of HPS in our region, low access to liver transplant specialists or low screening of HPS in some transplant centers. Future studies of Latin American populations are needed to confirm our results and evaluate whether there may be different genetics at play in this population.
A surprising finding in our series was that patients without IPVDs, and thus without HPS or other identifiable cardiopulmonary diseases, presented with an elevated A-a gradient (≥ 15). This finding may reflect undiagnosed cardiopulmonary disease despite negative testing (i.e., pulmonary function tests), HPS that could not be detected by echocardiography, and/or false-positive ABGs (falsely elevated A-a gradient due to specimen handling and/or processing). We also described similar clinical outcomes among patients with IPVD or HPS as previously reported. However, the question whether IPVD is a “pre-HPS state” has not been completely answered. In patients with HPS, a 12 month interval to assess worsening of oxygenation with ABG has been recommended.12,16 In our cohort, we found that 35% of patients with serial ABG measures subsequently developed HPS. In this line, Gupta, et al. also followed patients with IPVDs and HPS with at least two ABGs describing a large temporal variability in oxygenation, ranging from a drop to an increase in PaO2 over 1 year follow up.7 These results and ours provide evidence based data to support current recommendations to follow oxygenation in patients with IPVD.
Our study does have limitations. First, it is a single center study, which may limit the generalizability to other regions. Second, the sample size for these analyses was small, however; this is the first study to exclusively evaluate patients with isolated IPVD. Finally, ABG measurements were not performed at standardized time intervals and the follow-up period was variable due to patients being referred from all over the country.
In summary, HPS and IPVD are common complications in patients with portal hypertension who are candidates for LT. In our population, the majority of the HPS cases were mild or moderate. Patients with IPVD can evolve with PaO2 deterioration. However, over the time frame studied this did not influence outcome. Future prospective studies with longer follow-up will be necessary to confirm these findings.
Abbreviations- •
A-a: alveolar-arterial oxygen gradient.
- •
ABG: arterial blood gas.
- •
AUC: area under the curve.
- •
CE: contrast-enhanced transthoracic echocardiography.
- •
HPS: hepatopulmonary syndrome.
- •
IPVD: intrapulmonary vascular dilatations.
- •
MELD: Model for End-Stage Liver Disease.
- •
LT: liver transplantation.
- •
PaO2: partial pressure of oxygen.
- •
ROC: receiving operating characteristic.
- •
SpO2: pulse oximetry.