Currently, there is no standardized treatment regimen for non-alcoholic steatohepatitis.
AimWe performed a meta-analysis of high quality randomized controlled trials that evaluated treatment response to metformin, thiazolidinediones (TZDs), and vitamin E in adult patients with non-alcoholic steatohepatitis. Outcome measures were improvement in liver histology, biochemical, and anthropometric measures.
Material and methodsNine trials met inclusion criteria (3 with TZD, 3 with Metformin, 2 with Vitamin E and 1 with both TZD and Vitamin E.).
ResultsWith metformin, weighted liver histologic scores for steatosis, ballooning, and fibrosis did not demonstrate significant improvement and lobular inflammation worsened significantly (weighted mean increase 0.21, 95% CI 0.11 to 0.31, P < 0.0001). The liver histology score including steatosis (OR 3.51, 95% CI 2.14 to 5.78) and lobular inflammation (OR 2.65, 95% CI 1.69 to 4.15) improved with TZDs. Hepatic fibrosis (OR 1.58, 95% CI 0.98 to 2.54) and ballooning scores (OR 1.84, 95% CI 0.94 to 3.58) did not demonstrate significant improvement. With Vitamin E, weighted liver histologic scores for steatosis (weighted mean decrease -0.60, 95% CI -0.85 to -0.35, P < 0.0001), lobular inflammation (weighted mean decrease -0.40, 95% CI -0.61 to -0.20, P = 0.0001) and ballooning (weighted mean decrease -0.30, 95% CI -0.54 to -0.07, P = 0.01) demonstrated significant improvement compared to placebo. Fibrosis did not significantly change.
ConclusionIn patients with NASH, TZDs and Vitamin E improve liver histologic scores but metformin does not. Insulin resistance also improves with both TZDs and metformin. Fibrosis does not improve with any of the agents.
Non-alcoholic fatty liver disease (NAFLD) encompasses a spectrum of disease ranging from steatosis to steatohepatitis and cirrhosis. It is considered the most common cause of elevated liver enzymes with an estimated twenty percent of the population affected worldwide.1-4 Non-alcoholic steatohepatitis (NASH) refers to a subset of patients with NAFLD who demonstrate histological characteristics of hepatic steatosis, lobular inflammation and hepatocellular ballooning with progression to cirrho-sis estimated in up to fifteen percent of patients within a ten year period.1,2,5 In comparison to matched controls patients with either NAFLD or NASH have an increased mortality rate likely secondary to the prevalence of cardiovascular disease present in this population.1,2 Also, NASH cirrhosis has been associated with the development of hepatocellular carcinoma.7 Given the increasing incidence of NAFLD and the long-term consequences of this disease it is important to identify the risk factors and therapeutic measures, which can help curtail the progression of this aggressive illness.
The pathogenesis of NAFLD is not well understood, however, insulin resistance and metabolic syndrome have been associated with NAFLD.8 Also, lipid peroxidation and its’ byproducts have been found to be elevated in NASH patients and correlate directly to increasing necroinflammatory activity and fibrosis.9 Consequently, drugs that counteract these specific mechanisms have been used to facilitate the reduction of inflammation and fibrosis found in the liver of patients afflicted with NAFLD and NASH. Currently, there is no defined regimen for the treatment of NAFLD though many treatments have been purported.
Studies involving thiazolidinediones (TZDs), metformin, and antioxidants have been shown to improve biochemical parameters, glucose, and lipid metabolism.8,10-13 On the other hand, histological improvement with the same treatment measures has been difficult to interpret. A study by Bugianesi, et al. demonstrated metformin decreased the percentage of hepatic steatosis, necroinflammation, and fibrosis in non-diabetic patients, whereas a pilot study of metformin did not demonstrate a significant difference in any histopathological parameters when compared to placebo.14 Sanyal, et al. demonstrated improvement in lobular inflammation and hepatic steatosis but no significant improvement in fibrosis with pioglitazone or vitamin E.13 However, a placebo controlled trial of pioglitazone in non-diabetic patients’ demonstrated improvement in fibrosis over a twelve-month period.15 A recent meta-analysis by Singh, et al. aimed to compare the effectiveness of pharmacological interventions for NASH.16 However, their analysis excluded the use of metformin and included a Bayesian network analysis which compared agents that have never been tested head to head (Obeticholic acid from a single phase two trial and Pioglitazone). In addition, the pediatric population was also included by Singh, et al. which increases the heterogeneity of the studies used and makes analysis more difficult to compare.
Given the limited data and uncertainty regarding the superiority of any pharmacological agent in patients with NASH, we undertook meta-analysis of randomized place-bo controlled trials that examined metformin, TZDs such as pioglitazone and rosiglitazone, and vitamin E on adult patients with NASH.
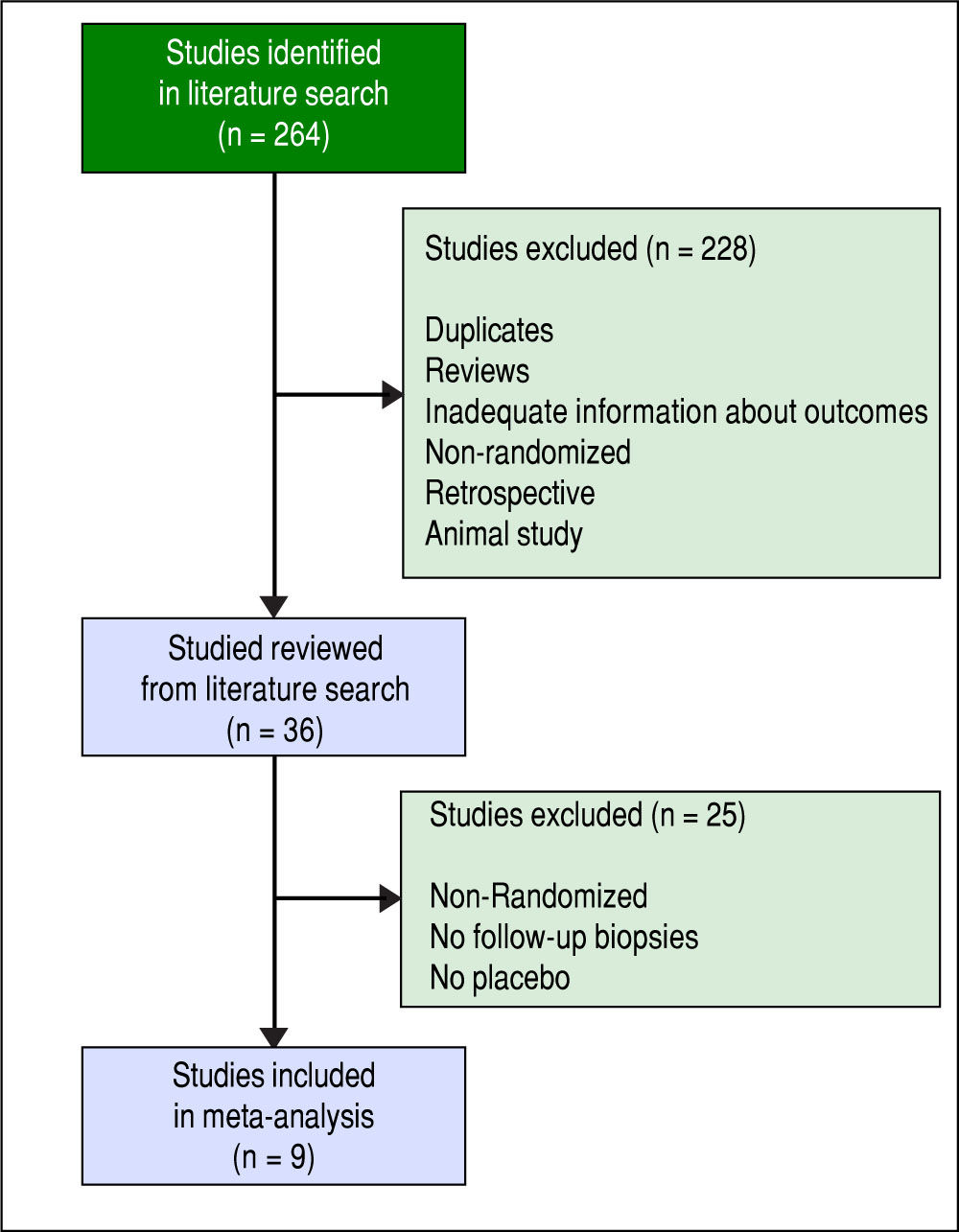
Material and MethodsStudy selectionWe selected studies using the following databases to Dec 31, 2014 (Figure 1): PubMed, Medline, clinicaltrials.gov, Cochrane central register of controlled trials. Key words used were the following: NASH (Non Alcoholic Steatohepatitis), NAFLD (Non Alcoholic Fatty Liver Disease), Rosiglitazone, Pioglitazone, Metformin, Vitamin E, and histology. We included human studies without language restrictions. Bibliographies or original articles were also searched to identify other relevant articles.
Inclusion criteria for meta-analysis were adult randomized placebo controlled trials in patients with NASH and a minimum duration of therapy of at least six months with reportable histology outcomes pre and post-treatment. Patients with diabetes and non-diabetics were included. Although changes in biochemical variables (AST ALT, hyperglycemia) and anthropometric parameters were also analyzed when present, we did not include trials that did not include liver histology outcomes. We did not include trials in children and trials without controls were also excluded. Two investigators independently carried out literature search and reviewed studies for inclusion and exclusion criteria. Data were abstracted independently by two investigators.
Initially, there were thirty-six studies examining the effects of the above pharmacological interventions in patients with NALFD or NASH. As such, only nine total trials fit all inclusion criteria including three trials of TZDs, three of metformin, two of vitamin E and one with both TZD and Vitamin E were included in the analysis.
Outcome measuresOutcome measures were changes in liver histology including steatosis, ballooning, lobular inflammation, fibrosis and NASH activity index using standardized histological criteria for NASH reported in the included studies. Also, evaluation of biochemical and anthropometric measures were examined including alanine aminotransferase (ALT), homeostatic model assessment of insulin resistance (HOMA-IR), fasting blood sugar, Hemoglobin A1c (HbA1c), body mass index (BMI), body weight, total cholesterol, high-density lipoprotein (HDL) and triglycerides.
TZD- histology was expressed in dichotomous format (improvement versus not) with Odds ratio and Confidence intervals calculated for each histologic parameter versus placebo.
Metformin and Vitamin E - Histologic parameters were expressed as continuous variables using weighted mean differences and confidence intervals calculated for each histologic parameter versus placebo/controls. Biopsy specimens in the included studies were assessed using the following scoring systems: Brunt (3), Promrat (2), Kleiner (4).
Quality assessmentWe assessed the methodological quality of the articles using The Cochrane Collaboration's Tool for Assessing Risk of Bias. We also used the Jadad 3 point scale for assessing the quality of each randomized trial.
Meta-analysisA meta-analysis of data from randomized controlled trials was performed using Stats Direct Software. For liver histologic parameters, treatment effects for dichotomous data (improvement in liver histology Yes vs. No) were expressed by Odds ratio and 95% confidence intervals. For liver histologic parameters, treatment effects for continuous data (change in liver histology parameter scores) were expressed by weighted mean differences (and confidence interval) in each histologic parameter.
Random effects models with the Mantel-Haenszel method were used for combining data from trials. Heterogeneity of trials was assessed using the I2 measure of inconsistency and the Cochran Q statistic. Publication bias was assessed using funnel plot analysis and the Egger test.
Continuous variablesFor continuous biochemical and anthropometric data weighted averages were estimated utilizing study means, sample size, and standard deviations. The Fisher exact method was used to combine the individual study P values and calculate an overall P value for comparison of each of these parameters.
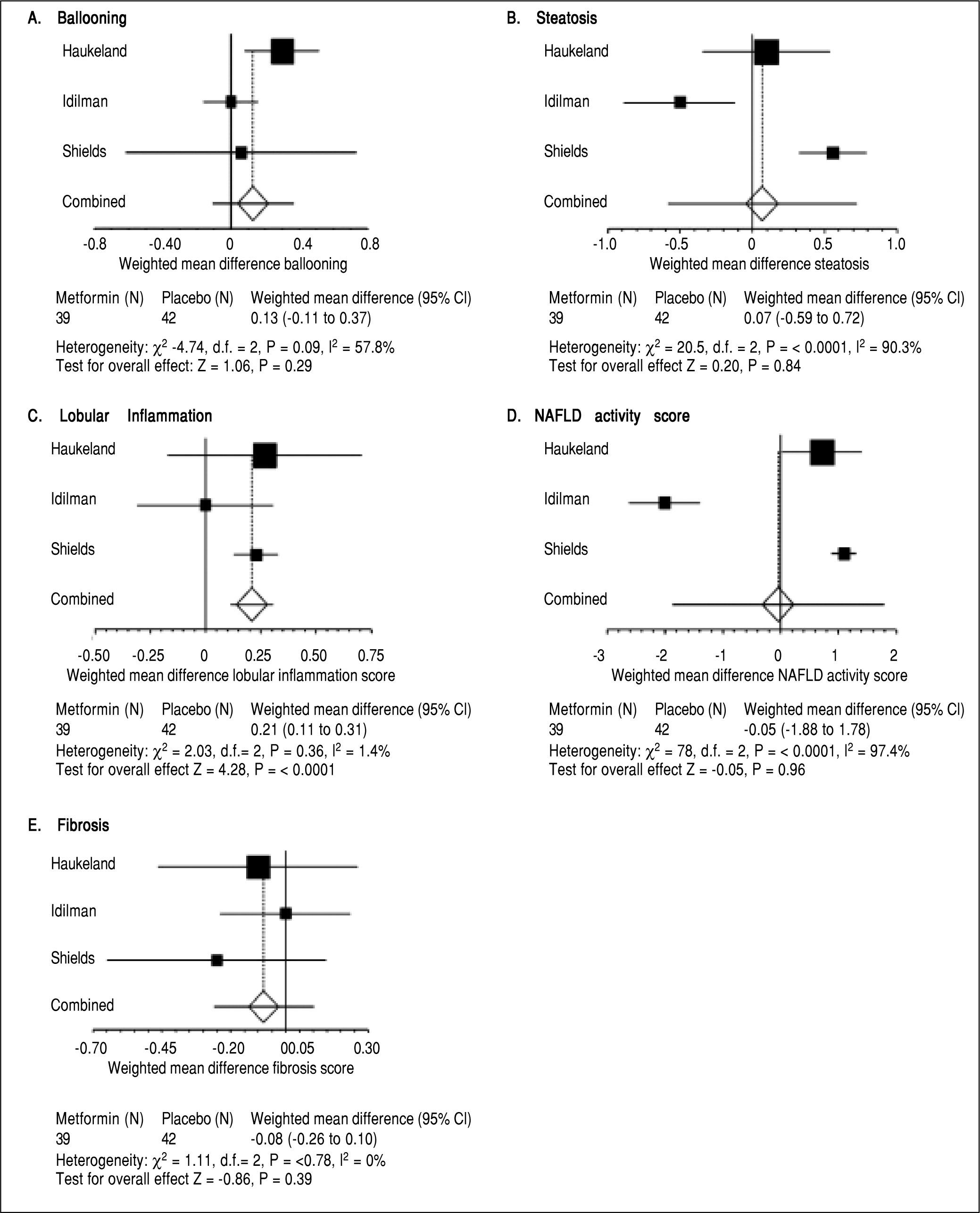
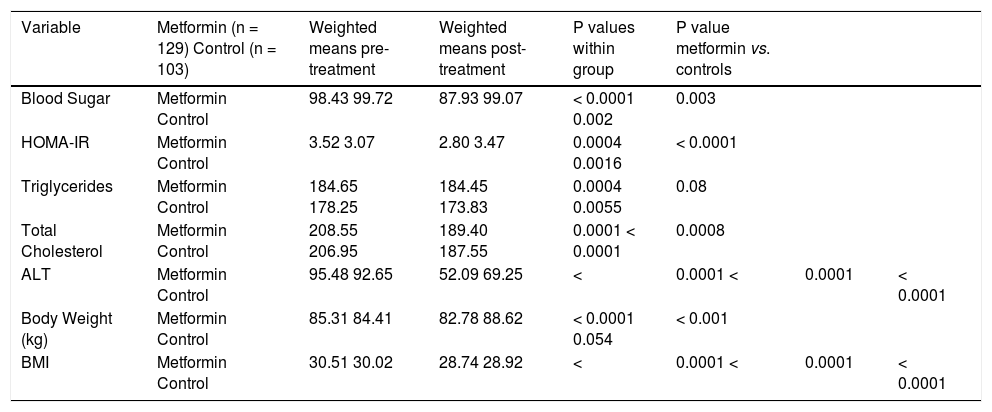
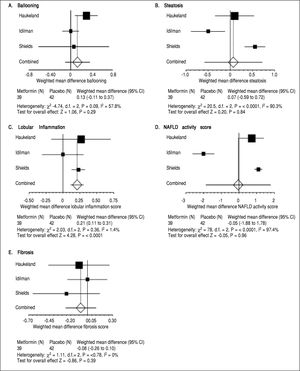
ResultsMetforminThree trials analyzing metformin therapy in patients with NASH were included in our meta-analysis (Table 1) with only Haukeland, et al.17 including a subset of diabetic patients. 81 patients were analyzed in the metformin group with over half (54%) attributable to the Haukeland, et al. study.17 Therapy lasted between six to twelve months. Of note, therapy regimens varied with Metformin dosed between 1,000 mg to 3,000 mg daily. Histological parameters including ballooning, fibrosis, steatosis and NAFLD activity score (NAS) did not significantly change with metformin therapy (Figure 2). Lobular inflammation significantly worsened after therapy (Figure 2) (weighted mean increase 0.21, 95% CI 0.11 to 0.31, P < 0.0001). Biochemical parameters including fasting blood sugar, HOMAIR, total cholesterol, ALT, body weight and BMI all significantly improved with metformin therapy as compared to the control group (Table 2). Triglyceride levels did not improve significantly with metformin. Of note, blood sugar, triglycerides, total cholesterol, ALT, and BMI all significantly improved within the control group during the trial; HOMAIR significantly worsened within the control group.
Review of various randomized controlled trials for non-alcoholic fatty liver disease.
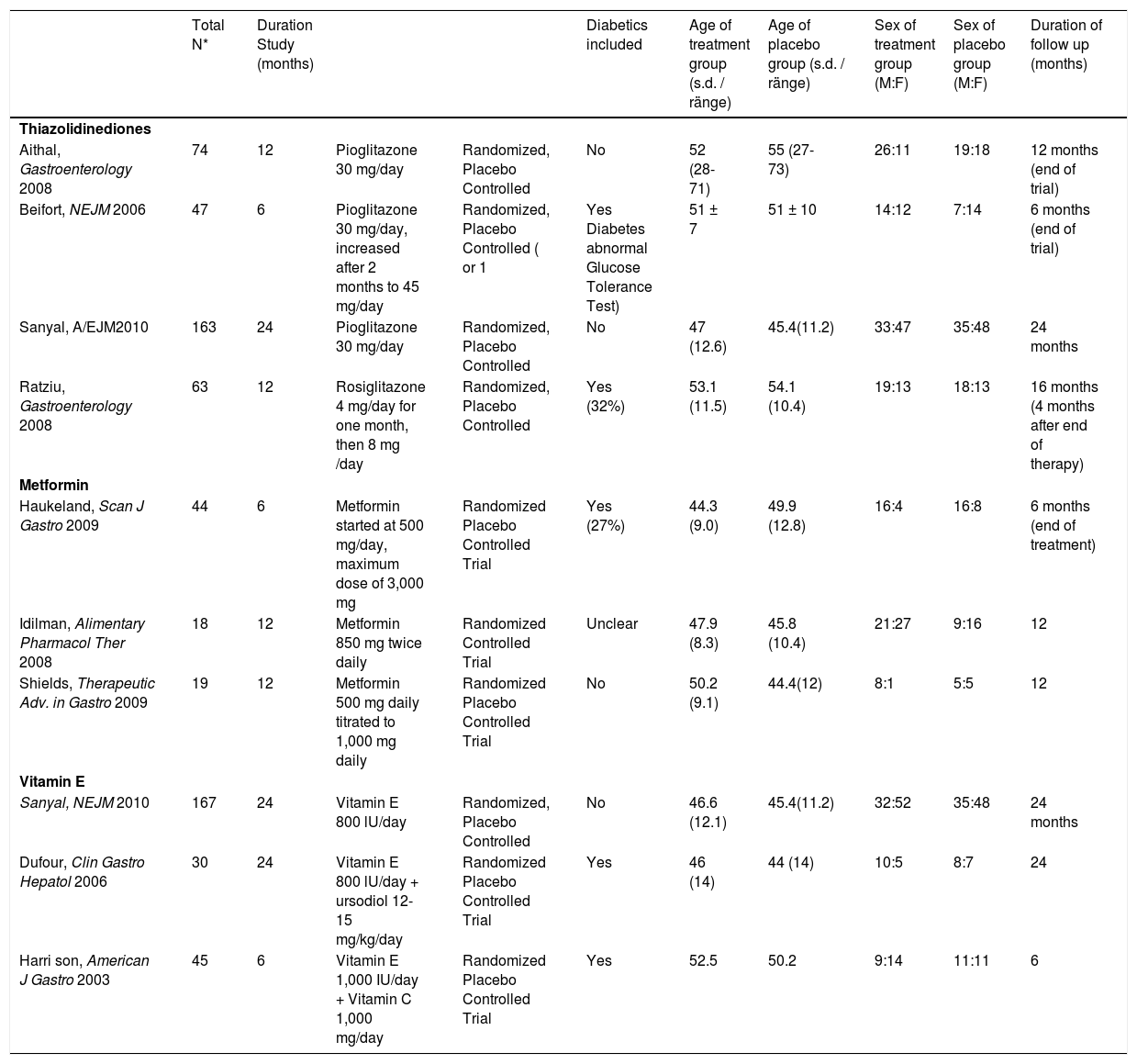
| Total N* | Duration Study (months) | Diabetics included | Age of treatment group (s.d. / ränge) | Age of placebo group (s.d. / ränge) | Sex of treatment group (M:F) | Sex of placebo group (M:F) | Duration of follow up (months) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Thiazolidinediones | ||||||||||
| Aithal, Gastroenterology 2008 | 74 | 12 | Pioglitazone 30 mg/day | Randomized, Placebo Controlled | No | 52 (28-71) | 55 (27-73) | 26:11 | 19:18 | 12 months (end of trial) |
| Beifort, NEJM 2006 | 47 | 6 | Pioglitazone 30 mg/day, increased after 2 months to 45 mg/day | Randomized, Placebo Controlled ( or 1 | Yes Diabetes abnormal Glucose Tolerance Test) | 51 ± 7 | 51 ± 10 | 14:12 | 7:14 | 6 months (end of trial) |
| Sanyal, A/EJM2010 | 163 | 24 | Pioglitazone 30 mg/day | Randomized, Placebo Controlled | No | 47 (12.6) | 45.4(11.2) | 33:47 | 35:48 | 24 months |
| Ratziu, Gastroenterology 2008 | 63 | 12 | Rosiglitazone 4 mg/day for one month, then 8 mg /day | Randomized, Placebo Controlled | Yes (32%) | 53.1 (11.5) | 54.1 (10.4) | 19:13 | 18:13 | 16 months (4 months after end of therapy) |
| Metformin | ||||||||||
| Haukeland, Scan J Gastro 2009 | 44 | 6 | Metformin started at 500 mg/day, maximum dose of 3,000 mg | Randomized Placebo Controlled Trial | Yes (27%) | 44.3 (9.0) | 49.9 (12.8) | 16:4 | 16:8 | 6 months (end of treatment) |
| Idilman, Alimentary Pharmacol Ther 2008 | 18 | 12 | Metformin 850 mg twice daily | Randomized Controlled Trial | Unclear | 47.9 (8.3) | 45.8 (10.4) | 21:27 | 9:16 | 12 |
| Shields, Therapeutic Adv. in Gastro 2009 | 19 | 12 | Metformin 500 mg daily titrated to 1,000 mg daily | Randomized Placebo Controlled Trial | No | 50.2 (9.1) | 44.4(12) | 8:1 | 5:5 | 12 |
| Vitamin E | ||||||||||
| Sanyal, NEJM 2010 | 167 | 24 | Vitamin E 800 lU/day | Randomized, Placebo Controlled | No | 46.6 (12.1) | 45.4(11.2) | 32:52 | 35:48 | 24 months |
| Dufour, Clin Gastro Hepatol 2006 | 30 | 24 | Vitamin E 800 lU/day + ursodiol 12-15 mg/kg/day | Randomized Placebo Controlled Trial | Yes | 46 (14) | 44 (14) | 10:5 | 8:7 | 24 |
| Harri son, American J Gastro 2003 | 45 | 6 | Vitamin E 1,000 IU/day + Vitamin C 1,000 mg/day | Randomized Placebo Controlled Trial | Yes | 52.5 | 50.2 | 9:14 | 11:11 | 6 |
Effect of metformin on biochemical and anthropometric variables in NAFLD.
| Variable | Metformin (n = 129) Control (n = 103) | Weighted means pre-treatment | Weighted means post-treatment | P values within group | P value metformin vs. controls | ||
|---|---|---|---|---|---|---|---|
| Blood Sugar | Metformin Control | 98.43 99.72 | 87.93 99.07 | < 0.0001 0.002 | 0.003 | ||
| HOMA-IR | Metformin Control | 3.52 3.07 | 2.80 3.47 | 0.0004 0.0016 | < 0.0001 | ||
| Triglycerides | Metformin Control | 184.65 178.25 | 184.45 173.83 | 0.0004 0.0055 | 0.08 | ||
| Total Cholesterol | Metformin Control | 208.55 206.95 | 189.40 187.55 | 0.0001 < 0.0001 | 0.0008 | ||
| ALT | Metformin Control | 95.48 92.65 | 52.09 69.25 | < | 0.0001 < | 0.0001 | < 0.0001 |
| Body Weight (kg) | Metformin Control | 85.31 84.41 | 82.78 88.62 | < 0.0001 0.054 | < 0.001 | ||
| BMI | Metformin Control | 30.51 30.02 | 28.74 28.92 | < | 0.0001 < | 0.0001 | < 0.0001 |
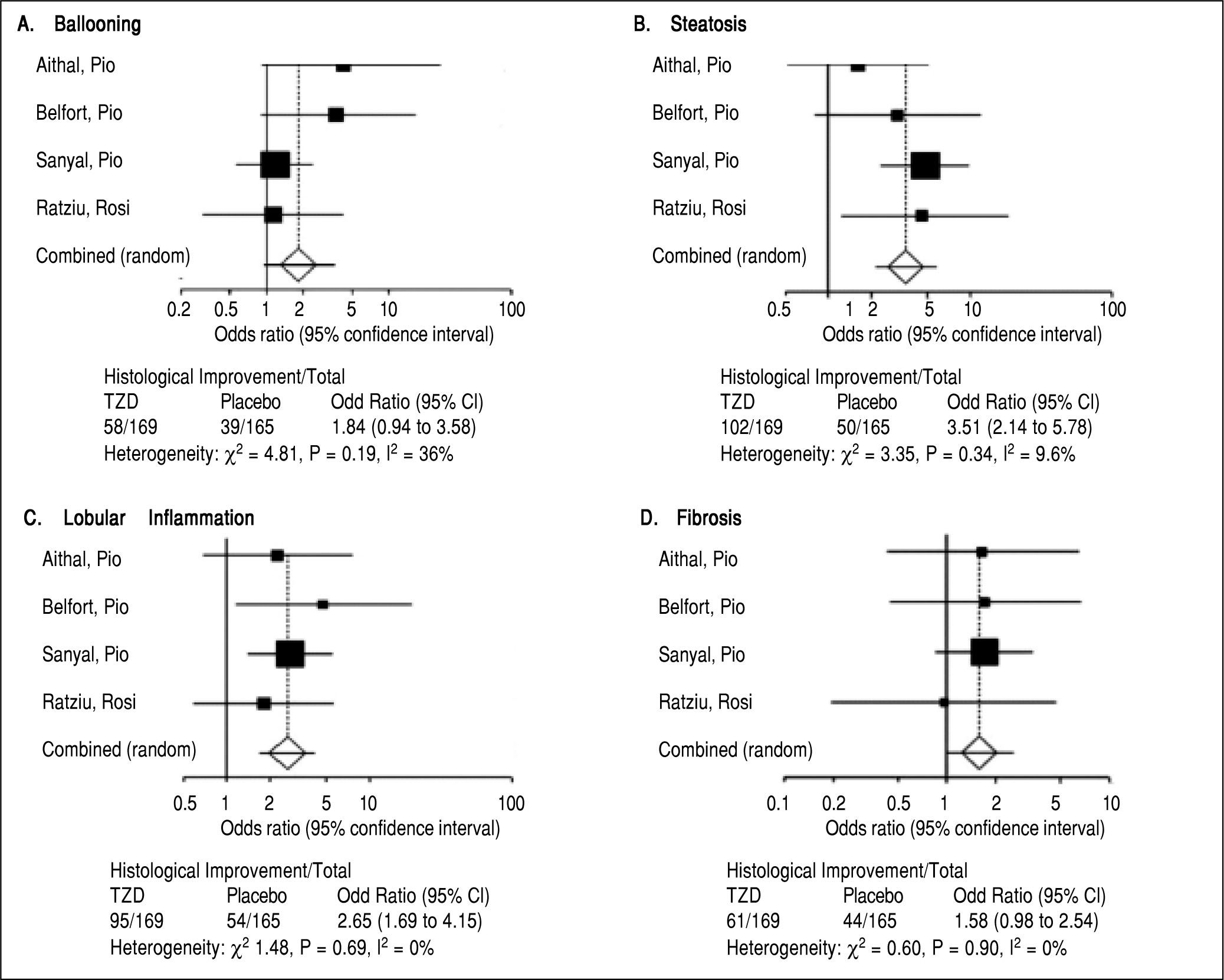
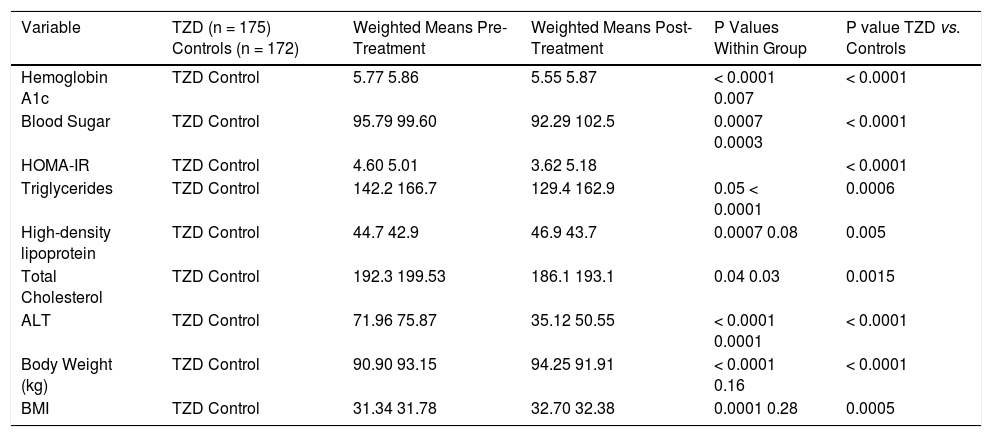
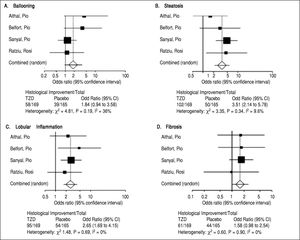
Four trials analyzing TZD (3 pioglitazone and 1 rosiglitazone) therapy in patients with NASH were included in our meta-analysis (Table 1) with Belfort, et al.18 and Ratziu, et al.19 including a subset of diabetic patients. 347 patients were analyzed in the TZD group with studies ranging from 47 to 163 patients. Therapy lasted between six to twenty four months. Of note, therapy regimens varied including Pioglitazone 30 mg daily with uptitration to 45 mg daily as well as Rosiglitazone up to 8 mg daily. Histological parameters including ballooning and fibrosis did not significantly change with TZD therapy (Figure 3). Steatosis and lobular inflammation significantly improved after therapy (Figure 3). Biochemical parameters including HbA1c, fasting blood sugar, HOMAIR, triglycerides, total cholesterol, high-density lipoprotein, and ALT all significantly improved with TZD therapy as compared to controls (Table 3). High-density lipoprotein, body weight, and BMI significantly worsened with TZD therapy as compared to controls. Of note, triglycerides, total cholesterol, and ALT all significantly improved within the control group. HbA1c and fasting blood sugar significantly worsened with the control group. High-density lipoprotein, body weight, and BMI did not significantly change in the control group.
Effect of Thiazolidinediones on Biochemical and Anthropometric Variables in NAFLD.
| Variable | TZD (n = 175) Controls (n = 172) | Weighted Means Pre-Treatment | Weighted Means Post-Treatment | P Values Within Group | P value TZD vs. Controls |
|---|---|---|---|---|---|
| Hemoglobin A1c | TZD Control | 5.77 5.86 | 5.55 5.87 | < 0.0001 0.007 | < 0.0001 |
| Blood Sugar | TZD Control | 95.79 99.60 | 92.29 102.5 | 0.0007 0.0003 | < 0.0001 |
| HOMA-IR | TZD Control | 4.60 5.01 | 3.62 5.18 | < 0.0001 | |
| Triglycerides | TZD Control | 142.2 166.7 | 129.4 162.9 | 0.05 < 0.0001 | 0.0006 |
| High-density lipoprotein | TZD Control | 44.7 42.9 | 46.9 43.7 | 0.0007 0.08 | 0.005 |
| Total Cholesterol | TZD Control | 192.3 199.53 | 186.1 193.1 | 0.04 0.03 | 0.0015 |
| ALT | TZD Control | 71.96 75.87 | 35.12 50.55 | < 0.0001 0.0001 | < 0.0001 |
| Body Weight (kg) | TZD Control | 90.90 93.15 | 94.25 91.91 | < 0.0001 0.16 | < 0.0001 |
| BMI | TZD Control | 31.34 31.78 | 32.70 32.38 | 0.0001 0.28 | 0.0005 |
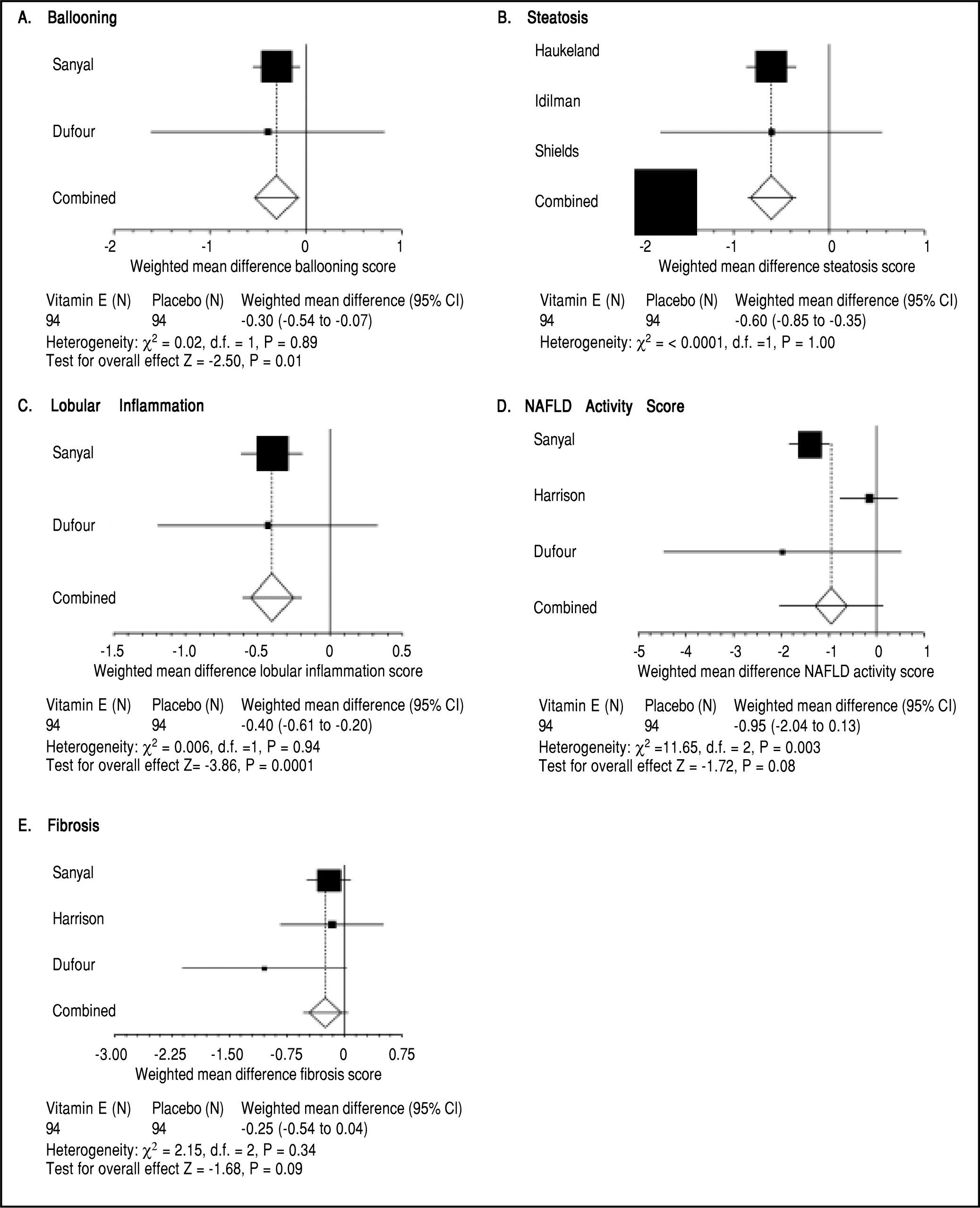
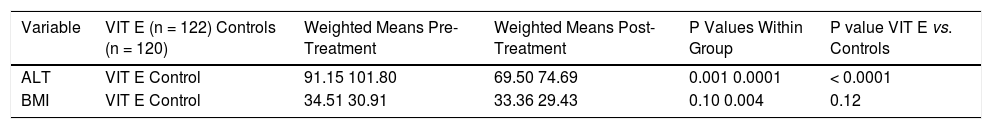
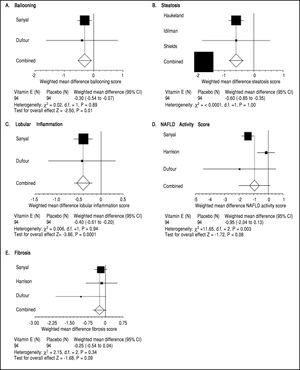
Three trials analyzing vitamin E therapy in patients with NASH were included in our meta-analysis (Table 1) with Harrison, et al.20 and Dufour, et al.21 including a subset of diabetic patients. 242 patients were analyzed in the Vitamin E group with studies ranging from 30 to 167 patients, with the predominant number of patients coming from the Sanyal, et al. study.13 Therapy lasted between six to twenty four months. Of note, therapy regimens varied with the use of Vitamin E 800 to 1000 IU/day. Histological parameters including fibrosis and NAS did not significantly change with vitamin E therapy (Figure 4). Ballooning, steatosis, and lobular inflammation significantly improved with vitamin E (Figure 4). Analysis of biochemical parameters within the vitamin E group was limited secondary to reported data, as such, only ALT and BMI changes were evaluated (Table 4). ALT significantly improved with vitamin E therapy as compared to the control group. BMI did not significantly change with vitamin E therapy as compared to the control group. Of note, ALT and BMI did significantly improve within the control group as well.
Effect of Vitamin E on Biochemical and Anthropometric Variables in NAFLD.
| Variable | VIT E (n = 122) Controls (n = 120) | Weighted Means Pre-Treatment | Weighted Means Post-Treatment | P Values Within Group | P value VIT E vs. Controls |
|---|---|---|---|---|---|
| ALT | VIT E Control | 91.15 101.80 | 69.50 74.69 | 0.001 0.0001 | < 0.0001 |
| BMI | VIT E Control | 34.51 30.91 | 33.36 29.43 | 0.10 0.004 | 0.12 |
Our meta-analysis of high quality randomized controlled trials demonstrates that metformin, TZDs, and Vitamin E therapy do not significantly improve fibrosis. However, both TZDs and Vitamin E significantly improve steatosis and lobular inflammation with Vitamin E also significantly improving hepatocyte ballooning. All of the agents studied significantly improved ALT. Metformin and TZDs both showed a significant improvement in fasting blood sugar, HOMAIR and total cholesterol. Despite improvement in histology with TZD therapy BMI and body weight significantly worsened with treatment. Vitamin E therapy did not significantly alter BMI.
Lifestyle modification including an intense exercise program has shown that a > 7% weight loss is associated with significant improvement in steatosis, necrosis, and inflammation but not fibrosis.22 However, our meta-analysis reveals that histological response may not necessarily correlate with weight loss given the adverse effects of TZD therapy on body weight. The importance of weight loss has been challenged and a study examining an exercise program without dietary modification revealed liver fat content could decrease without a significant change in body weight.23,24 As such, our analysis provides further evidence that the pathophysiology involved in the development and progression of NASH may be much more complicated than simply the development of insulin resistance.
Our study also illustrates the limited data and number of RCTs that include histological outcomes in adult patients for the treatment of NASH. In addition, the conclusions arrived by the current studies are limited due to the number of patients involved with only the Sanyal, et al. study including more than one hundred patients. Also, pharmaceutical agents studied for efficacy in NAFLD are focused on liver histologic outcomes and do not use cardiovascular disease, malignancy, or mortality as end points although these are important in patients with NAFLD. Though adverse effects are not studied as primary end points and thus, are not easily evaluated in a meta-analysis, all agents studied have been associated with adverse effects which need to be further elucidated prior to implementation. The use of metformin has been associated with the development of lactic acidosis, TZDs may cause an increased incidence of congestive heart failure,25 and long-term vitamin E may increase risk of prostate cancer.26 It is unknown whether the changes or improvement seen in histology reverses after cessation of therapy or even translates to an improvement in liver related mortality. In addition, only five trials studied included a diabetic population with the largest trial including non-diabetic subjects. As such, evidenced based guidelines regarding therapy are challenging to construct for the current and future patient population given the increasing incidence of diabetes.
We found that insulin sensitizers and vitamin E play a major role in improving biochemical parameters and over the short term can have a beneficial effect on histologic markers of liver injury. However, it is unknown whether these improvements predict improved clinical outcomes. Future trials should include histological out-comes with longer duration of treatment and follow-up to determine whether or not our current proposed theories of the treatment of NASH are validated. In addition, there may be value in examining if a combination of insulin sensitizers and antioxidants provide a synergistic response on histology.