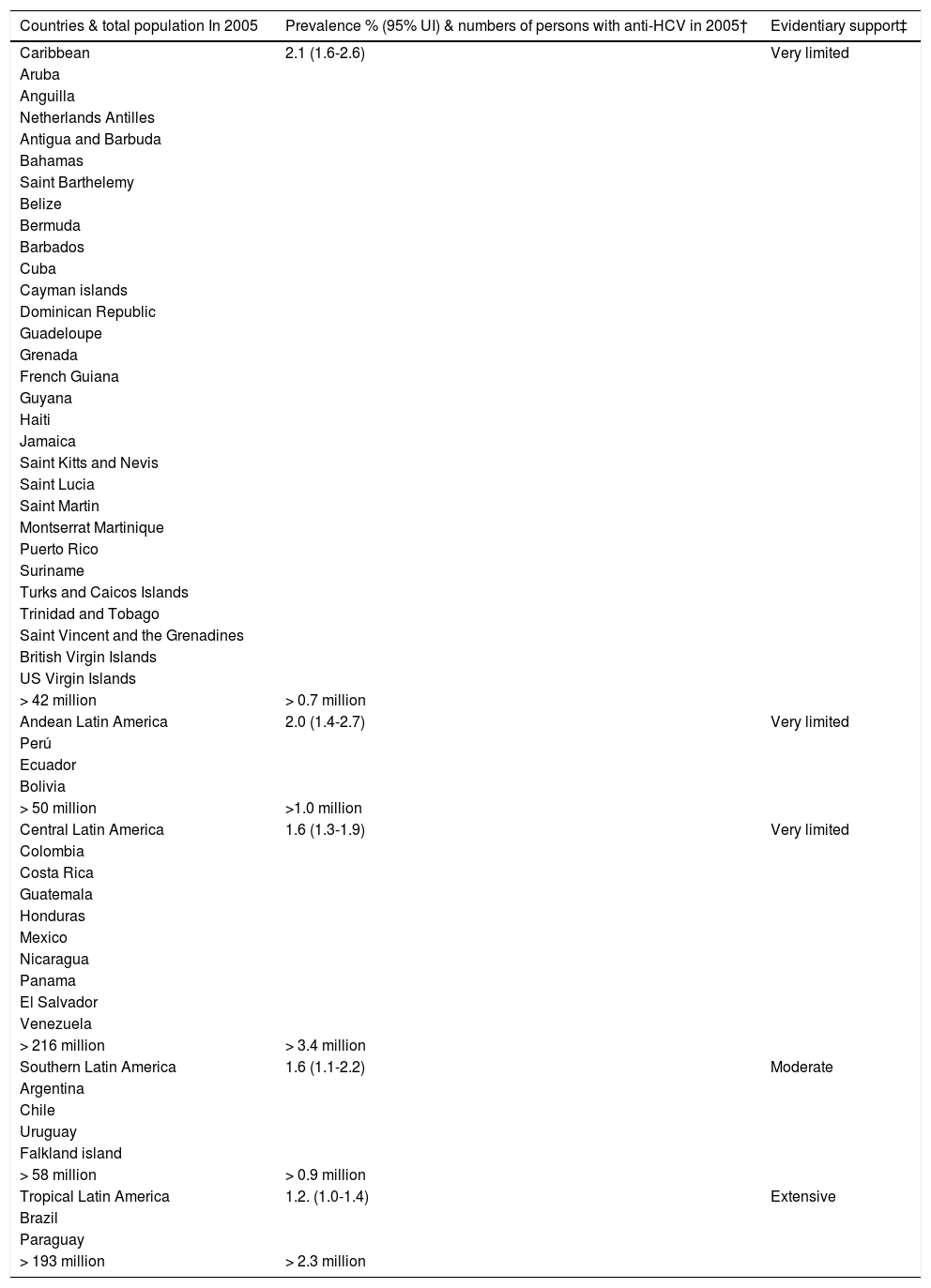
Chronic hepatitis C virus (HCV) infection is a major public health concern. It is estimated that more than 185 million people, around 3% of the world population, are currently living with chronic hepatitis C.1 About one-third of these individuals will develop cirrhosis and hepatocellular carcinoma (HCC), leading to approximately 350,000 deaths each year.2,3 The prevalence of HCV infection in Latin American countries is heterogeneous, as illustrated in table 1.
Prevalence and evidentiary support.
| Countries & total population In 2005 | Prevalence % (95% UI) & numbers of persons with anti-HCV in 2005† | Evidentiary support‡ |
|---|---|---|
| Caribbean | 2.1 (1.6-2.6) | Very limited |
| Aruba | ||
| Anguilla | ||
| Netherlands Antilles | ||
| Antigua and Barbuda | ||
| Bahamas | ||
| Saint Barthelemy | ||
| Belize | ||
| Bermuda | ||
| Barbados | ||
| Cuba | ||
| Cayman islands | ||
| Dominican Republic | ||
| Guadeloupe | ||
| Grenada | ||
| French Guiana | ||
| Guyana | ||
| Haiti | ||
| Jamaica | ||
| Saint Kitts and Nevis | ||
| Saint Lucia | ||
| Saint Martin | ||
| Montserrat Martinique | ||
| Puerto Rico | ||
| Suriname | ||
| Turks and Caicos Islands | ||
| Trinidad and Tobago | ||
| Saint Vincent and the Grenadines | ||
| British Virgin Islands | ||
| US Virgin Islands | ||
| > 42 million | > 0.7 million | |
| Andean Latin America | 2.0 (1.4-2.7) | Very limited |
| Perú | ||
| Ecuador | ||
| Bolivia | ||
| > 50 million | >1.0 million | |
| Central Latin America | 1.6 (1.3-1.9) | Very limited |
| Colombia | ||
| Costa Rica | ||
| Guatemala | ||
| Honduras | ||
| Mexico | ||
| Nicaragua | ||
| Panama | ||
| El Salvador | ||
| Venezuela | ||
| > 216 million | > 3.4 million | |
| Southern Latin America | 1.6 (1.1-2.2) | Moderate |
| Argentina | ||
| Chile | ||
| Uruguay | ||
| Falkland island | ||
| > 58 million | > 0.9 million | |
| Tropical Latin America | 1.2. (1.0-1.4) | Extensive |
| Brazil | ||
| Paraguay | ||
| > 193 million | > 2.3 million |
In 2010, the Latin American Association for the Study of the Liver (LAASD) developed its own guidelines for the diagnosis and treatment of HCV. Until 2011, the standard of care for patients with HCV genotype (GT)1 was pegylated interferon (PEG-IFN) plus ribavirin (RBV). The sustained virologic response (SVR) rates were 40-50%.4,5 The standard of care for patients with either HCV GT2 or GT3 was PEG-IFN plus RBV for 24 weeks with SVR rates ranging from 69% to 74%.6 At that time, first-in-class protease inhibitors (PIs) [boceprevir (BOC) and telaprevir (TVR)] were the first direct-acting antiviral (DAA) therapies approved for patients with GT1, given in conjunction with both PEG-IFN and RBV for 24-48 weeks, depending on whether the patient had a robust response. The first-generation DAAPIs inhibit the NS3/4A protease, which in turn diminishes viral replication. The SVR rates in pivotal phase 3 studies of treatment-naïve patients with GT1 receiving PEG-IFN plus RBV plus a PI ranged from 63 to 75%. In patients who previously received PEG-IFN plus RBV but did not achieve SVR, superior SVR rates of 75-83% were achieved in relapsers, 52-59% in partial responders, and 29-38% in nonresponders.7–10
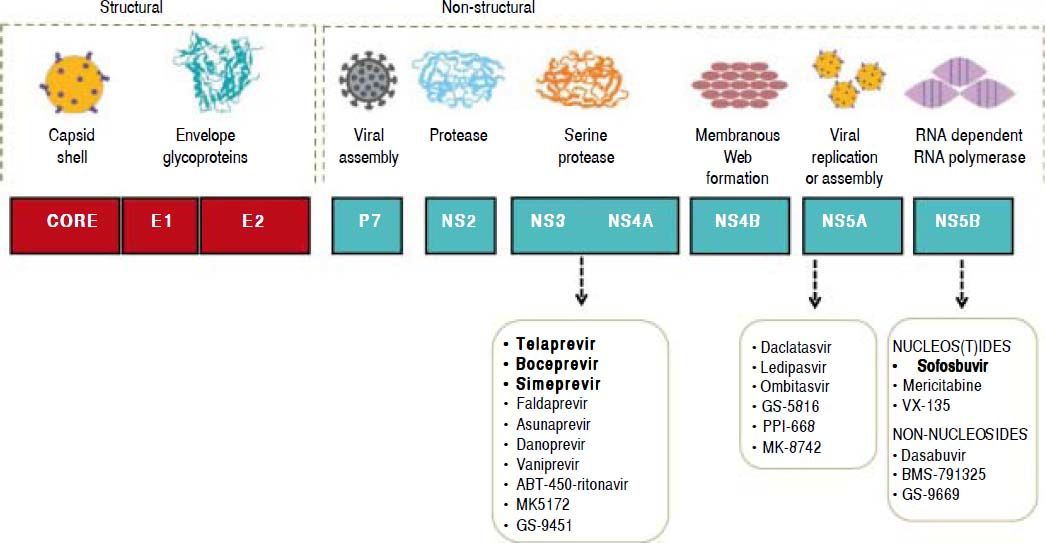
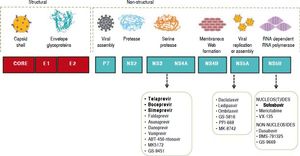
In 2013, the LAASD reviewed and updated the guidelines to include the first-generation DAAs for treatment and laboratory tests for the diagnosis, monitoring and evaluation of patients with chronic HCV infection.11 Fortunately, thanks to ongoing research, in vitro systems to culture HCV became available, and these tools have allowed the development of DAAs that are specifically designed to target HCV proteins, particularly the nonstructural proteins. In fact, the efforts have focused on the six nonstructural (NS) proteins (NS2, NS3, NS4A, NS4B, NS5A, and NS5B) that play critical roles in HCV entry, replication, and proliferation, and serve as possible targets for development of the new DAA therapies (Figure 1).
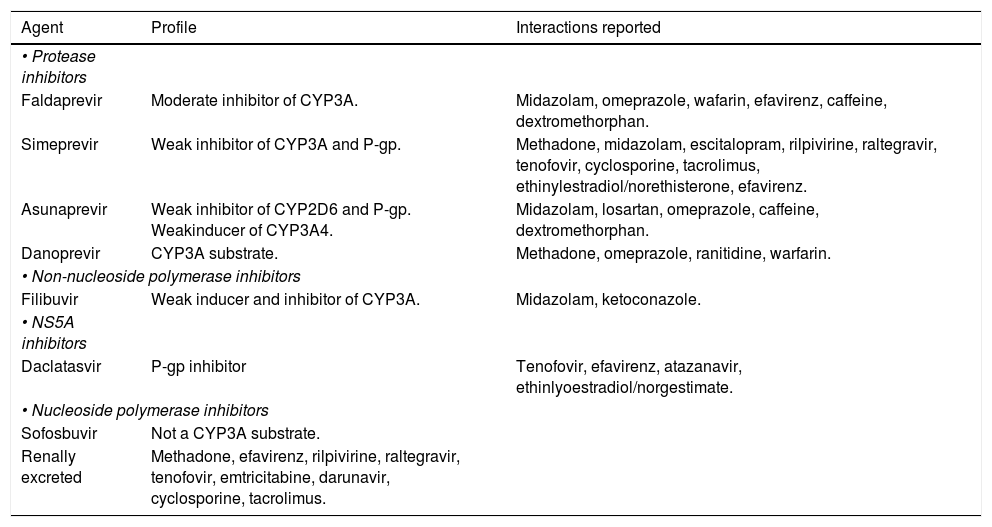
The NS3/4A inhibitors target the serine protease NS3/4A, which cleaves the HCV polyprotein at four sites. As mentioned above, the first DAAs available were TVR and BOC. The protease inhibitor simeprevir(SMV) has recently been licensed, and others, such as faldaprevir, asunaprevir, vaniprevir, and ritonavir-boosted ABT-450, are currently in the process of being approved. The newer drugs have easier dosing regimens and seem to have a lower propensity for toxicity and drug-drug interactions. In addition, these new DAAs have activity against GTs other than GT1, particularly GT2, GT4, GT5, and GT6. For GT1 infection, PIs can increase the SVR from 45% with standard PEG-IFN-based treatment to as high as 80%-90%, with lower responses typically seen in those with advanced cirrhosis or other markers of poor outcome.
Resistance to PIs occurs mainly through newly acquired resistance mutations in the gene encoding the NS3 protease, at codons 36, 54, 155, 156, 168, and 170.12 It has also been suggested that the existence of polymorphisms in some viruses, such as the Q80K polymorphism that is present in the GT1a viruses, is associated with a reduced response. Although worldwide prevalence of this polymorphism has been calculated to be 25%,13 it is associated with about a threefold reduction in response to SMV and a significant reduction in treatment response.14 The PIs are currently licensed for use in conjunction with PEG-IFN and RBV, although IFN-free regimens (such as combined SMV and sofosbuvir (SOF) and asunaprevir or ABT-450-based treatment) will soon be available.
NS5AThe NS5A protein is essential for both viral assembly and replication. Inhibitors of NS5A are potent antivirals that act at picomolar concentrations, although the response differs between GT1a and GT1b viruses.15 Daclatasvir(DCV), ledipasvir (LDV), ABT-267, GS-5816, and MK-4782 are NS5A inhibitors that may be licensed within the next year. These agents seem to have minimal adverse reactions, and no serious drug-drug interactions are yet known. Resistance mutations in the NS5A protein encountered in clinical trials to date include M28T, L31M/V, and Y93C/N.
NS5B inhibitorsThe NS5B RNA-dependent RNA polymerase is responsible for replication of HCV RNA. As with inhibitors of the HIV reverse transcriptase enzyme, there are two main classes of NS5B inhibitors. These are the nucleos(t)ide inhibitors (nucleoside or nucleotide inhibitors), which bind to the active site of the enzyme and cause premature chain termination, and the nonnucleoside inhibitors, which bind outside the active site but cause a conformational change that inhibits RNA polymerase activity.
Several agents are currently in advanced stages of development, and the nucleos(t)ide inhibitor SOF-recently became the first NS5B inhibitor to be li- censed for treatment of HCV infection. These agents seem to have pangenotypic activity and minimal toxicity or drug interactions. In vitro resistance to SOF seems to occur with the development of an S282T mutation in the NS5B gene, although this has yet to be seen in large numbers of patients. This is in contrast to PI-based therapy, where resistance mutations are commonly seen when treatment fails.16
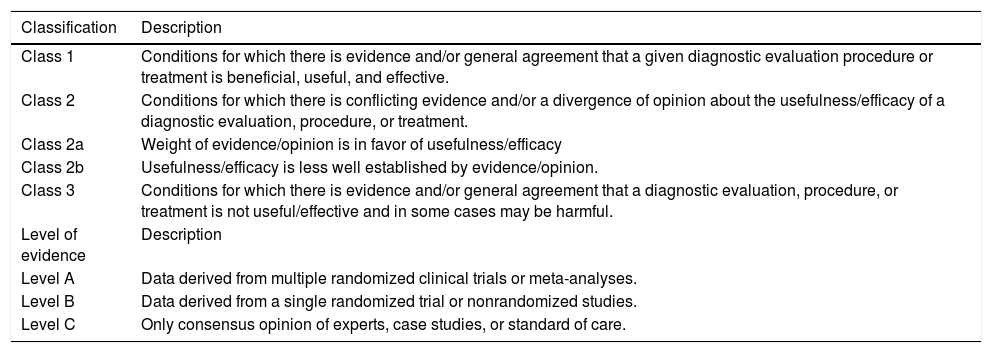
The LAASD recommendations have been updated in 2014 by a panel of experts chosen by the Governing Board. The Recommendations have been based as far as possible on evidence from existing publications. The evidence and recommendations in these guidelines have been graded according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system. The strength of recommendations thus reflects the quality of underlying evidence. The principles of the GRADE system have been enunciated. The quality of the evidence in the clinical practice guidelines has been classified into one of three levels: high (A), moderate (B) or low (C). The GRADE system offers two grades of recommendation: strong (1) or weak (2) (Table 2).
Grading system for recommendations.
| Classification | Description |
|---|---|
| Class 1 | Conditions for which there is evidence and/or general agreement that a given diagnostic evaluation procedure or treatment is beneficial, useful, and effective. |
| Class 2 | Conditions for which there is conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of a diagnostic evaluation, procedure, or treatment. |
| Class 2a | Weight of evidence/opinion is in favor of usefulness/efficacy |
| Class 2b | Usefulness/efficacy is less well established by evidence/opinion. |
| Class 3 | Conditions for which there is evidence and/or general agreement that a diagnostic evaluation, procedure, or treatment is not useful/effective and in some cases may be harmful. |
| Level of evidence | Description |
| Level A | Data derived from multiple randomized clinical trials or meta-analyses. |
| Level B | Data derived from a single randomized trial or nonrandomized studies. |
| Level C | Only consensus opinion of experts, case studies, or standard of care. |
The guidelines are intended for use by hepatologists, gastroenterologists and infectious disease doctors who are in charge of the treatment of people with hepatitis C in the Latin American countries. Also is important to mention that these guidelines might change as new therapies will be introduced in different countries. For that reason, we are planning to review and update them at least one or two times a year.
2. Public Policies for Facing Hepatitis C in Latin American CountriesDeveloping countries face substantial barriers to screening, including low political, provider, and community awareness of hepatitis C as a significant health threat, that leads to deprioritization of testing and other preventive health services. In addition, public health officials in many developing countries do not understand the true burden of disease within their borders because their surveillance infrastructure may be inadequate: one-third of World Health Organization (WHO)member countries do not collect prevalence data for viral hepatitis.17 Robust surveillance for HCV infection, particularly serosurveillance, is critical for assessing this burden, because many newly infected people are asymptomatic and do not seek care for their infection until years, even decades, after they are infected. Tables 3 and 4 below list those patients eligible to receive antiviral treatment for HCV.
Patients eligible to receive antiviral treatment for HCV.
| • Age older than or equal to 18 years. |
| • HCV-RNA detectable onserum. |
| • Chronic hepatitis and significant fibrosis (grade 2 or 3) measured by liver biopsy or non invase methods. |
| • Compensated liver cirrhosis (Child Pugh 5-6 points without history of variceal bleeding, ascites or encephalopathy). |
| • No hematological and biochemical alterations that preclude the use of PEG-IFN and RBV. |
| • No contraindications. |
| • Motivated patient who understand the treatment and its implications. |
All treatment-naïve and experienced patients with compensated chronic liver disease related to HCV, who are willing to be treated and who have no contraindications to treatment, should be considered for therapy. Treatment should be prioritized in patients with advanced fibrosis (METAVIR score F3 to F4) and in those patients with clinically significant extra-hepatic manifestations (symptomatic cryoglobulinaemia or HCV immune complex nephropathy). Treatment is justified in patients with moderate fibrosis (METAVIR score F2).
In treatment IFN-free, ideally ribavirin-free therapy may also be considered in patients with decom-pensated cirrhosis.
The burden of disease is critical for decisions about national health policies, and therefore there is a need for accurate estimations globally, regionally and nationally. However, accurate data on the burden of chronic HCV infection are not available in the Latin American region. In the development of treatment programs for HCV infection, building in methods for data collection and recording that allow regular and routine program review will help to facilitate ongoing service feedback and improvement, and will also help to generate evidence regarding the relative benefits and cost-effectiveness of different program strategies.
Cost of treatmentAlthough hepatitis C is curable, most patients outside of the developed world, where hepatitis C is a major public health problem, are unable to access treatment. Treatment coverage should be improved not only in resource-limited countries but also in developed countries where less than 20% of HCV-infected patients receive antiviral therapy (AVT). Decreasing the cost of the drugs is urgently required for developing countries as well as developed countries that will not be able to cover all the HCV treatment-related expenses. This goal is feasible but will require the support of pharmaceutical companies, international health agencies and donors, governments and nongovernmental organizations, and the commitment of scientists and physicians. Mechanisms for accelerated access to simplified treatment of HCV infection should be prioritized. The simplification agenda for HCV management will need to take into account the different capacities of different settings. Governments, policymakers and the academic sector are critical to delivering HCV services, implementing surveillance programs, disseminating information and increasing public and provider awareness. Continued involvement of key stakeholders including advocacy and patient groups is also essential to ensure that vulnerable and underserved populations have appropriate representation. Although patient and provider factors receive the greatest attention, obstacles arising at the government and payer levels are likewise important. In an international study of HCV providers, lack of treatment promotion and insufficient funding were noted as significant government-level barriers. Lack of insurance coverage, high out-of-pocket expenses and excessive paperwork were cited as payer-level barriers.
3. Diagnosis of Chronic Hepatitis C (Screening Recommendations in General and for Special Populations)Hepatitis C is currently a public health problem world wide, recognized as a disease of global importance, affecting both industrialized and developing countries.18–20 To estimate the global consequences of chronic hepatitis C, knowledge of the prevalence of HCV in each country is required. This estimate should be made through population-based studies. However, because in many countries these are not available and the data are scarce, reference is made only to specific groups, including blood donors, illicit drug users, or individuals with high-risk sexual behavior, which do not represent the population as a whole.21
The direct determination of the incidence of HCV infection is difficult. Incidence is estimated using available data on the prevalence. Available data suggest that the prevalence of HCV infection is approximately 2-3% worldwide (130-170 million people). Approximately 15-25% of HCV-infected patients progress to cirrhosis, which can occur in about 20-30 years.22 On assessing the impact of hepatitis C in the United States of America (USA) in a systematic re-view,23 it was clear that screening was neglected (70% of those infected were unaware of their status), and it became clear that the prevalence of cirrhosis is increasing and will continue to increase in the next decade, and that HCV infection is a major cause of mortality and liver-related morbidity. HCV infection leads to significant loss of quality of life and is responsible for significant costs in healthcare.
In Europe, HCV is the major cause of cirrhosis, increasing the mortality rate to 1.5-5 times that of the general population, and in cohorts of hospitalized patients, morbidity/mortality is higher. It was also observed that screening is neglected: HCV is considered to be a huge public health problem.24
In a study that evaluated the projection of HCV infection in Latin America,25 it was observed that the prevalence of HCV varies between 1 and 2.3%. The number of diagnosed and treated cases is till low, while there are increasing rates of complications such as progression to cirrhosis and HCC.
In a recent systematic review of 25 articles, in which the burden of hepatitis C in Latin America was evaluated26 from nine population-based studies, the estimated burden of the infection was 7.8 million individuals (prevalence of infection of 0.9-5.8%). The biggest challenge appears to be located in Mexico and Brazil, where around 4 million people are infected. Specifically in Brazil, a population-based prevalence study of 19,503 individuals, conducted in the major cities of the country and funded by the Ministry of Health/Bureau of Health Surveillance, revealed an overall prevalence of anti-HCV antibodies of 1.38%.27
With respect to the costs of HCV, a US study estimated the cost of a patient with HCV to be US $20,961 compared with US $5,451 for controls.28 The most recent study that assessed the future burden of HCV in the USA, using a model with a dynamic system involving 36 cohorts, indicates that despite a decrease of two-thirds in the prevalence of infection in 2030, there will be an increase in the incidence of cirrhosis (626,500 in 2015), the incidence of decompensated cirrhosis (107,400 in 2019), the incidence of HCC (23,800 in 2018), mortality from liver disease (29,695 in 2019) and cost (9.1 billion dollars in 2024).29
Chronic hepatitis C is a disease with high costs for health care institutions, so efforts are needed in screening and early treatment before progression to cirrhosis-actions that reduce costs in managing this condition. In view of this, the reduction in overall mortality and morbidity related to chronic hepatitis C, especially in settings where resources are scarce, should be considered to be a high priority by public health authorities.21 It isimportant to emphasize thatin mostcountries of Latin America, the true prevalenceof HCVis not known, and screeningis also neglected. In this document, we attempt to providea suggested course of action for the countries of this continent.
The approach to detecting HCV infections is to screen people with a history of exposure to the virus and to test individuals who have an identifiable risk factor. The main risk factors are the following: illicit injecting drug useat present orin the past, including intranasal drug users who share contaminated devices; receipt of blood products before the screening of blood supply started in 1992, although screening was not mandatory until 1996 in Chile; receipt of clotting factor concentrates before 1987 (after which viral inactivation procedures were implemented); healthcare exposure to long-term he-modialysis, needlestick injuries among health care workers, and patient-to-patient transmission resulting from poor infection control practices. Other modes of transmission include children born to HCV-infected mothers and sexual transmission, mainly among HIV-infected men who have unprotected sex with men. Other risk factors include in- carceration, exposure to an infected sexual partner or multiple sexual partners, and living with HCV-in-fected people, sharing a razor or toothbrush, and tattooing or piercing in an unregulated setting. Because of shared transmission modes, people with HIV infection are at risk for HCV infection. Recent data also support testing of all cadaveric and living solid-organ donors because of the risk that HCV infection poses to the recipient. Individuals with unexplained elevations of aminotransferases should be tested for the presence of HCV infection.30–33 Generally, it is accepted that these risk groups should be screened for HCV. In 1998, the Centers for Disease Control and Prevention (CDC) issued recommendations for identifying HCV-infected people.34 Testing for HCV was recommended for people most likely to be infected, including those who had ever had at least one risk factor. In 1999, HCV testing was recommended for people with HIV.35
Given that Brazil is the Latin American country with the largest number of HCV carriers, the analysis of the previously cited population-based study becomes important in evaluating the major risk factors.27 In this study, the multivariate model showed the following to be predictors of HCV infection: age, injecting drug use (OR = 6.65), inhaled drug use (OR = 2.59), hospitalization (OR = 1.90), groups socially deprived by a lack of sewage disposal (OR = 2.53), and injections with a (reusable) glass syringe (OR = 1.52, with a borderline p value). In another study36 that had the objective of obtaining data on acute hepatitis C in Brazil, among 133 nonuremic patients, the main risk factors were hospital procedures, whereas in 37 hemodialysis patients, dialysis was the single risk factor in 95% of cases. Also of interest is a study that assessed the prevalence of hepatitis C markers in patients with HIV infection and found almost 40% positivity.37 Thus, we can infer that the main risk factors described in the literature are also important in Latin American countries, suggesting the importance of screening in these risk population.
However, in the Brazilian population-based study, the known risk factors explain fewer than 50% of the infected cases,27 limiting the application of prevention strategies. In a study that evaluated participants in the National Health and Nutrition Examination Survey, only 3.7% of HCV-infected people reported having been tested based on known HCV-related risk factors.38 Thus, the success of risk-based testing strategies has been limited.
It is important to recognize the impact of HCV on liver disease progression, which will impact the health system.39 In a multicohort natural history model for predicting disease outcomes and benefits of therapy, it was concluded that prevalence of hepatitis C cirrhosis and its complications will continue to increase through the next decade and will mostly affect those older than 60 years of age.40 Assuming that 30% of cases of HCV are diagnosed and that up to 25% of those are treated, we would expect just a 1% reduction in cirrhosis by 2020, with a 15.6% reduction if all patients were treated. If the success of therapy increased to 80%, treatment of all infected individuals would reduce cirrhosis by 30.4%. This makes it urgent to define innovative public health policies to improve HCV screening, which is the only way to allow more HCV patients access to therapy. Other wise, without screening, HCV patients remain undiagnosed until they develop advanced liver disease. Only with increasing AVT(more diagnoses) and with a higher response rate (a reality in the present era) will we observe a reduction in disease impact in the coming years.
It is estimated that 45%-85% of adults in the USA who are chronically infected with HCV are unaware of their condition.41 Higher percentages have been reported in European countries,24 and the figure in Latin America is unknown. However, the reality in Latin America is likely to be similar. Because of the limited effectiveness of the testing recommendations, the CDC, after searching multiple data bases to identify studies pertinent to the question, considered a birth-year-based strategy to increase the proportion of infected individuals detected: one-time HCV testing of all people born during 1945-1965 (“baby boomers”). These people account for around 75% of all prevalence of those with anti-HCV antibodies.42 European health authorities should encourage innovative approaches, such as those proposed recently by the CDC, to increase the proportion of HCV-infected people aware of their condition.24 A review that studied 110,223 cases of past or current HCV infection showed that 68% of people would have been identified through a one-time birth-year-based HCV testing strategy, whereas only around 27% would have been screened with the risk-based approach.43 The cost-effectiveness of birth-cohort testing is comparable to that of current risk-based screening strategies.41,42
In the Latin American region, the age-specific prevalence of HCV infection shows the increase progressive with age above 35 years old, with a peak prevalence at age 55-65.1 This is in concordance with the data from Pereira, et al. showing a progression of HCV prevalence with age.27 In addition, the prevalence of infection did not vary significantly between 1990 and 2005, suggesting that age (rather than year of birth) is associated with the risk of infection.1
Although there is a lack of direct evidence that HCV testing positively affects related morbidity and mortality, targeted testing of people belonging to risk groups and those with high HCV preva- lence is likely to increase the number of HCV-infected people identified, referred to a specialist, and provided access to treatment, resulting in a higher likelihood of treatment success. An additional benefit is that knowing one’s HCV infection status provides the opportunity to reduce transmission of the disease.
Thus our screening recommendations for general and special populations are as follows.
RecommendationsScreening recommendationsfor general and special populations.
- 1.
Individuals who have an identifiable risk factor
- •
Illicit injecting drug users at present or in the past and intranasal drug users.
- •
Individuals who received blood products (or underwent an organ transplant) before 1992 and who received clotting factor concentrates before 1987.
- •
- 2.
Individuals with a history of comorbidities
- •
Long-term hemodialysis.
- •
HIV infection.
- •
Unexplained elevations of aminotransferases.
- •
- 3.
Individuals with a history of exposure to the virus.
- •
All people who have undergone a medical procedure.
- •
Needlestick injuries among health care workers.
- •
Needlestick injury and children born to HCV-infected mothers.
- •
Sexual transmission, mainly among HIV-infected men who have unprotected sex with men.
- •
Having been incarcerated.
- •
Exposure to an infected sexual partner or multiple sexual partners.
- •
Living with HCV-infectedpeople, sharing a razor or toothbrush.
- •
Having undergone tattooing or piercing in an unregulated setting.
(Rating: Class I, Level B).
- •
- 4.
Given the need to reduce the proportion of infected patients who are unaware of their status, especially in countries with more resources, we also recommend the following.
- •
One-time HCV testing of people 45 years and older.
(Rating: Class I, Level B).
- •
Staging of liver fibrosis is important in the management of patients with chronic liver diseases, because the severity of fibrosis influences the prognosis and treatment options.44,45
Liver biopsy is still the “gold standard” in the diagnosis and staging of chronic hepatitis C because it provides data on staging and disease activity, concomitant liver disease and associated metabolic processes, prognostic assessment and therapeutic monitoring. As liver biopsy is an invasive method, it presents certain risks, including mortality and morbidity (the risk of severe complications is 1/4,000 to 1/10,000). Moreover, it has some limitations, including sampling errors and interobserver disagreement, especially for intermediate degrees of fibrosis.46–49
Noninvasive methods used to evaluate the staging of fibrosis have shown good accuracy, and several methods or combinations have been validated and can replace biopsy in clinical practice.
Mechanical noninvasive methodsThe four mechanical methods currently available are: transient elastography (liver assessed by Fibro-Scan®), acoustic radiation force elastography (ARFI), shear wave elastography (SWE) and MRI elastography. FibroScan®, ARFI and SWE have in common the fact that they are unable to discriminate between intermediate stages of fibrosis, their best application being for the diagnosis of cirrhosis and advanced fibrosis (F3, F4).49,50
Of the four methods mentioned, transient elastography (liver assessed by FibroScan®) is the one associated with the greatest number of publications, especially in chronic hepatitis C, and accordingly is the most validated and standardized for almost all liver diseases.51–55 It can be performed at bedside with a rapid learning curve, and it has a validated prognostic value in cirrhosis. However, the equipment is expensive, obesity and the presence of ascites are limitations for the procedure, and acute hepatitis, extrahepatic cholestasis, and congestion can lead to false positive results.50
ARFI and SWE are more recent and very promising methodologies, associated with the propagation of acoustic waves. In a recent meta-analysis, ARFI gave results comparable to FibroScan® for the diagnosis of cirrhosis and advanced fibrosis,56 and SWE can have a superior performance for significant fibrosis (≥ F2).50 Despite this potential, these two methods, compared with FibroScan®, still need better standardization and better knowledge of the confounding factors, and have a longer learning curve.50 MRE is the least studied and standardized, and the most expensive, of the mechanical methods, but it can have great sensitivity in differentiating intermediate degrees of fibrosis.57
Biochemical (biomarkers) and combined methodsSeveral biochemical tests have been investigated in hepatitis C in an attempt to evaluate the staging of chronic hepatitis C.Among these, the most validated are undoubtedly the noncommerical APRI (AST-to-platelet ratio index) and FIB4 (AST, ALT, age and platelets) and the patented Fibrotest® and Fibrometer®. Fibrometer®, and especially Fibrotest®, have been extensively used in France and other countries, and are validated for use in various liver diseases. These two tests are patented and must be performed in laboratories that meet certain quality standards, and thus are more expensive and less readily available than other tests. The APRI score and FIB4 are simple, reproducible, lower cost and more reliable. Comparative independent studies could not demonstrate significant differences between the different biochemical methods and also pointed out that their performance alone is not adequate to replace liver biopsy58,59 and that none of them should be recommended as a sole method for staging disease. The exception would be in low- and medium-income countries where the WHO guideline2 suggested the utilization of APRI and FIB4 for staging of advanced and significant fibrosis. For this purpose, there are three main cutoff values for APRI: < 0.5 for the exclusion and ≥ 1.5 for the confirmation of the presence of significant fibrosis, and < 1.0 and ≥ 2 for the diagnosis of cirrhosis.2,60 For FIB4, the threshold value would be < 1.45 for excluding significant fibrosis and > 3.25 for confirming cirrhosis.2,58 The staging strategy proposed by WHO experts uses a combination of the low cutoff to rule out the presence of a particular stage of fibrosis and the high cutoff to confirm that the patient has fibrosis that is greater than or equal to a particular stage (e.g. > F2 or F4).61
Although this strategy could have some application, as stated by WHO experts, a significant number of patients will fall in the indeterminate range of test results (i.e., their score will be between the low and the high cutoffs), and such patients will need an additional method to predict liver fibrosis.2
To increase the sensitivity and specificity of noninvasive methods, attempts have been made to combine the methods. The first successful combination was of Fibrotest® with APRI (SAFE-biopsy) for the diagnosis of both cirrhosis and a significant biopsy.60 Alternatively Fibrometer®, Fibrotest® and APRI can be combined with elastography using FibroScan® in diagnostic algorithms.61–63 With the use of these algorithms, there is an important reduction in the need for a liver biopsy, and a high percentage of cases can be correctly classified.64,65
Recommendations- 1.
Whenever possible, use noninvasive methods. Liver biopsy in the staging of hepatitis C is reserved for cases of clinical suspicion of association with other liver disease, cases of disagreement between the results of noninvasive methods, or cases where the use of indirect methods is clinically or technically impossible (Class 1, Level A).
- 2.
The assessment of advanced liver fibrosis (F3, F4 of METAVIR classification) and cirrhosis (F4) in patients with chronic hepatitis C can be made indirectly by mechanical methods, preferably by elastography by FibroScan® (Class 1, Level B).
- 3.
The highest accuracy and greatest reduction in the need for liver biopsy is achieved with the combination of two biomarkers or with the combination of a biomarker with a mechanical method (Class 1, Level B).
- 4.
In the setting of low-income countries, the combination of a low and high cutoff level for the APRI and FIB4 levels can be indicated (recommendation 2B) but a significant number of patients will not be properly classified (outside the cutoff values).
Sustained eradication of HCV RNA is possible and is associated with higher overall survival, even for patients who already have cirrhosis.66–70 Remarkably, the success of therapy has increased exponentially with the arrival of new DAAs. The downside is that these new agents have a high cost and are not uniformly available in different parts of the world.71 Any HCV-infected patient is a potential candidate for antiviral treatment, but the priority should be for those with more advanced fibrosis (METAVIR ≥ F2) and/or clinically significant extra-hepatic manifestations associated with HCV.56,66 Patients with milder disease and no compelling reason to eradicate HCV should probably wait for the IFN-free therapies that will be available in the near future.
Fortunately in some Latin American countries the new DAAs are in the process to be approved a we expect that they can be use in this year.
Finally, it is important to be familiar with all nomenclature and definitions in the medical treatment of hepatitis C (Table 5).
Treatment response in hepatitis C virus (HCV) infection
| • Rapid viral response: undetectable HCV RNA at four weeks. |
| • Early viral response: = 2 log reduction in HCV RNA at 12 weeks. |
| • End of treatment response: undetectable HCV RNA at the end of treatment. |
| • Sustained virologic response (at 12 or 24 weeks): undetectable HCV RNA 12 or 24 weeks after completion of treatment. |
| • Null response: early viral response not achieved. |
| • Partial response: early viral response achieved, but virus not completely suppressed by week 24. |
| • Virologic breakthrough: HCV RNA undetectable during treatment, but virus re-emerges while still on treatment. |
| • Relapse: reappearance of HCV RNA after cessation of treatment. |
In resource-limited countries, treatment-naïve patients with HCV GT1 usually have access to PEG-IFN/RBV plus one of the first-generation PIs: BOC or TVR. There are no head-to-head trials comparing both agents; however, most recent meta-analyses indicate similar efficacy and safety of both agents (Grade 1A).72,73 Furthermore, about half of the treated patients achieve an extended rapid viral response (eRVR) and are able to use response-guided therapy (RGT) to shorten the treatment duration to 24 weeks of triple therapy without loss of SVR, pro-vided that they are not cirrhotic (Grade 1A).7,8,74 Definitions of eRVR differ for BOC (HCV RNA < 15 IU/mL between weeks 8 and 24) and TVR (HCV RNA < 15 IU/mL at week 4 and 12). Stopping rules also differ for BOC (HCV RNA > 100 IU/mL at week 12 or detectable at week 24) and TVR (HCV RNA above 1,000 IU/mL at week 4 or 12, or detectable at week 24). Thus, the therapeutic scheme recommended with BOC for a noncirrhotic treatment-naïve patient is 4 weeks of PEG-IFN/RBV alone (lead-in) followed by BOC plus PEG-IFN/RBV for 24 weeks in those with eRVR or 44 weeks in those without eRVR.7 For a noncirrhotic treatment-naïve patient treated with TVR, the recommendation is to start directly with 12 weeks of TVR plus PEG-IFN/RBV followed by 12 weeks of PEG-IFN/RBV in those with eRVR or 36 weeks in those without eRVR.8,74 Registration trials in HCV GT1 treatment-naïve patients show that triple therapy with either BOC7 or TVR8 plus PEG-IFN/RBV has a higher SVR rate than PEG-IFN/RBV alone (66-75% vs. 38-44%, respectively) (Grade 1A). Overall, the safety of triple therapy was similar to that of PEG-IFN/RBV, with around 10-15% of severe adverse events (SAEs) and < 1% of deaths in both regimens. However, there was a higher incidence of the following adverse events compared with PEG-IFN/RBV alone:7,8,74
- 1.
Anemia with TVR and BOC (39-49% vs. 19-29%);
- 2.
Disgeusia with BOC (43 vs. 18%);
- 3.
Skin rash with TVR (61 vs. 48%).
Pruritus and anal discomfort were seen more often with TVR than with BOC.
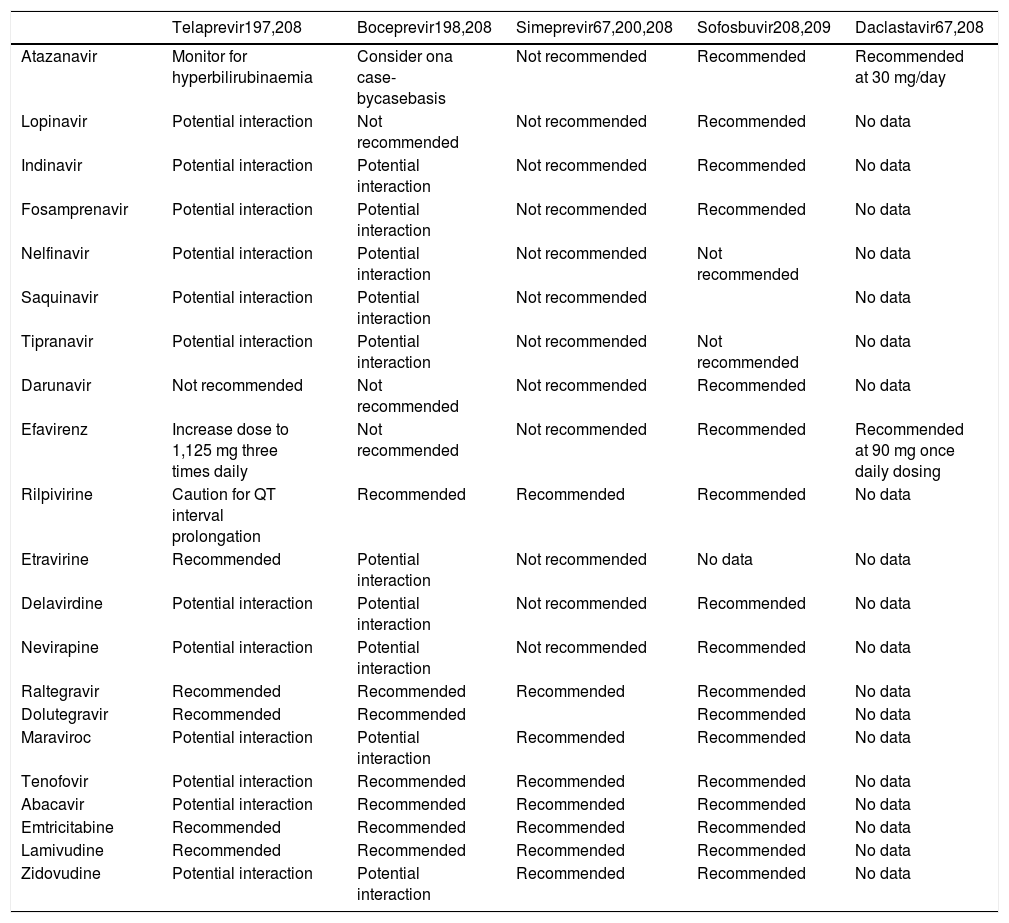
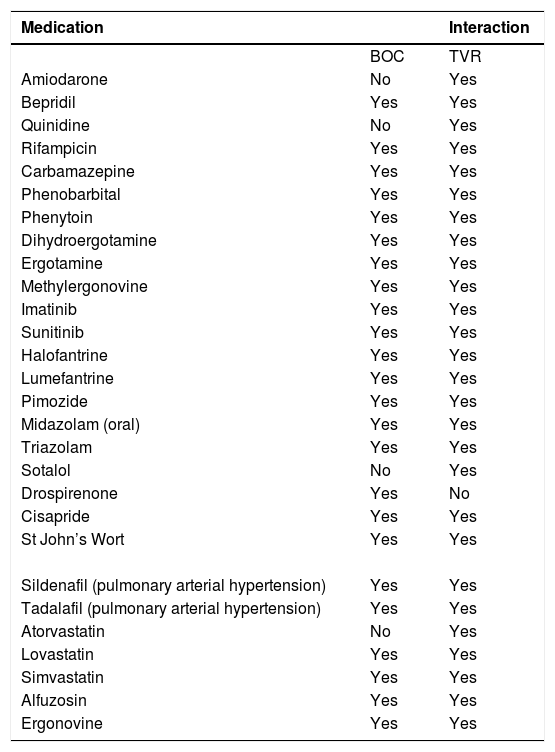
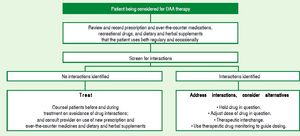
Pill burden was more of an issue with BOC (4 pills every 8 h) than TVR (2 pills every or 3 pills every 12 h75). Anemia is the primary concern with the first-generation DAAs and should be carefully looked for. It is important to assess the cardiovascular status of patients before starting therapy, especially individuals above 50-60 years of age. Those with lower baseline hemoglobin might need weekly follow-up. RBV dose reduction to 500-600 mg/day does not impact SVR, even if carried out when HCV RNA is still detectable. Erythropoietin can be started if hemoglobin falls to < 10 mg/dL. Transfusion can become necessary in around 5% of patients.7,8,74 TVR-associated rash occurs in approximately 50% of patients and is usually mild or moderate, frequently managed only with antihistamines and topical steroids. Severe rashes or lesions involving > 50% of the body surface require treatment interruption. Once stopped, neither TVR nor BOC can be restarted. Patients should be advised to inform health care personnel about all concomitant medications, and a list of potentially harmful drug-drug interactions is available on internet sites such as Hep-Drug Interactions from the University of Liverpool (http://www.hep-druginteractions.org), among others.
Recently, two second-wave DAA agents were approved in the USA and European Union (EU) and as we mentioned above the new DAAs in some Latin American countries are in the process to be approved soon: the polymerase inhibitor SOF and the PI SMV. The recommended therapeutic scheme with SOF for HCV GT1 treatment-naïve patients is one pill (400 mg) of SOF once daily plus PEG-IFN/RBV for a fixed duration of 12 weeks, with an SVR of 89% in GT1 patients vs. 60% estimated for the historical control group in the NEUTRINO trial.76The SVR dropped to 82% in HCV cirrhotics (84% in GT1a and 67% in GT1b) (Grade 1A). Only 2% interrupted treatment because of SAEs.
The recommended therapeutic scheme with SMV for HCV GT1 treatment-naïve patients consists of one pill (150 mg) of SMV once daily plus PEG-IFN/ RBV for 12 weeks, followed by PEG-IFN/RBV for 12 weeks in those with eRVR, defined as HCV RNA < 25 IU/mL at week 4 and undetectable (< 15 IU/ mL) at week 12.69,70 Overall, in the QUEST 177 and QUEST 278 trials, the SVR was around 80% with triple therapy vs. 50% with PEG-IFN/RBV (Grade 1A). Almost 90% of patients achieved eRVR and stopped therapy at 24 weeks, with an SVR of about 88%. SVR was < 30% in patients without eRVR, which is probably too low to justify continuing therapy (Grade 3). Cirrhotics treated with SMV had a lower SVR rate, between 58 and 65%, in the QUEST 177 and QUEST 278 trials, respectively. Among HCV GT1a patients with the Q80K variant present at baseline, SVR with SMV plus PEG-IFN/RBV has the same efficacy as PEG-IFN/RBV alone.77,78 This mutation occurs in around one-third of GT1a patients in North America but seems to be much less frequent in other parts of the world including South America.79 Current guidelines advise not to use SMV in GT1a patients with the Q80K variant2,66,67 (Grade 3). Discontinuation for adverse events was < 3% in the QUEST-1 and QUEST-2 trials.77,78 Triple therapy with SMV was associated with some pruritus, mild rash, mild photosensitivity, and a transient and mild elevation in indirect bilirubin levels, without a concomitant rise in aminotransferases.77,78 Recent guidelines consider SOF plus PEG-IFN/RBV for 12 weeks, if available, to be the treatment of choice for treatment-naïve HCV GT1.2,66,67 Treatment with SMV but not TVR or BOC66 plus PEG-IFN/RBV is considered to be a suitable alternative.2,66,67(Grade 3).
Treatment-experienced patientsAmong treatment-experienced patients with HCV GT1, a phase3 trial showed that a 4-week lead-in with PEG-IFN/RBV followed by 34-44 weeks of BOC plus PEG-IFN/RBV (depending on eRVR) had a higher SVR than PEG-IFN/RBV for 48 weeks, both in relapsers (69-75% vs. 29%, respectively) and partial responders (40-52% vs. 7%, respectively) (Grade 1A).9 Null responders were not included in this study. Similarly, TVR plus PEG-IFN/RBV for 12 weeks followed by PEG-IFN/RBV for 36 weeks showed a higher SVR rate compared with PEG-IFN/RBV for 48 weeks in all groups of patients, including relapsers (83 vs. 24%, respectively), partial responders (59 vs. 15%, respectively) and null responders (29 vs. 5%, respectively) (Grade 1A).80 A lead-in arm was tested in this study and did not show a higher SVR rate compared with no lead-in (Grade 1A). The same stopping rules used for treatment-naïve patients were applied for the treatment-experienced patients ( Grade 1A). Lead-in could be used in the management of nonresponder patients who are not willing to wait for better therapies. Indeed, if HCV RNA drops > 1 log IU/mL compared with baseline at the end of the lead-in (week 4), the chance of an SVR increases to about 50%, vs. only 5% in those with < 1 log IU/mL drop (Grade 2).9,80 Overall, the safety of triple therapy in treatment-experienced patients was similar to that reported in treatmentnaïve patients.
Regarding the second-wave agents, there are no phase 3 data available exploring the use of SOF plus PEG-IFN/RBV. Even though SOF + PEG-IFN/RBV has not been studied in patients who previously failed PEG-IFN/RBV(and probably never will be), an exploratory analysis by the FDA shows that approximately 78% of HCV GT1 patients who had previ- ously failed PEG-IFN/RBV would have responded to SOF plus PEG-IFN/RBV. Alternatively, SMV plus PEG-IFN/RBV for 12 weeks followed by 12 or 36 weeks of PEG-IFN/RBV (depending on eRVR) showed around 80% SVR in relapsers and 50% in previous nonresponders to PEG-IFN/RBV (Grade 1A).10,81 Safety was similar to that reported in treat-ment-naïve patients (Grade 1A).10,80
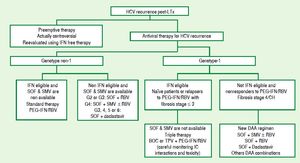
Treatment of HCV GT1 with IFN-free therapyThe COSMOS study is a phase 2 trial that explored the use of 12 or 24 weeks of fixed therapy with SMV (150 mg once daily) plus SOF (400 mg once daily) ± RBV, in two cohorts of HCV GT1-infected patients: prior null responders with META- VIR F0-2 (Cohort 1)81 and prior null responders and treatment-naïve patients with METAVIR F3-4 (Cohort 2).82–83 In Cohort 1, the SVR rate was similar in all treatment arms, ranging from 79 to 96%, with no significant advantage of RBV use or longer treatment duration (Grade 2A). In Cohort 2, SVR was also similar among treatment arms, ranging from 93 to 100%, with no significant advantage of RBV use or longer treatment duration (Grade 2A). Safety was remarkably good, with less than 2% SAEs. This regimen, although based on phase2 trials with low numbers of patients, is being currently recommended in the most recent guidelines as the treatment of choice for patients with HCV GT1 who are ineligible for or previous nonresponders to IFN-based therapy (Grade 2A).2,67
Recommendations for HCV GT1 TreatmentA) Current standard of care with PEG-IFN-based therapy- 1.
Dual therapy with PEG-IFN/RBV is suboptimal for most patients with genotype 1, except for a small subgroup of patients with IL28B CC, minimal fibrosis and RVR. Therefore, triple therapy is superior to dual therapy with PEG-IFN/RBV and should be preferred in countries where it is available (Class I, Level A).
- 2.
Patients with mild fibrosis and without extrahepatic manifestations could wait for IFN-free therapy and should be followed closely to make sure that there is no rapid disease progression (Class II, Level B).
- 3.
Treatment with TVR plus PEG-IFN/RBV should be stopped if HCV RNA is > 1,000 IU/mL at weeks 4 or 12 or detectable at week 24 (Class I, Level A).
- 4.
Treatment with BOC plus PEG-IFN/RBV should be stopped if HCV RNA is > 1,000 IU/mL at week 8 or > 100 IU/mL at week 12 or detectable at week 24 (Class I, Level A).
- 5.
Giving TVR or BOC to treatment-naïve patients who have eRVR and fibrosis METAVIR stage < F3 could shorten triple therapy to 24 weeks based on response-guided therapy (Class I, Level B).
- 6.
SOF plus PEG-IFN/RBV for 12 weeks is superior to triple therapy with TVR or BOC and should be preferred in countries where it is available (Class II, Level B).
- 7.
SMV plus PEG-IFN/RBV for 24 weeks in patients with eRVR is superior to triple therapy with TVR or BOC in patients with GT1b or GT1a without the Q80K variant and could be an alternative option in countries where SOF is not available (Class II, Level B).
- 8.
Treatment-experienced patients with null or partial response to PEG-IFN/RBV have low rates of SVR with PEG-IFN-based therapies, especially if METAVIR F3/F4. Therefore, patients should preferably wait for IFN-free therapy. If treatment with TVR or BOC is contemplated, it is recommended to start with a lead-in phase and to proceed with triple therapy only if HCV RNA drops > 1 log at week 4 of dual therapy (Class II, Level B).
- 9.
Phase 3 results with SOF plus SMV for 12 weeks or SOF plus DCV for 12 weeks are still pending. Based on phase 2 data, these regimens could be recommended for patients who either are IFN ineligible or have had null or partial response to PEG-IFN/RBV (Class II, Level A).
- 10.
SOF plus DCV for 12-24 weeks is preferable in patients who failed triple therapy with TVR or BOC, because there is no reliable evidence that SOF plus SMV can be used in patients that failed a regimen with a protease inhibitor (Class III, Level B).
- 11.
Phase 3 data showing SVR above 80% with short duration therapy are already available for several combinations of new DAA compunds, such as SOF plus LDV co-formulated in a single pill, ABT-450/r/ombitasvir plus dasabuvir, and asunaprevir plus daclatasvir (for genotype 1b);however, at the time of this writing, these have not been approved, so they will be reviewed in the future when this guideline is updated.
HCVGT2 accounts for nearly 10% of the patients with chronic HCV worldwide. Until recently, the combination of PEG-IFN and RBV was considered to be the standard therapy for patients chronically infected with GT2 HCV.84 This regimen is associated with the best rates of SVRcompared with other GT, reaching 85%. However, this protocol has many adverse effects, and there are patients who are unable to be treated with PEG-IFN and patients who have previously failed to obtain an SVR with standard therapy. The DAAs TVR and BOC are approved for use only for GT1. An alternative treatment is necessary, and one potential option is the second-generation DAAs, which showed activity across all GTs in in vitro studies.
DAAs TVR is an oral nucleotide analogue inhibitor of the HCV-specific NS5B polymerase enzyme, which has shown pangenotypic activity in vitro. Two randomized, phase 3 studies were conducted in patients with chronic hepatitis C GT2 or GT3 infection. In both studies, SOF and RBV were administered orally at a dose of 400 mg once daily and 800-1,200 mg twice daily, respectively. In the first trial, named POSITRON, the safety and efficacy of SOF+RBV over 12 weeks was compared blind with that of placebo in patients unable to receive PEG-IFN. The overall SVR rate was 78 vs. 0% (p < 0.001). The SVR rate was 93% among patients with GT2 infection. This high SVR rate was similar when cirrhosis was diagnosed. In the second study (FUSION), 201 patients who had failed prior treatment were randomized to receive 12 or 16 weeks of treatment. HCV GT2 infections were significantly associated with a high SVR rate with both treatment durations (86 and 94% respectively). Cirrhotic patients had 60 SVR when they received 12 weeks of treatment and 78% SVR with 16 weeks (compared with 96 vs. 100% in the patients without cirrhosis).85
No patient receiving SOF in either study had virologic breakthrough, and among the patients who had a relapse, sequencing analysis of samples collected at the time of relapse showed no resistance-associated variants (RAVs).
Regarding safety, the rates of SAEs in the POSITRON trial were 5% in the SOF plus RBV group and 3% in the placebo group; in the FUSION study, the rates were 5% in the 12-weeks group and 3% in the 16-weeks group. Patients treated with SOF and RBV had higher rates of fatigue, insomnia and anemia compared with those who received placebo.
Zeuzem, et al.86 conducted a study involving HCV GT2 and GT3 (treatment-naïve and previously treated patients) that confirmed the efficacy described above. The HCV GT2 group was randomized to receive SOF plus RBV or placebo for 12 weeks. AnSVR was obtained in 68 of 73 treated patients. The rates of response were consistently high across subgroups. The absence of virological breakthrough during treatment and the absence of RAVs in relapse confirm thatthe SOF plus RBV regimen has a high barrier to resistance.The reasons for the higher rates of response among patients with HCV GT2, also observed among patients treated with PEG-IFN/RBV, remain unclear.
LDV demonstrated a high potency for HCV GT1a, GT1b, GT4a, and GT6a but lower activity against GT2a and GT3a.87
In vitro, DCV is an oral highly selective NS5A inhibitor of HCV replication with broad coverage of HCV GTs. The combination of DCVplus SOF given for 24 weeks achieved an SVR in 91% of treatment-naïve patients infected with HCV GT2/GT3. Addition of RBV had no effect on the SVR rate.88
This treatment is well tolerated, has comfortable administration,short treatment duration and excellent efficacy. The expected high cost of this treatment will preclude its prompt and wider use, allowing room for alternative cheaper options in this easier-to-treat population. Access is currently the most important limitation on this treatment.
Recommendation- 1.
Combination of daily SOF (400 mg) and daily RBV (1,000 or 1,200 mg in patients < 75 kg or > 75 kg, respectively) for 12 weeks is recommended in treatment-naïve patients and treatment-experienced noncirrhotic patients (Class 1, Level A).
- 2.
Extended treatment should be considered in cirrhotic nonresponder patients (Class 1, Level B).
- 3.
If there are no contraindications, PEG-IFN/RBV may be considered to be an acceptable treatment until SOF becomes available and accessible (Class 1, Level A).
Overall, it is estimated that about 10-15% of the world HCV reservoir is accounted for by GT3.89 The approved treatment for chronic HVC GT3 in South America is still PEG-IFN/RBV for 24 weeks with a reported SVR rate before the addition of PIs of 69%, far lower than for GT2-infected patients (82%) but higher than for those with GT1 (45%-50%).4,90,91 A better understanding of the HCV life cycle has led to the development of a number of new DAAs.92
DAAs associated with IFN-containing regimensTVR and BOC are an important breakthrough for hepatitis C GT1 treatment, increasing SVR rates in treatment-naïve patients from 44 to 70%.93,94 Unfortunately, in GT3 patients, BOC monotherapy achieved only a modest drop in HCV RNA levels, while the activity of TVR was negligible.95,96
Patients treated with DCV plus PEG-IFN for 12 or 16 weeks achieved numerically higher SVR rates than those treated with PEG-IFN/RBV alone, with the SVR rate being lower in GT3 than GT2 patients (68 vs. 83%, respectively).97 Because this difference was not statistically significant, this combination was not studied further for GT3.
In the ELECTRON study, a combination of SOF (400 mg once daily) and RBV for 12 weeks plus PEG-IFN (4, 8 or 12 weeks of therapy) resulted in a 100% SVR at week 12 in a small group of noncirrhotic GT2 and GT3 patients.98 In a similar study (PROTON), patients with GT2 or GT3 without cirrhosis who received SOF plus PEG-IFN/RBV for 12 weeks achieved an SVR12 rate of 92% (23/25 patients).99 The LONE-STAR-2 study evaluated SOF plus standard of care for 12 weeks in GT3 treatment-experienced individuals: the reported SVR was 83% (20/24), including 10/ 12 patients with cirrhosis.100
DAAs with IFN-free regimensA noninferiority phase 3 study, the FISSION trial, included treatment-naïveGT2 and GT3 patients and compared SVR rates between SOF and RBV for 12 weeks with standard treatment with PEG-IFN/ RBV for 24 weeks.101 Although the SVR12 rates were similar for both groups (67%), SVR rates were significantly lower for GT3 than for GT2 (58 vs. 97%; respectively). Furthermore, in GT3 patients, SVR rates in the SOF arm were even lower than in the standard-of-care arm (58 vs. 62%, respectively, p = NS).101
Similar findings were described in the FUSION and POSITRON trials. These studies evaluated SOF and RBV for 12 or 16 weeks in prior nonresponders (FUSION) and patients intolerant to IFN (POSI-TRON).102 Again, SVR rates were consistently lower in GT3 than in GT2 patients. Cirrhosis was associated with even lower SVR12 rates: 60 and 19% in GT2 and GT3, respectively.102 Extending therapy with SOF and RBV from 12 to 16 weeks increased overall SVR rates from 86 to 94% in GT2 patients and from 30 to 62% in GT3 patients. Notably, in the subgroup of patients with cirrhosis and GT3, prolonging therapy from 12 to 16 weeks tripled SVR12 rates from 19 to 61%.102 Thus, with the intention of improving SVR rates in this difficult-to-treat population, the VALENCE study evaluated SOF/RBV therapy for 24 weeks in GT3 patients. The overall SVR12 was 84% and was higher among treatment-naïve patients than among treatment-experienced patients (93 vs. 77%, respectively). In treatment-experienced noncirrhotic and cirrhotic patients, the SVR12 rates were 87 and 60%, respectively.103
In more recent open-label study,GT2 and GT3 patients who had failed 12- or 16-week SOF/RBV regimens (FISSION, FUSION and POSITRON) were offered either SOF/RBV for 24 weeks or SOF/ PEG-IFN/RBV for 12 weeks. Retreatment with SOF regimens of longer duration or with the addition of PEG-IFN resulted in SVR12s of 63% (24/38) and 91% (20/22), respectively.103
Two phase 2 trials evaluated the association of SOF with two different NS5A inhibitors. Firstly, the ELECTRON-2 trial evaluated the combination of SOF with LDV ± RBV for 12 weeks in treatmentnaïve GT3 patients. The addition of RBV to SOF/ LDV resulted in a 100% SVR12, while the SOF/LDV group showed 64% SVR12.104 Secondly, GS-5816 25 mg or 100 mg was associated with SOF in GT1-GT6 treatment-naïve noncirrhotic patients. The SVR12 in GT3 patients was 93% in both groups (25/27).105
Recently, a study evaluated the combination of DCV and SOF in an IFN-free regimen in previously untreated patients with GT1, GT2 or GT3.106 The patients were randomly assigned to receive DCV plus SOF ± RBV for 24 weeks. A total of 89% (16/18 patients) with GT3 infection had an SVR12. The most common adverse events were fatigue, headache and nausea. The addition of RBV did not affect the virological response rate and increased the frequency of anemia.106
In summary, hepatitis C GT3 infection has become one of the most difficult to treat. It is now debatable whether GT2 and GT3 patients should be combined in clinical trials because of their distinct characteristics. Few data are available to define the best treatment option for this population. In Latin America, the combination of SOF with RBVfor 24 weeks seems to be the best alternative for noncirrhotic HCV GT3 patients, once SOF becomes approved. In IFN-toler- ant patients who have failed a previous SOF-RBV regimen and in treatment-naïve patients with cirrhosis, therapy with SOF/PEG-IFN/RBV may be considered to be the best alternative. More effective approaches such as SOF/DCV or SOF/LDV plus RBV may not be alternatives because of their prohibitive cost. In the meantime, while we wait for approval of new DAAs, the combination of PEG-IFN/RBV remains an acceptable standard of care.67
Recommendations for GT3 HCV InfectionA) Current standard of care with PEG-IFN and RBV- 1.
Treatment duration should be personalized according to the on-treatment virological response at weeks 4 and 12 and eventually week 24 (Class I, Level B).
- 2.
Treatment should be stopped at week 12 if the HCV RNA decrease is < 2 log10 IU/mL and at week 24 if HCV RNA is still detectable (Class I, Level B).
- 3.
In patients with an RVR and low baseline viral load (< 400,000-800,000 IU/mL) and absence of negative predictors of response (advanced fibrosis, metabolic syndrome, insulin resistance or hepatic steatosis), treatment for 12-16 weeks can be considered (Class II, Level B).
- 4.
Patients who have an early virologic response (HCV RNA detectable at week 4 but undetectable at week 12) should be treated for 48 weeks (Class II, Level C).
- 1.
Weight-based RBV (1,000 or 1,200 mg in patients < 75 kg or ≥ 75 kg, respectively), and daily SOF (400 mg) for 24 weeks. This alternative should be proposed in treatment-naïve noncirrhotic patients (Class II, Level A).
- 2.
PEG-IFN-a, weight-based RBV (1,000 or 1,200 mg in patients < 75 kg or ≥ 75 kg, respectively), and daily SOF (400 mg) for 12 weeks (Class II, Level A). This regimen is especially recommended in treatment-experienced and cirrhotic patients.
- 3.
Combination of daily SOF (400 mg) and change to “new combinations of SOF plus NS5A inhibitors such as DCV and or LDV with or without RBV should also be considered in the future”.
- 4.
PEG-IFN/RBV remains an acceptable standard of careuntil SOF and new direct antiviral agents are approved.
Although they account for more than 20% of all HCV cases worldwide, GT4, GT5 and GT6 have generally been neglected or underrepresented in most large multinational clinical trials.107
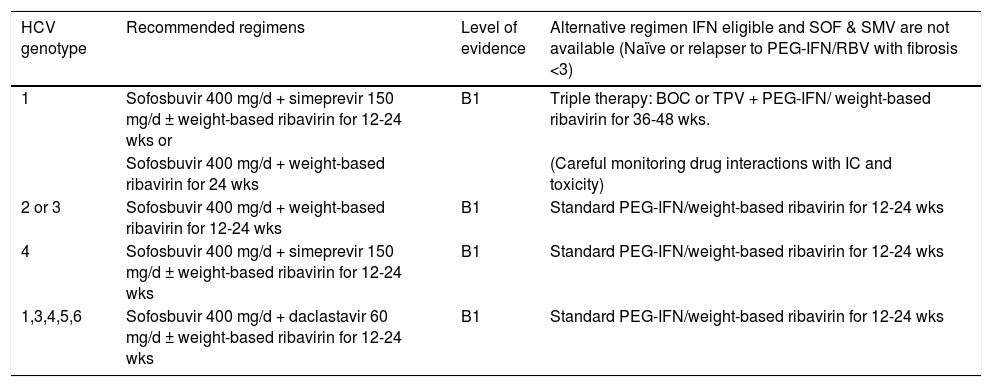
New treatment options for HCV GT4(Table 6)Four to six treatment options are suggested for the management of patients infected with HCV GT4.66,67
- •
Treatment-naïve patients can be managed with a combination of weekly PEG-IFN, daily weight-based RBV (1,000 or 1,200 mg in patients < 75 kg or ≥ 75 kg, respectively) and daily SOF (400 mg) for 12 weeks (recommendation B1; Class IIa, Level B).
- •
Patients who are PEG-IFN intolerant/ineligible can be treated with daily SOF (400 mg) and daily weight-based RBV (1,000 or 1,200 mg in patients < 75 kg or ≥ 75 kg, respectively) for 24 weeks (recommendation C2; Class IIb, Level B).
- •
One alternative consists of a combination of weekly PEG-IFN, daily weight-based RBV 1,000 or 1,200 mg in patients < 75 kg or ≥ 75 kg, respectively) and daily SMV (150 mg) (recommendation B1; Class IIb, Level B).
- •
SMV should be administered for 12 weeks in combination with PEG-IFN and RBV, followed by PEG-IFN + RBV for an additional 12 weeks (total treatment duration 24 weeks) in treatment-naïve and prior relapser patients. However, an additional 36 weeks with PEG-IFN + RBV (total treatment duration 48 weeks) should be administered in prior partial and null responders, including cirrhotics (recommendation B1). HCV RNA levels should be monitored on treatment because therapy could be shortened if HCV RNA level is < 25 IU/mL at treatment week 4, week 12 and week 24 (recommendation A2).
- •
Although there are no data with the next combination, but extrapolating the results of the COSMOS trial, in patients with HCV GT4, an IFN-free combination of daily SOF (400 mg) and daily SMV (150 mg) for 12 weeks (recommendation B2), adding daily weight-based RBV (1,000 or 1,200 mg in patients < 75 kg or ≥ 75 kg, respectively), should be considered in patients with predictors of poor response to anti-HCV therapy, especially prior nonresponders and/or patients with cirrhosis) (recommendation B2).67
- •
We can consider that patients infected with GT4 can be treated with an IFN-free combination of daily SOF (400 mg) and daily DCV (60 mg) for 12 weeks in treatment-naïve patients or 24 weeks in treatment-experienced patients (pending data with 12 weeks of therapy in treatment-experienced patients) (recommendation B2). Adding daily weight-based RBV (1,000 or 1,200 mg in patients < 75 kg or ≥ 75 kg, respectively) should be considered in patients with predictors of poor response to anti-HCV therapy, especially prior nonresponders and/or patients with cirrhosis (recommendation B2).67
- •
An alternative option is the combination of PEG-IFN, daily weight-based RBV (1,000 or 1,200 mg in patients < 75 kg or ≥ 75 kg, respectively) and daily DCV (60 mg) for 12 weeks followed by 12 weeks of PEG-IFN/RBV alone or a further 12 weeks of PEG-IFN/RBV + DCV (response- guided therapy) (recommendation B1).
- •
DCV should be administered for 12 weeks in combination with PEG-IFN/RBV. DCV should be continued in combination with PEG-IFN/ RBV for an additional 12 weeks (total duration of 24 weeks) in patients who do not achieve an HCV RNA level < 25 IU/mL at week 4 and un-detectable at week 10. PEG-IFN/RBV should be continued alone between week 12 and 24 (total duration of 24 weeks) in patients who achieve an HCV RNA level < 25 IU/mL at week 4 and undetectable at week 10 (recommendation B1).67
- •
For previously nonresponsive GT4 patients, daily SOF (400 mg) plus weekly PEG-IFN and daily weight-based RBV (1,000 or 1,200 mg in patients < 75 kg or ≥ 75 kg, respectively) for 12 weeks has been recommended for retreatment of IFN-eligible subjects (Class IIa, Level C). The alternative retreatment regimen for this type of patients could be daily SOF (400 mg) and weight-based RBV (1,000 or 1,200 mg in patients < 75 kg or ≥ 75 kg, respectively) for 24 weeks (Class IIa, Level B).66
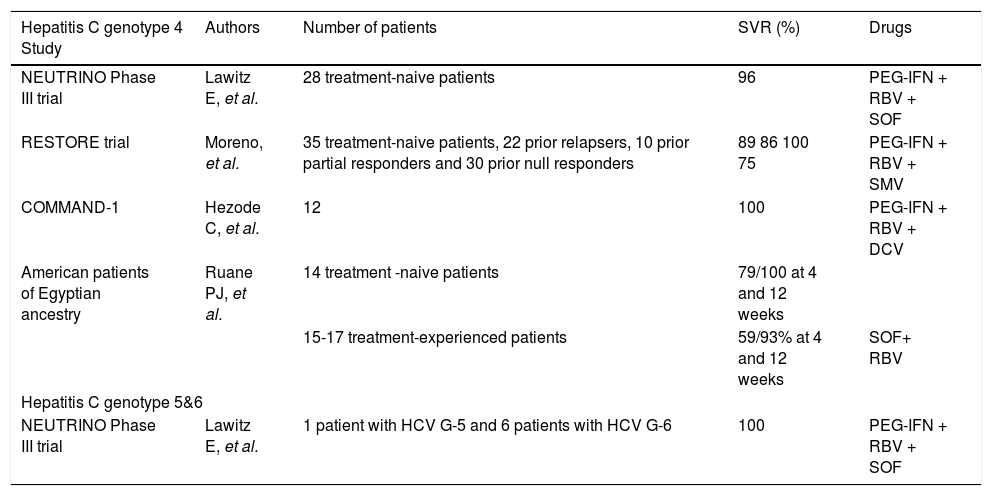
Trials of treatment for HCV genotypes 4, 5 & 6.
| Hepatitis C genotype 4 Study | Authors | Number of patients | SVR (%) | Drugs |
|---|---|---|---|---|
| NEUTRINO Phase III trial | Lawitz E, et al. | 28 treatment-naive patients | 96 | PEG-IFN + RBV + SOF |
| RESTORE trial | Moreno, et al. | 35 treatment-naive patients, 22 prior relapsers, 10 prior partial responders and 30 prior null responders | 89 86 100 75 | PEG-IFN + RBV + SMV |
| COMMAND-1 | Hezode C, et al. | 12 | 100 | PEG-IFN + RBV + DCV |
| American patients of Egyptian ancestry | Ruane PJ, et al. | 14 treatment -naive patients | 79/100 at 4 and 12 weeks | |
| 15-17 treatment-experienced patients | 59/93% at 4 and 12 weeks | SOF+ RBV | ||
| Hepatitis C genotype 5&6 | ||||
| NEUTRINO Phase III trial | Lawitz E, et al. | 1 patient with HCV G-5 and 6 patients with HCV G-6 | 100 | PEG-IFN + RBV + SOF |
Although the prevalence of this GT in Latin America is very low, and the experience is limited to isolated cases, the recommendations that could be followed according to the most available drugs in our region are as follows.26
- •
The standard regimen for treatment-naïve patients with GT4 is a combination of subcutaneous weekly PEG-IFN (PEG-IFN-α2a at a dose of 180 μg/week or PEG-IFN-α2b at a dose of 1.5 μg/kg/week) plus RBV at 15 mg/kg/day in two divided doses for 48 weeks (recommendation A1);26 however, in patients who achieve RVR and who do not have predictors of poor response (baseline viral load > 800,000 IU/mL, advanced fibrosis or cirrhosis and insulin resistance), an international panel of experts suggests that treatment can be shortened to 24 weeks.26
- •
Patients with a complete early virological response (EVR) at week 12 have a high probability of achieving an SVR with a 48-week regimen. Patients with a partial or slow EVR (no RVR and detectable HCV RNA but > 2 log10 drop at week 12 and virus negative at week 24) may be considered for treatment prolongation to 72 weeks, if they can tolerate this.26
The following treatment regimens for GT5 and GT6 can be suggested.
- •
Treatment-naïve patients infected with HCV GT5 or GT6 must be treated with a combination of weekly PEG-IFN, daily weight-based RBV (1,000 or 1,200 mg in patients < 75 kg or ≥ 75 kg, respectively), and daily SOF (400 mg) for 12 weeks (recommendation B1; Class IIa, LevelB).66,67
- •
Patients who are PEG-IFN intolerant or ineligible can be treated with daily SOF (400 mg) and daily weight-based RBV (1,000 or 1,200 mg in patients < 75 kg or ≥ 75 kg, respectively) for 24 weeks (recommendation C2).67
- •
The recommended regimen for HCV GT5 or GT6PEG-IFN/RBV nonresponder patients is daily SOF (400 mg) for 12 weeks and daily weight-based RBV (1,000 or 1,200 mg in patients < 75 kg or ≥ 75 kg, respectively) plus weekly PEG-IFN for 12 weeks also recommended for retreatment of IFN-eligible people (Class IIa, Level C).67
As with GT4, experience with GT5 and GT6 is very limited in our region. However, we can use the following recommendations.25,26
9. Treatment of Acute Hepatitis in AdultsAcute hepatitis C infection is defined as the presence of clinical signs and symptoms of hepatitis within 6 months of presumed HCV exposure.108 The majority of these patients go undetected. Acute HCV infection accounts for 15% of symptomatic cases of acute liver disease.109,110 Early treatment is appropriate for patients who do not spontaneously clear the virus, and is associated with high SVR-rates.
DiagnosisA newly positive HCV RNA polymerase chain reaction (PCR), followed by the development of HCV antibodies within 12 weeks, is considered to be definitive proof of acute infection with HCV. However, this requires documentation of a recent serum sample with a negative HCV RNA PCR and anti-HCV antibodies. In its absence, distinguishing between an acute and a newly discovered chronic infection is difficult, because both cases may have detectable HCV RNA andanti-HCV antibodies. Any patient with symptoms of, or exposure to, HCV should be tested for HCV RNA and anti-HCV antibodies.
HCV RNAThis can be detected by PCR within a period between a few days and 8 weeks postexposure, depending upon the size of the inoculum.111,112 The minimal interval after which a persistently negative HCV PCR test excludes infection has not been established. In a study of 14 patients with needlestick injuries, a negative HCV PCR at 2 weeks post exposure had a 100% negative predictive value.113 Most experts recommend testing at baseline, week 4, week 12, and 6 months.
Anti -HCV antibodiesMost patients seroconvert between 2 and 6 months after exposure. The rate is higher in symptomatic infection, where up to half have detectable antibodies at presentation, while in subclinical infection,it may take a year for antibodies to be de-tectable.112,114 People with suspected acute HCV or known exposure to HCV must have HCV RNA testing by PCR, because a negative antibody test does not rule out infection.115 A positive anti-HCV antibody test does not distinguish acute or early infection from chronic infection or from a prior infection that has spontaneously cleared. Some patients with prior infection may have negative antibody tests because anti-HCV antibody levels may drop to unde-tectable levels in patients who have cleared the infection.116–118
AminotransferaseThe level of aminotransferase can fluctuate; elevations of greater than 10-20 times the upper limit of normal are seen, but not all patients will have these at the time of presentation, and normalization of aminotransferase levels after acute infection does not necessarily mean that the infection has cleared.119,120
Acute vs. chronic infectionThis distinction is important because it has treatment implications, as patients with acute HCV infection who do not spontaneously clear the virus should receive treatment with an IFN-based regimen. Treatment decisions and regimen in patients with chronic hepatitis C are very different.
Spontaneous viral clearanceBetween 14% and 50% of patients with HCV may spontaneously clear the virus.112,121 Recent studies report spontaneous clearance rates of around 50%.36,122–125 Most patients who are destined to clear HCV viremiaspontaneously do so within 12 weeks, and usually no later than 20 weeks, after the onset of symptoms.121,122 However, clearance after follow-up (12 months) has also been described.123 Symptomatic acute HCV infection is associated with a higher rate of spontaneous clearance than asymptomatic infection.121,123–125 Other factors associated with spontaneous clearance include a rapid decline in HCV RNA,126–130 female sex,131 and polymorphisms in the IL28B gene. Patients who clear HCV should have subsequent HCV RNA determinations at 3-month intervals for 1 year.
TreatmentMost patients with acute HCV will develop chronic infection if left untreated. Treatment with an IFN-based regimen during the acute infection leads to SVR rates over 80%.132 Not all patients need treatment, and treatment efficacy depends on several factors.
Who to treatTreatment should be administered to patients with acute HCV who have a high likelihood of being compliant with treatment, as noncompliance is associated with significantly decreased SVR rates,133–135 and to those patients who do not have any comorbid illnesses that are contraindications to treatment.
When to treatThe treatment for symptomatic acute HCV should be delayed for 12 weeks from the time of suspected inoculation, or from the time of diagnosis if the time of inoculation is uncertain, to allow spontaneous clearance to occur. One meta-analysis of 1,075 patients suggested overall SVR rates greater than 80%,132 while a second meta-analysis of 12 trials concluded that delaying therapy by 8-12 weeks did not decrease the SVR rate.136
Patients infected via a blood transfusion and patients with asymptomatic acute HCV should be offered immediate treatment upon diagnosis, because chronic infection appears to be highly likely. The Hep-Net Acute HCV-III study demonstrated that the efficacy of therapy initiated after waiting 12 weeks to evaluate potential HCV clearance might not be inferior to immediate therapy. However, this strategy requires strict compliance of patients with a follow-up test and, if needed, with therapy. In an intention-to-treat analysis of symptomatic patients, the SVR rate (including sustained spontaneous clearance in the delayed group) was higher with immediate treatment than with delayed treatment (67 vs. 54%). This difference was not statistically significant. Among those that completed the treatment and follow-up, the SVR rates were 90 and 93% for the immediate-treatment and delayed-treatment groups, respectively.135
What to treat with- •
PEG-IFN. Patients should receive weekly PEG-IFN-α, either PEG-IFN-α2a 180 μg/week or PEG-IFN-a2b 1.5 μg/kg/week. The reported efficacy is from 57 to 95%.134,137–140
- •
Standard IFN. Standard IFN, 5 million units per day for the first 4 weeks then 5 million units 3 times a week for the remainder of the treatment is an alternative, with an efficacy of 22-98%.128,132,141 PEG-IFN may be preferable because is easier to use and more tolerable, but head-to-head comparative studies are lacking.
- •
RBV. RBV does not appear to be beneficial in patients who are not coinfected with HIV,142,143 unless is not clear whether their infection is acute or chronic,or in patients with acute infection with positive HCR RNA at the end of IFN monotherapy. Patients who are coinfected with HIV should receive PEG-IFN as well as weight-based RBV (< 75 kg, 1,000 mg; ≥ 75 kg, 1,200 mg) divided into two daily doses, provided there is no contraindication to using RBV. The efficacy of monotherapy in coinfected patients ranges from 0% to 10%.144,145 The addition of RBV increases the SVR rates to 47-80%.146–149
- •
DAAs are the standard of care, in combination with RBV with or without PEG-IFNdepending on the GT, for chronic HCV infection in those countries where these agents are available. It is not standard of care for acute HCV infection to use them as first-line therapy because of the high SVR rates with IFN-based monotherapy, the risk of additional side effects with the additional agent, especially with the first generation of DAAs, and the limited data available for the use of these agents in acute infection. A study of the use of TVR in patients coinfected with HIV was published recently.150 Other studies evaluating the use of IFN-free antiviral regimens are underway.
GT and RVR are the most important factors determining the length of treatment. The duration for GT1 should be 24 weeks, but 12 weeks is a reasonable alternative in patients who have achieved RVR and are not tolerating therapy. For GT2, GT3, and GT4, the duration of therapy is 12 weeks.151 In patients with GT1 who achieve RVR, the SVR rates are 46, 75, and 92% with 8, 12, and 24 weeks of treatment,respectively, whereas response rates are 0, 0, and 33%, respectively, among those who failed to achieve an RVR. Similar results were seen in patients with GT4.
Recommendations- 1.
Symptomatic patients should wait 12 weeks from the time of suspected inoculation or time of diagnosis if the time of inoculation is unknown before starting therapy, to allow time for spontaneous viral clearance to occur (Grade 2B). Asymptomatic patients, those infected by blood transfusion and those who are not willing to wait for follow-up testing should be offered immediate therapy (Grade 2B). IFN-based monotherapy is the treatment of choice for those HIVnegative patients who fail to clear the virus spontaneously after 12 weeks of follow-up, rather than following these patients closely (Grade 1A).
- 2.
Patients should receive PEG-IFN(α2a or α2b) rather than standard IFN (Grade 2C).
- 3.
HIV-negative patients with acute HCV who fail to clear the virus spontaneously and are treatment candidates should receive treatment with an IFN-based regimen rather than combination therapy with IFN and RBV (Grade 2B). The addition ofRBV is a reasonable alternative if it is not clear whether the patient’s infection is acute or chronic, or if they are HCV RNA-positive after 12 weeks of therapy (Grade 2B). The addition of a DAA should be considered in those places where it is available.
- 4.
HIV-positive patients with acute HCV who fail to clear the virus spontaneously and are treatment candidates should receive treatment rather than being followed closely (Grade 2C). The treatment should be with IFN-based therapy combined with weight-based RBV (Grade 2C).
- 5.
Patients with GT2, GT3, or GT4 and RVR should be treated for 12 weeks rather than 24 weeks (Grade 2B). Those patients with GT1 who do not achieve RVR should be treated for 24 weeks (Grade 2B), and those who do achieve RVR should also be treated for 24 weeks rather than 12 weeks (Grade 2B).
Patients with HCV-related cirrhosis face a high risk of developing HCC, end-stage liver disease and the necessity of liver transplantation (LT). Therefore, patients with compensated cirrhosis need to be cured of their chronic HCV infection with some degree of urgency.
In a large and heterogeneous region like Latin America, where in most countries the new-generation DAAs have not yet been approved, we have to consider the use of triple therapy with first-generation PIssuch as BOC and TVR for patients with compensated cirrhosis.
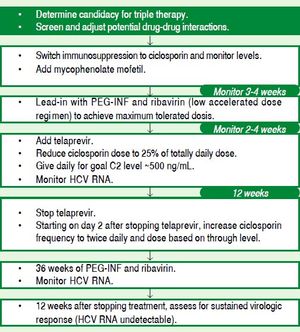
GT1Real-life studies with the first-generation PIs have demonstrated that GT1 cirrhotic patients, usually nonresponders to previous PEG-IFN/RBV treatment, have high adverse event rates and poor SVR rates.152,153 In the CUPIC study, among patients given TVR, 74.2% of relapsers, 40.0% of partial responders, and 19.4% of null responders achieved SVR12. Among those given BOC, 53.9% of relapsers, 38.3% of partial responders and none of the null responders achieved SVR12. In a multi-variate analysis, factors associated with SVR12 included prior treatment response, no lead-in phase, HCV GT1b (vs. GT1a), and baseline platelet count greater than 100,000/mm3. SAEs occurred in 49.9% of cases, including liver decompensation, severe infections in 10.4%, and death in 2.2%. In multivariate analysis, a baseline serum albumin level less than 35 g/L and baseline platelet counts of 100,000/mm3 or less predicted severe side effects or death.152
In another cohort of 160 GT1 cirrhotic patients, 47% with Child-Pugh (CP) ≥ 6 cirrhosis (CP range 6-10), and 35% previous null/partial responders, received triple therapy for a targeted duration of 48 weeks. SVR12 was achieved by 35% of patients with CP ≥ 6 vs. 54% of those with CP = 5. CP = 5, RVR and GT1b independently predicted SVR12. Compared with those with CP = 5, patients with CP ≥ 6 had more PEG-IFN dose reductions, eltrombopag use, transfusions and hospitalizations to manage adverse events. Overall, 42% discontinued treatment early. Nine patients on the waiting list were treated for a median of 97 days prior to LT, and five achieved post-LT SVR.153
In addition, many cirrhotic patients are poor candidates for IFN-based regimens.
GT2 and GT3Refer to the Latin American Association for the Study of the Liver Practice Guidelines: Diagnosis, management, and treatment of Hepatitis C, 2010.154
Countries where SOF and SMV are availableRecently, the first nucleotide analogue NS5B polymerase inhibitor, SOF, and a second-generation once-daily dosing HCV NS3/4A inhibitor SMV, were approved in Europe and the USA.
In the phase 3 NEUTRINO study, the SVR12 in treatment-naïveGT1 patients treated with SOFplus PEG-IFN/RBV for 12 weeks was 90%.76 In the subgroup of cirrhotic patients (17%) included in the study, the SVR rate was 80%, a good response rate compared with previous studies with first-generation DAAs. Unlike observations with the PI-based regimens, the SVR rate in GT1a patients was 98% compared with 82% in GT1b patients. The NEUTRINO study did not include previous null responders, but an FDA analysis estimated an SVR rate in such patients of approximately 70%.
In the phase 3 QUEST 1 study, treatmentnaïveGT1 patients were randomized to receive SMV (150 mg) or placebo for 12 weeks plus PEG-IFN/RBV for 24 or 48 weeks according to RGT. The SVR12 rate for cirrhotic patients (12% of the total population) was 58%, compared with 80% in the overall population. The SVR12 rate for GT1b patients was 90%, compared with 71% in GT1a patients. 155
QUEST 2 was a phase 3 trial with the same design,based on the European population. The rate of SVR12 for cirrhotic patients (11.2% of the total population) was 64.7% compared with 81.3% in the overall population.156 The SVR12 rate in GT1b patients was 82% compared with 80.4% in GT1a patients. The presence of the Q80K mutation detected in the GT1a subtype reduced the SVR rate from 84% to 58%. Although very common in the US and European population, this RAV appears to be less common in the PI-naïve population in Latin America.157
In a phase 2 study of relapsers from previous PEG-IFN/RBV treatment (PROMISE), 260 patients (15.6% cirrhotics) were treated with SMV (150 mg) or placebo for 12 weeks plus PEG-IFN/RBV for 24 or 48 weeks according to RGT. The SVR12 rate for cirrhotic patients was 74.4% compared with 79.2% in the overall population. The SVR12 rate for GT1b patients was 85.3% compared with 70.3% in GT1a patients.158
The combination of SOF plus SMV with or without RBV for 12 and 24 weeks was compared in the COSMOS study in 87 treatment-naïve patients and previous null responders with GT1 HCV infection and advanced (METAVIR F3-F4) fibrosis.159 SVR12 was seen in 100% of treatment-naïve patients. In the null responders group, SVR12 was 100% with triple therapy and 93% in the group without the addition of RBV.
In the near future, the best chance for a potential cure for patients with cirrhosis is an oral combination regimen of potent DAAs. A new class of HCV DAAs called NS5A inhibitors will be an important part of two potent IFN-free regimens: the once-daily, single tablet, fixed-dose combination of SOF/LDV and a three-drug regimen that includes a fixed-dose combination of a ritonavir-boosted HCV PI (ABT-450) plus ombitasvir (NS5A inhibitor) plus dasabuvir (a nonnucleoside polymerase inhibitor).
Three studies evaluated GT2 treatment-naïve patients with cirrhosis with SOF and RBV for 12 weeks. In the POSITRON study, 207 patients in whom IFN treatment was not an option received SOF with RBV for 12 weeks.102 Overall, SVR12 occurred in 92% of GT2 patients and in 94% of cirrhotic patients, suggesting that cirrhosis was not a negative predictive factor in this subgroup of patients.
The FUSION study compared 12 and 16 weeks of SOFwith RBV for treatment-experienced patients.102 Among patients with cirrhosis who received 12 weeks of treatment, the rate of response in GT2 patients was 60%, compared with 96% in noncirrhotic patients. In the arm in which patients received 16 weeks of treatment, the SVR12 was 78% for cirrhotic patients, compared with 100% for patients without cirrhosis.
In the VALENCE study, 73 GT2 patients were treated for 12 weeks with SOF and RBV.86 Overall, an SVR12 was seen in 93% of these patients, with no significant difference between patients with or without cirrhosis.
An open-label, single-arm phase 2 trial (LONESTAR) evaluated the use of SOF with PEG-IFN and RBV in treatment-experienced patients with HCV GT2 or GT3.160 Cirrhosis was present at baseline in 61% of patients. AnSVR12 was seen in 96% of 23 patients with GT2. SVR12 occurred in 93% of patients with cirrhosis and in 100% without cirrhosis. Despite the limitations of this small study, combination PEG-IFN plus SOF and RBV is an alternative 12-week regimen for GT2 patients with cirrhosis.
In the POSITRON study, among patients with cirrhosis who received 12 weeks of treatment with SOF and RBV, the rate of response was 21%, compared with 68% among patients without cirrho-sis.102 Among patients with cirrhosis who received 16 weeks of treatment, the rate of response was 66% (78% with HCV GT2 infection and 61% with HCV GT3 infection) compared with 76% among patients without cirrhosis (100% with HCV GT2 infection and 63% with HCV GT3 infection).
In the FUSION study, among patients with cirrhosis who received 12 weeks of treatment, the rate of response in GT3 patients was 19%, compared with 37% in noncirrhotic patients.102 In the arm in which patients received 16 weeks of treatment, the SVR12 was 61% for cirrhotic patients, compared with 63% among patients without cirrhosis.
In the VALENCE study, 250 GT3 patients were treated for 24 weeks with SOF and RBV.86 Overall, anSVR12 was seen in 85% of these patients, in 61% of patients with cirrhosis andin 91% of patients without cirrhosis.
In the same single-arm phase 2 trial (LONESTAR) evaluated the use of SOFwith PEG-IFN/RBV in treatment-experienced patients with HCV GT2 or GT3.160 Cirrhosis was present at baseline in 61% of patients. SVR12 was seen in 83% of 24 patients with GT3. SVR12 occurred in 83% of patients with cirrhosis. Despite the limitations of this small study, a combination of PEG-IFN plus SOF and RBV is an alternative 12-week regimen for GT3 patients with cirrhosis.
RecommendationsPatients with hepatitis C GT1-current
TVR for 12 weeks plus PEG-IFN/RBV for 48 weeks (recommendation A). BOC for 44 weeks plus PEG-IFN/RBV for 48 weeks (Class I, Level A, Class I, Level A).
GTl-Current and future
SOF plus PEG-IFN/RBV for 12 weeks (recommendation A). SMV for 12 weeks plus PEG-IFN/ RBV for 24-48 weeks (recommendation A). SOFplus RBV for 12 weeks (recommendation B). SOF plus SMV and RBV for 12 weeks (Class II, Level A, Class II, Level A, Class II, Level B, Class II, Level B).
GT2
SOF plus RBV for 12 weeks (recommendation A). SOF plus RBV for 24 weeks (recommendation B). SOF plus PEG-IFN/RBV for 12 weeks (Class II, Level A, Class II, Level B, Class II, Level B).
GT3
SOF plus RBV for 24 weeks (recommendation A). SOF plus PEG-IFN/RBV for 12 weeks(Class II, Level A).
11. Treatment of Hepatitis C in Patients Waiting for Liver TransplantationCurrent AVT in patients awaiting Liver Transplantation IFN-free regimens in patients awaiting Liver Transplantation.
Infection of the graft with HCV after LT is universal in patients who are transplanted for HCV cirrhosis. The course of the HCV recurrence is accelerated, with development of cirrhosis in approximately 30 % of recipients at 5 years.161 There is a need to treat hepatitis C infection in patients on the waiting list to prevent HCV infection of the graft. Therapy for a short period may achieve undetectable levels of HCV RNA at the time of LT. This strategy may prevent graft infection following LT.162 Moreover, a second potential benefit of AVT in these patients is to improve liver function (which in some cases might lead to the patient’s being delisted). Although this has been clearly shown in patients with HBV-related cirrhosis treated with nucleo(s)tide analogues,163 information on HCV-infected cirrhotics is lacking.
Patients with advanced cirrhosis awaiting an LT are one of the most difficult populations to treat. Current data on AVT before LT, including the role of new DAA agents, will be reviewed.
Current AVT in patients awaiting LTCurrent IFN-based treatments are not optimal in patients with advanced liver disease. PEG-IFN/ RBV is indicated for patients on the waiting list and can prevent graft infection in patients who achieve undetectable levels of HCV RNA.164–166 Response rates are higher in individuals infected with HCV GT2 and GT3 compared with GT1, or in those with the IL28B CC GT. In those patients who achieve viral clearance, a longer duration of treatment is associated with lower rates of HCV recurrence after LT. Nevertheless, IFN-based therapy can only be administered in cirrhotics with good liver function. Good candidates are patients with CP < 7 in whom the indication for transplantation is HCC. In patients with more advanced disease, SAEs (e.g., bacterial infections including spontaneous bacterial peritonitis) can be life-threatening. Thus, only a small proportion of HCV-infected patients can undergo IFN-based therapy, and fewer than 30% will achieve a virological response that is maintained after LT.
The development of the two first-generation PIs, BOC and TVR, has been a major step forward in the treatment of chronic hepatitis C.9,10,167,168 Unfortunately, response rates are lower in cirrhotic patients, particularly in those who are previous null responders (a frequent situation in patients awaiting LT).
Verna et al. reported the results of triple therapy in 20 HCV GT1 cirrhotic patients on the waiting list.169 Most of them were previous nonresponders and had HCC. Patients underwent triple therapy (90% with TVR) for a median time of 14 weeks; at week 12, up to 77% of patients had undetectable HCV RNA. Seven of the eight patients transplanted by the time of the analysis reached LT with undetectable HCV RNA, and six patients remained RNAnegative 12 weeks after transplantation.
From a safety point of view, 25% of patients discontinued therapy, and two patients were hospitalized because of liver decompensation.
PI-based regimens in patients with compensated cirrhosis may be associated with SAEs such as severe infections, clinical decompensation and even death. These SAEs were not reported in the registration trials because patients included in these studies were compensated cirrhotics without significant portal hypertension. The main predictive factors for severe complications in cirrhotics undergoing triple therapy are a low platelet count (< 100,000/mm3) and low serum albumin levels (< 35 g/L). The risk for severe complications is 50% in patients with both factors.168 Overall, the data reported in these studies indicate that the proportion of patients on the transplant waiting list that may benefit from triple therapy is very small.
In summary, current IFN-based regimens are only indicated in patients with compensated liver disease with a good chance of achieving a virological response (i.e., GT2/3 or GT1 IL28B CC, preferably those who are treatment naïve or relapsers from previous PEG-IFN/RBV therapy).
IFN-free regimens in patients awaiting LTRecently, the first data on the safety and efficacy of IFN-free regimens in patients awaiting LT have been presented. In most phase 2 and registration trials, the proportion of patients with cirrhosis included is relatively small, and most of these are treatment naïve. A significant proportion of patients on the transplant waiting list are treatment experienced (some with a first-generation PI in triple ther- apy), and most of them have clinically significant portal hypertension. Despite these differences, we decided that it was relevant to review the efficacy data for IFN-free regimens, including those for patients with cirrhosis.
The first oral IFN-free regimen studied in patients awaiting LT combined SOF and RBV.170 In this phase-2 open-label study, 61 patients received therapy until the time of transplant, or up to 48 weeks of treatment before LT while on the waiting list (median duration 17 weeks). Forty patients underwent LT, and of these, 37 (92%) had HCV RNA < 25 IU/mL before LT. Of these, 26 individuals reached 12 weeks of follow-up after transplantation, and 18 (69%) achieved SVR12. Seven patients (27%) had a virological relapse. Safety and tolerance of this regimen was good. The probability of relapse after LT was closely related to the length of virus undetectability before LT was performed. The most frequently reported adverse events were mild and were attributed to RBV. These results are encouraging and suggest that most likely longer treatment duration and/or the addition of a second DAA, or other combinations, will be able to prevent graft infection in most patients.
Other ongoing studies in GT1 patients with compensated and decompensated cirrhosis will provide results soon.171 These studies are being performed in treatment-naïve and treatment-experienced patients, combining SOF plus LDV, SOF plus SMV, SOF plus DCV or ABT-450 boosted with ritonavir plus ABT-267 and ABT-333. Some of these combinations are coadministered with RBV, and the duration of therapy is 12-24 weeks. Despite the small sample size, the results are excellent, with SVR12 rates ranging between 90 and 100%. Therefore, the future for these patients is highly promising.
There are some issues that should be taken into consideration in patients awaiting LT. First, the goal in these patients is to achieve undetectable HCV RNA at the time of transplantation. Be- cause the main source of viral production will be removed (liver explant), a short treatment course may be enough to prevent graft infection. In any case, a minimum duration of undetectable HCV RNA before transplantation will be necessary to prevent graft infection, and this will depend on viral kinetics. In most of these treatment combinations, RVR rates ranged from 90 to 100%. These studies are limited by their small size, but they support the potential efficacy of a short-course treatment before LT to prevent graft infection. Nevertheless, studies in patients with significant portal hypertension are crucial, because first- and second-phase HCV RNA decay in these patients may differ from that in patients with early cirrhosis.
A second distinct feature of patients with advanced liver disease is the impact of liver function on drug pharmacokinetics (PK). Liver metabolic functions are significantly involved in the clearance of several drugs. As an example, when SOF is administered, patients with moderate and severe hepatic impairment experience a less profound viral decline than those with normal liver function. These data might have clinical consequences and might explain why, in patients with advanced liver disease, longer treatment duration can reduce the rates of virological relapse.
A third distinct feature of patients awaiting LT is the potential risk of viral breakthrough or relapse during or after treatment, which may theoretically induce flares that could lead to liver decompensation. It is thus very important to choose the best treatment combination (high potency and high genetic barrier to resistance) to minimize the possibility of virological relapse or the selection of RAVs.
Finally, another aim of AVTin patients with decompensated cirrhosis should be improvement of liver function. Preliminary data from the post-LT compassionate use program using SOF and RBV strongly suggest that viral clearance is associated with a rapid improvement in liver function.
Recommendations- •
In patients awaiting LT, AVT is highly recommended, because it may prevent graft infection if HCV RNA has been undetectable prior to transplantation (recommendation A1).
- •
In patients with preserved liver function (Child-Pugh A with HCC), therapy with PEG-IFN/ RBV might be indicated in patients with GT2 or GT3. Triple therapy including BOC or TVR should only be used in patients with platelets > 100,000/mm3 and albumin levels > 3.5 g/dL.
- •
Treatment including weight-based RBV (1,000 or 1,200 mg in patients < 75 kg or ≥ 75 kg, respectively) and SOF (400 mg) until LTis indicated if SOF is available (recommendation A1).
- •
Patients with preserved liver function (Child-Pugh A) can also be treated with a combination of weekly PEG-IFN-α, daily weight-based RBV (1,000 or 1,200 mg in patients < 75 kg or ≥ 75 kg, respectively) and daily SOF (400 mg) for 12 weeks (recommendation B1).
- •
Patients with preserved liver function (Child-Pugh A) and preserved renal function with GT1-4 infection can be treated with daily SOF (400 mg) and daily DCV (60 mg) for 12 weeks prior to transplantation (recommendation B1).
HCV infection is one of the leading causes of endstage liver disease and the main indication for LT in most countries.172 All patients who undergo LT with detectable serum HCV RNA experience graft reinfection. Between 20 and 30% of patients have developed cirrhosis at 5 years post-LT.173 The outcome for transplant patients with cirrhosis of the graft is severe, with a rate of decompensation at 1 year of approximately 40%.174 Meanwhile 2-8% of patients experience a severe HCV recurrence known as cholestatic hepatitis (CH).175 In these conditions, the prognosis is very poor for patients who do not respond to AVT, retransplantation being the only option in patients with decompensated liver disease. Because preventive therapy is lacking, the prognosis of HCV-infected LT patients, whose survival is shorter than other recipients, can only be changed by the treatment of recurrent infection.161 An SVR is associated with better long-term outcomes, improved graft fibrosis and survival.176 Two approaches can be considered for the timing of AVT after LT: treatment before the development of injury to the graft in the early phase within 1 month after transplantation (preemptive strategy), or treatment when chronic hepatitis has been diagnosed. At present, the preemptive strategy with PEG-IFN/RBV is not recommended, because several studies have shown that it is difficult to initiate AVT with IFN during the postoperative period and that efficacy is poor.177–181 Patients with severe end-stage liver disease prior to LT are frequently ineligible for this approach. However, the preemptive strategy should be reassessed with the availability of new DAAs. It is generally accepted that AVT should be initiated in the presence of histologically proven HCV recurrence. However, this decision must also take into account the patient’s age and general condition, and the stage of fibrosis, usually > F1 on the METAVIR scale. AVT should be initiated in the presence of severe fibrosis and rapid progression of fibrosis with a higher risk of graft loss, especially CH. If a liver graft biopsy is not performed, other noninvasive markers can help to make the treatment decision. A cutoff value of 8.7 kPa for liver stiffness had a sensitivity and a negative predictive value > 0.90 for significant fibrosis and portal hypertension in all cases.182 Also, it is possible to use the measurement of the hepatic venous pressure gradient, where a gradient > 6 mmHg indicates significant fibrosis.183 Although noninvasive markers can discriminate the stage of fibrosis, scheduled protocol biopsies of the graft before AVT are essential for obtaining crucial data such as the progression of graft fibrosis, the presence of rejection or biliary obstruction, or the degree of steatosis. However, the tolerance to therapy decreases significantly in patients with fibrosis stage > 3, suggesting that AVT should be initiated before advanced fibrosis develops.176 Systematic reviews of dual therapy have shown that dose reductions of RBV and/or PEG-IFN were necessary in around 70% of patients, and the rate of treatment discontinuation was approximately 30%.184–186 Liver recipients are particularly exposed to the hematological toxicity of PEG-IFN/RBV and infections. Although a first-generation PI can be used after LT in GT1 patients, these regimens are associated with serious toxicity and drug-drug interactions, especially with tacrolimus and cyclosporine, which limit their potential benefit. Triple AVT with TVR or BOC was less effective in patients with GT1a, IL-28B polymorphism CT or TT and those who were nonresponders to a previous PEG-IFN/RBV regimen. During triple therapy, the risk of biopsy-proven acute rejection seems to be similar to that in control groups and varies from 4% to 6%.187–189 In 2014, SOF and SMV are now recommended as part of the preferred or alternative regimens for the treatment of recurrent HCV infection in posttransplant patients. DCVhas also been in- cluded in some regimens, depending on HCV GT, but published efficacy data are limited. SMV has not been studied with SOF in the posttransplant setting; however, drug interaction studies in noninfected participants indicate that SMV can be given safely in conjunction with calcineurin inhibitors. The combination of SOFand RBV yielded an SVR rate of 77%, 4 weeks after the end of therapy in 40 patients with posttransplant HCV recurrence.190 One liver transplant recipient with severe recurrent HCV was reported to have been treated successfully witha combination of SOF and DCV.191 No clinically significant drug-drug interactions have been reported between SOF, SMV, DCV and calcineurin inhibitors. In Table 7,we have included the recommended and alternative regimens (Figure 2).
ALEH recommended regimens for treatment of recurrent HCV in liver transplant recipient
| HCV genotype | Recommended regimens | Level of evidence | Alternative regimen IFN eligible and SOF & SMV are not available (Naïve or relapser to PEG-IFN/RBV with fibrosis <3) |
|---|---|---|---|
| 1 | Sofosbuvir 400 mg/d + simeprevir 150 mg/d ± weight-based ribavirin for 12-24 wks or | B1 | Triple therapy: BOC or TPV + PEG-IFN/ weight-based ribavirin for 36-48 wks. |
| Sofosbuvir 400 mg/d + weight-based ribavirin for 24 wks | (Careful monitoring drug interactions with IC and toxicity) | ||
| 2 or 3 | Sofosbuvir 400 mg/d + weight-based ribavirin for 12-24 wks | B1 | Standard PEG-IFN/weight-based ribavirin for 12-24 wks |
| 4 | Sofosbuvir 400 mg/d + simeprevir 150 mg/d ± weight-based ribavirin for 12-24 wks | B1 | Standard PEG-IFN/weight-based ribavirin for 12-24 wks |
| 1,3,4,5,6 | Sofosbuvir 400 mg/d + daclastavir 60 mg/d ± weight-based ribavirin for 12-24 wks | B1 | Standard PEG-IFN/weight-based ribavirin for 12-24 wks |
- A.
The recommended standard of care for liver transplant recipients is treatment of confirmed recurrent liver disease. Significant fibrosis or portal hypertension 1 year after transplantation predicts rapid disease progression and graft loss, and indicates the need for more urgent antiviral treatment (Class B, Level 2).
- B.
Dose adjustment is not required for tacrolimus or cyclosporine with any of these news AVT combinations. However careful monitoring is important because of the absence of safety data in this population (Class B, Level 1).
Patients with HIV-HCV coinfection have a faster rate of fibrosis progression, resulting in more frequent occurrences of cirrhosis, end-stage liver disease and HCC.192,193 Therefore, hepatitis C treatment is an urgent need for this population.
Optimal conditions for treatment are not always possible or similar in all regions of the world, including Latin America. Optimal conditions may involve high-cost medications, maintenance of appropriate facilities, and assurance of adequate numbers and training of staff. Therefore, it is necessary to adapt current recommendations for hepatitis C treatment within the economic capacity of each particular region.2,67 Taking into account these different aspects, the aim of this section is to discuss the indications for hepatitis C treatment in HIV-HCV coinfected patients in Latin America and to present treatment options for this group of patients.
Who Should be Treated?In general, indications for HCV treatment in HCV-HIV coinfected people are identical to those in patients with HCV monoinfection.2,67 Treatment should be prioritized for patients with moderate or significant fibrosis (METAVIR score F2-F4).67,194–196 In patients with no or mild disease (METAVIR score F0-F1), the indication for, and timing of, therapy an be individualized.
Treatment of HCVDifferent drug combinations are available for patients with chronic hepatitis C infection. Indications will depend mainly on the availability of such drugs in different settings.2
GT1 Option 1-Treatment with PEG-IFN/RBVPEG-IFN in combination with RBV is recommended for the treatment of chronic HCV infection rather than standard non-PEG-IFN with RBV.2,67,194,196
The published SVR from the five largest controlled studies performed with PEG-IFN/RBV range from 14 to 35 % for GT1 and GT4.196
Both PEG-IFN-a molecules can be used. It is recommended that weight-based RBV (1,000 mg/day for < 75kg and 1,200 mg/day for ≥ 75 kg) should be used in coinfected patients. Carriers of HCV GT1 and GT4 with EVR (week 12) but not RVR (week 4) might benefit from extended (72-week) courses of therapy.196
Option 2-Treatment with TVR or BOCPeople treated with these DAAs had an estimated SVR almost twice that of people receiving only PEG-IFN/RBV. The recommendation for the use of TVR or BOC for HIV-HCV coinfected patients should primarily involve patients with the best chances of SVR and safety. The overall treatment duration of TVR-or BOC-based HCV therapy is 48 weeks.194 Dosage and futility rules for TVR and BOC should follow their label indications.197,198
Dual therapy with PEG-IFN/RBV may be appropriate for selected treatment-naïve patients who may achieve high SVR rates. Treatment-naïve patients with a fibrosis score ≤ F2 and RVR after 4 weeks of treatment with PEG-IFN/RBV may obtain a high rate of SVR, similar to rates obtained with triple therapy including TVR or BOC. This approach could avoid the cost and additional side-effects associated with PI treatment.67
Option 3-Treatment with SMVThis combination has been evaluated in coinfected patients in the C212 study. An SVR was achieved in 79% of treatment-naïve patients (42/53), in 87% (13/ 15) of prior relapsers and in 57% (16/28) of prior null responders.199 Dosage and futility rules for SMV should follow its label.200
Option 4-Treatment with SOF and PEG-IFN/RBVFor HIV-HCV coinfected patients, the SVR rate in a Phase 2 trial was 87% for GT subtype 1a and 89% (17/19) for subtype 1b (13/15).201 However, this treatment strategy has not been formally investigated in clinical studies of GT1 IFN-experienced patients. In addition, relatively small numbers of patients with cirrhosis were included.67
SOF should be administered with both PEG-IFN-α and RBVfor 12 weeks. The recommended dose of SOF is one 400 mg tablet taken once daily.
Option 5-Treatment with SOF and SMVThis recommendation is based on preliminary results from the COSMOS Phase IIb trial.202 Patients infected with HCV GT1 can be treated with a combination of daily SOF (400 mg) and daily SMV (150 mg) for 12 weeks.
Option 6-Treatment with SOF and DCVBoth treatment-naïve and treatment-experienced patients infected with HCV GT1 can be treated with a combination of SOF and DCV, including those who failed on a triple combination of PEG-IFN-α, RBV and either TVR or BOC. This recommendation is based on preliminary results from a Phase IIb trial recently published.19 Patients should be treated with daily SOF (400 mg) and daily DCV (60 mg) for 12 weeks. This combination should be considered especially in patients with predictors of poor response to anti-HCV therapy, prior nonresponders and/or patients with cirrhosis.
GT2 Option 1-Treatment with PEG-IFN and RBV (recommendation B1)2The SVR rate in controlled studies performed with PEG-IFN/RBV ranges from 44 to 73% for GT2 and GT3. Drug doses are the same as for GT1.
Patients with HCV GT2-3 with a RVR-as long as HCV load is low, there is good compliance with treatment, there is not advanced hepatic fibrosis, and weight-based RBV dosing is provided-could benefit from shorter (24 weeks) courses of therapy.196 For other patients with HCV GT2 or GT3, 48 weeks of therapy could still be advisable.196
Option 2-Treatment with SOF and RBVPatients infected with HCV GT2 should be treated with the combination of SOF and RBV.67 This recommendation is based on preliminary results from Phase III trials.67 During the PHOTON 1 trial, SVR24 was 88% for treatment-naïve patients treated for 12 weeks and 92% for treatment-experienced patients treated for 24 weeks.203 Patients infected with HCV GT2 must be treated with daily weight-based RBV and SOF (400 mg) for 12 weeks.67
Therapy should be prolonged to 20 or 24 weeks in patients with cirrhosis, especially if they are treatment experienced (recommendation B1).67
Option 3-Treatment with SOF and PEG-IFN/RBV (recommendation B1)67This recommendation is based on preliminary results from Phase II trials.201 SOF should be administered with both PEG-IGN-α and RBV for 12 weeks. The recommended dose of SOF is 400 mg daily. The dose of RBV should be weight based. This combination could be an alternative for cirrhotic and/or treatment-experienced patients.67
GT3 Option 1-Treatment with PEG-IFN/RBV (recommendation B1)2
See GT2 treatment with PEG-IFN/RBV.
Option 2-Treatment with SOF, PEG-IFN-α and RBV (recommendation A2)67This recommendation is based on results from Phase II trials.100,201 Patients infected with HCV GT3 should be treated with a combination of weekly PEG-IFN-α, daily weight-based RBV, and daily SOF (400 mg) for 12 weeks.
Option 3-Treatment with SOF and RBV (recommendation A2)67This recommendation is based on results from Phase III trials.67,203 Patients infected with HCV GT3 should be treated with daily weight-based RBV and daily SOF(400 mg) for 24 weeks. This therapy is suboptimal in treatment-experienced cirrhotics, for whom an alternative treatment option should be considered.67
Option 4-Treatment with SOF and DCVPatients infected with HCV GT3 could be treated with SOF and DCV.67 This recommendation is based on preliminary results from a Phase IIb trial recently published,106 but few data are available with this combination in patients infected with GT3. Patients infected with HCV GT3 should be treated with daily SOF (400 mg) and daily DCV (60 mg) for 12 weeks in treatment-naïve patients or 24 weeks in treatment-experienced patients.67
GT4 Option 1-Treatment with PEG-IFN/RBV(recommendation B1)2Drug doses and treatment duration the same as for GT1.
Option 2-Treatment with SOF, PEG-IFN and RBVPatients infected with HCV GT4 can be treated with weekly PEG-IFN-α, RBV and SOF.67 Very few data have been presented in HIV-coinfected patients.201 Drug doses and treatment duration are the same as for GT1.
Other options are also possible, although very few data are available:67
GT5 AND GT6 Option 1-Treatment with PEG-IFN/RBVThere are no published data regarding the SVR rate for coinfected patients treated with this regimen.196 Drug doses and treatment duration are the same as for GT1.
Option 2-Treatment with SOF and PEG-IFN/RBVPatients infected with HCV GT5 or GT6 could be treated with PEG-IFN-α, RBV, and SOF.67 There are no published data regarding the SVR rate for HIV coinfected patients treated with this regimen. Drug doses and treatment duration are the same as for GT1.
Drug Interactions Between Antiretrovirals and Daas for Hepatitis C TreatmentRelevant drug-drug interactions between the DAAs and antiretroviral drugs occur during hepatitis C treatment in HIV coinfected patients. Data on current recommendations regarding the use of antiretroviral drugs during HCV treatment are summarized in table 8.
Recommendations regarding the use of antiretroviral drugs during hepatitis С treatment.
| Telaprevir197,208 | Boceprevir198,208 | Simeprevir67,200,208 | Sofosbuvir208,209 | Daclastavir67,208 | |
|---|---|---|---|---|---|
| Atazanavir | Monitor for hyperbilirubinaemia | Consider ona case-bycasebasis | Not recommended | Recommended | Recommended at 30 mg/day |
| Lopinavir | Potential interaction | Not recommended | Not recommended | Recommended | No data |
| Indinavir | Potential interaction | Potential interaction | Not recommended | Recommended | No data |
| Fosamprenavir | Potential interaction | Potential interaction | Not recommended | Recommended | No data |
| Nelfinavir | Potential interaction | Potential interaction | Not recommended | Not recommended | No data |
| Saquinavir | Potential interaction | Potential interaction | Not recommended | No data | |
| Tipranavir | Potential interaction | Potential interaction | Not recommended | Not recommended | No data |
| Darunavir | Not recommended | Not recommended | Not recommended | Recommended | No data |
| Efavirenz | Increase dose to 1,125 mg three times daily | Not recommended | Not recommended | Recommended | Recommended at 90 mg once daily dosing |
| Rilpivirine | Caution for QT interval prolongation | Recommended | Recommended | Recommended | No data |
| Etravirine | Recommended | Potential interaction | Not recommended | No data | No data |
| Delavirdine | Potential interaction | Potential interaction | Not recommended | Recommended | No data |
| Nevirapine | Potential interaction | Potential interaction | Not recommended | Recommended | No data |
| Raltegravir | Recommended | Recommended | Recommended | Recommended | No data |
| Dolutegravir | Recommended | Recommended | Recommended | No data | |
| Maraviroc | Potential interaction | Potential interaction | Recommended | Recommended | No data |
| Tenofovir | Potential interaction | Recommended | Recommended | Recommended | No data |
| Abacavir | Potential interaction | Recommended | Recommended | Recommended | No data |
| Emtricitabine | Recommended | Recommended | Recommended | Recommended | No data |
| Lamivudine | Recommended | Recommended | Recommended | Recommended | No data |
| Zidovudine | Potential interaction | Potential interaction | Recommended | Recommended | No data |
- 1.
All treatment-naïve and treatment-experienced patients with compensated disease because of HCV and HIV should be considered for therapy (recommendation A1)67
- 2.
Patients with contraindications to use of IFN or patients intolerant to IFN should be considered for IFN-free therapy (recommendationA1).67
PEG-IFN/RBV is recommended for the treatment of chronic HCV infection rather than standard non-PEG-IFN with RBV (recommendation B1).2,67,194,196
Option 2-Treatment with TVR or BOCTreatment with TVR or BOC, given in combination withPEG-IFN-α and RBV, is suggested for GT1 chronic HCV infection, rather than PEG-IFN/RBV alone (recommendation B2).2,194
This category includes patients with F2-F3 METAVIR scores. Cirrhotic patients should also be selected, excluding those with platelets < 100,000/ mm3 in combination with serum albumin < 35 mg/ dL (recommendation B2).67,204–207
Option 3-Treatment with SMVSMV, given in combination with PEG-IFN-α and RBV, is recommended for people with HCV GT1b infection and for people with HCV GT1a infection without the Q80K polymorphism, rather than PEG-IFN/RBV alone (recommendation A1).2,67
Option 4-Treatment with SOF and PEG-IFN/RBVSOF, given in combination with PEG-IFN-α and RBV, is recommended in GT1 infection rather than PEG-IFN/RBV alone or PEG-IFN/RBV and TVR or BOC (recommendation A1).2,67
The dose of RBV should be weight based. SOF in combination with daily weight-based RBV for 24 weeks can be considered for patients with HCV GT1 infection who are IFN ineligible (recommendation B2).67
Option 5-Treatment with SOF and SMVThis combination should be considered especially in patients with predictors of poor response to anti-HCV therapy, prior nonresponders and/or patients with cirrhosis (recommendation A1).67
Option 6-Treatment with SOF and DCVBoth treatment-naïve patients and treatment-experienced patients infected with HCV GT1 can be treated with a combination of SOF and DCV, i ncluding those who failed a triple combination of PEG-IFN-α, RBV and either TVR or BOC (recommendation B1).67 This recommendation is based on preliminary results from a Phase IIb trial recently published.106 Patients should be treated with daily SOF (400 mg) and daily DCV (60 mg) for 12 weeks. This combination should be considered especially in patients with predictors of poor response to anti-HCV therapy, prior nonresponders and/or patients with cirrhosis (recommendation A1).67
GT2 Option 1-Treatment with PEG-IFN/RBV2SVRs from controlled studies performed with PEG-IFN/RBV range from 44 to 73% for GT2 and GT3. Drug doses the same as for GT1 (recommendation B1).2
Option 2-Treatment with SOF and RBVPatients infected with HCV GT2 should be treated with the combination of SOF and RBV (recommendation A1).67
This recommendation is based on preliminary results from Phase III trials.67 During the PHOTON 1 trial, SVR24 was 88% for treatment-naïve patients treated for 12 weeks and 92% for treatment-experi- enced patients treated for 24 weeks.203 Patients infected with HCV GT2 must be treated with daily weight-based RBV and SOF (400 mg) for 12 weeks (recommendation A1).67
Option 3-Treatment with SOF and PEG-IFN/RBV (recommendation B1)67This recommendation is based on preliminary results from Phase II trials.201 SOF should be administered with both PEG-IFN-α and RBV for 12 weeks. The recommended dose of SOF is 400 mg daily. The dose of RBV should be weight based (recommendation B1).
GT3 Option 3-Treatment with SOF and RBV (recommendation A2)67This recommendation is based on results from Phase III trials.67,203 Patients infected with HCV GT3 should be treated with daily weight-based RBV and daily SOF(400 mg) for 24 weeks. This therapy is suboptimal in treatment-experienced cirrhotics, for whom an alternative treatment option should be considered (recommendation A2).
Few data are available with this combination in patients infected with GT3. Patients infected with HCV GT3 should be treated with daily SOF (400 mg) and daily DCV (60 mg) for 12 weeks in treat-ment-naïve patients or 24 weeks in treatment-experienced patients (recommendation B1).67
GT4 Option 2-Treatment with SOF and PEG-IFN/RBVPatients infected with HCV GT4 can be treated with weekly PEG-IFN-α, RBV and SOF (recommendation B1).67
Other options are also possible, although very few data are available.67
GT5 AND GT6 Option 2-Treatment with SOF and PEG-IFN/RBVPatients infected with HCV GT5 or GT6 could be treated with PEG-IFN-α, RBV, and SOF (recommendation B1).67
14. Treatment of Special Populations: HBV CoinfectionIntroductionHBV/HCV share the same pathways of viral transmission, and coinfection is frequent in several geographical areas where both infections show a high level of endemicity.210,211 Very few data have been published on the spread of HBV-HCV coinfection in these areas, although HBV-HCV coinfection is a very frequent finding in those populations associated with a high risk of acquiring both infections, such as injecting drug users,212 hemodialysis patients213 and HIV-infected people.214 It is noteworthy that some people may simultaneously acquire HBV and HCV infection from subjects replicating both HBV and HCV. Despite scanty data in the literature, several case reports showed a pattern of disease where a decreased HBV replication is associated with a clearly documented HCV disease progression215216.
The second pattern is a superinfection of HBV on chronic hepatitis C, or HCV on HBV chronic carriers. Although an inhibitory effect of HBV superinfection on chronic HCV replication has been clearly documented, the clinical course of acute HBV in these patients was described as severe.217 Despite the association with a more severe clinical course, chronic HBV-HCV coinfection is characterized by a reciprocal inhibition of viral replication;218 the strong inhibitory effect is exerted by the superinfecting virus on the preexisting one.219 In a single 1-year longitudinal study, the virological profile of chronic HBV-HCV coinfection was characterized by dynamic fluctuations in HBV and HCV viremia in one-third of cases, whereas in the remaining cases, it remained constant. Despite the virological evidence of viral interference in patients with HBV-HCV coinfection, the interaction between these viruses remains to be fully understood, and further studies using in vitro models are needed. No direct reciprocal interference was found in one in vitro model, and indirect mechanisms likely to be mediated by innate and/or adaptive host immune responses have been suggested.220
The current international guide lines do not suggest first-line treatment for these patients.65,221 However, recently other international associations using GRADE have suggested PEG-IFN-α, RBV, and PIs following the same rules as in monoinfected patients (recommendation B2).67 The expert panel also stated that if HBV replication is at significant levels before, during, or after HCV clearance, concurrent HBV nucleoside/nucleotide analogue therapy may be indicated (recommendation C2). Latin American guidelines (ALEH 2011) suggested treating the dominant virus without using a rating system of recommendation levels.222 It seems rational to hypothesize that effective treatment may eradicate HCV infection and inhibit HBV replication without severe adverse effects. The careful monitoring of disease progression, viral replication, viral suppression, possible predominance of one virus over the other, comorbidities and cofactors (e.g., metabolic syndrome, alcohol or drug intake), presence of hepatitis delta virus (HDV) or HIV infection, host genetic factors and type of response to previous antiviral treatments is warranted, to select the best therapy for patients with HBV-HCV coinfection. Treatment of chronic HBV-HCV may change according to HBV or HCV replication predominance. Different therapy options for these different viral scenarios will be discussed here.
Treatment in HCV RNA-positive/HBV DNA-negative patientsStudies published from the beginning of 2000 onwards showed poor efficacy of standard IFN-α plus RBV for the treatment of patients with chronic HBV-HCV coinfection and HCV replication.223 However, Liu et al.224 conducted a comparative, multicenter open-label study that showed the efficacy and safety of PEG-IFN-α2a plus RBV in 161 patients with chronic HBV-HCV coinfection, all with active HCV replication, and in 160 control patients with HCV monoinfection. No difference in the rate of HCV SVR was observed between patients with dual infection or monoinfection. Indeed, for HCV GT1, the SVR rate was 72.2% in patients with dual infection and 77.3% in HCV-monoinfected patients, whereas for patients with HCV GT2/3, the SVR rates were 82.8 and 84%, respectively. In a 5-year follow-up study published in 2013, the same group showed the durability of HCV SVR in HBV-HCV coinfected patients treated with PEG-IFN. No data have yet been published on the efficacy of DAAs in combination with PEG-IFN plus RBV or with IFN-free drugs for treating patients with chronic HBV-HCV coinfection. However, taking into account that in HCV GT1 monoinfection, triple therapy achieved SVR more frequently than dual therapy in theranaïve, relapser and previous nonresponder p atients,225 it seems that triple therapy may be also an option for patients with chronic HBV-HCV GT1 coinfection, a hypothesis that awaits confirmation in clinical trials. Whether IFN-free DAA-based therapy will be effective in eradicating HCV infection also in HBV-HCV coinfected patients is a very important issue that warrants investigation.226 An attempt at a treatment algorithm was proposed by Sagnelli, et al.227
Treatment in HBV DNA-positive/HCV RNA-negative patientsInformation on the use of anti-HBV drugs for patients with chronic HBV-HCV coinfection is scarce, most likely because HBV predominates less frequently than HCV. In the above mentioned study by Liu et al.,224 145 patients with HBV-HCV coinfection, all HCVRNA-positive and 68 (46.9%) HBVDNA-positive at baseline, were treated with PEG-IFN-α2a plus RBV. At the end of treatment, 55% of the 68 became HBVDNA-negative, and more interestingly, 11.2% of all 145 treated patients became HBV surface antigen (HBsAg)negative. In subsequent analyses, the same researchers described an association between lower HBsAg levels at baseline and a greater likelihood of clearing HBsAg during treatment (40% for HBsAg level < 20 IU/mL vs. 2.2% for HBsAg level > 20 IU/mL; p < 0.05),228 and a 30% cumulative HBsAg seroclearance rate at the end of a 5-year posttreatment follow-up. However, after a longer follow-up period of 4 years, the authors showed that HBV DNA became positive in 47 out of 76 cases (61.8%), with this reappearance being transient in 21 (44.7%), intermittent in 12 (25.5%) and sustained in 14 (29.8%).226
In line with the above mentioned studies, Yu, et al.229 observed that 11 of 46 (23.9%) patients with HBV-HCV coinfection and negative HBV DNA at baseline became HBVDNA-positive after anti-HCV PEG-IFN plus RBV treatment. The HBV reactivation rate was significantly higher in patients who achieved HCV SVR (33.3%) than in those who failed to achieve this favorable result (8.7%) (p = 0.036). An algorithm was also proposed by Sagnelli, et al. for treatment of this group of patients.227
Recommendations- 1.
An exhaustive analysis of the disease progression, virus predominance, comorbidities, presence of hepatitis delta virus or HIV infection, and response to previous antiviral treatments is crucial for a better selection of patients for treatment.
- 2.
Only the EASL Clinical Practice Guidelines (CPGs) have recommended HCV/HBV coinfection treatment using the GRADE recommendation system.
- 3.
Effective treatment should eradicate HCV infection and inhibit HBV replication. Peg IFN and ribavirin may be useful to treat HCV-RNA-positive/HBV-DNA-negative patients, and Peg IFN and nucleoside/nucleotide analogs (NUC) may be useful to treat HBV-DNA-positive/HCV-RNA-negative patients (Recommendation C2).
- 4.
No data on the efficacy of combining DAAs plus Peg-IFN and ribavirin treatments and interferon free molecules (sofosbuvir, simeprevir) in HBV/HCV chronic coinfection have been published, but in cases with HCV predominance, Peg-IFN plus ribavirin and a first-generation DAA, such as boceprevir or telaprevir, should provide satisfactory sustained response rates, and significantly reduce the risk of liver-related mortality, as well as all-cause mortality (Recommendation C2).
The impact of chronic HCV infection and the characteristics of the clinical course of the disease in predialysis renal patients are not very well identified.230–232 There is a large amount of information regarding hepatitis C in hemodialysis patients, but it is not known whether the clinical course and histopathological aspects of patients under hemodialysis can be extrapolated to predialysis patients.233–236 Additionally, few studies have evaluated specific aspects of hepatitis C treatment in predialysis patients. All these aspects contribute to the weaker evidence for the recommendations for this specific group of patients.
Treatment indicationThe decision to treat is fundamentally based on the stage of renal function, the rate of progression of renal dysfunction and the possibility of preemptive renal transplant, more than on the stage of liver disease.
Renal dysfunction in chronic kidney diseases (CKD) is classified in five stages based on glomerular filtration rate (GFR), as follows.
- •
GFR > 90 (normal function).
- •
GFR 60-89 mL/min (mild dysfunction).
- •
GFR 30-59 mL/min (moderate dysfunction).
- •
GFR 15-29 mL/min (severe dysfunction).
- •
RGF < 15 mL/min (end-stage renal disease, ESRD).
In this section, we will refer to patients in stages 2, 3 and 4 of renal dysfunction.237
For patients with mild or moderate renal dysfunction, it is important to evaluate the rate of pro- gression of renal disease. If renal function is stable, treatment is recommended. If renal function is unstable and the deterioration of renal function is rapid, it is better to wait and treat when the patient is under hemodialysis (rating 2C).
Type of treatmentThe treatment of choice is still the combination of IFN-α2a and RBV, depending on the HCVGT.238 IFN-α2a seems to be the preferred option because PEG-IFN-a2a is cleared by the liver and PEG-IFN-α2b via the kidneys. The recommended dose of PEG-IFN-α2a is 135 mg/week. RBV should be used with caution, and the dose should be adjusted according to creatinine clearance (Table 10). Impaired excretion of RBV occurs in patients with CKD, as RBV is mostly eliminated by the kidney. The accumulation of the drug can exacerbate the anemia in this population already at risk.238
Co-medications contraindicated with boceprevir (BOC) and telaprevir (TVR).
| Medication | Interaction | |
|---|---|---|
| BOC | TVR | |
| Amiodarone | No | Yes |
| Bepridil | Yes | Yes |
| Quinidine | No | Yes |
| Rifampicin | Yes | Yes |
| Carbamazepine | Yes | Yes |
| Phenobarbital | Yes | Yes |
| Phenytoin | Yes | Yes |
| Dihydroergotamine | Yes | Yes |
| Ergotamine | Yes | Yes |
| Methylergonovine | Yes | Yes |
| Imatinib | Yes | Yes |
| Sunitinib | Yes | Yes |
| Halofantrine | Yes | Yes |
| Lumefantrine | Yes | Yes |
| Pimozide | Yes | Yes |
| Midazolam (oral) | Yes | Yes |
| Triazolam | Yes | Yes |
| Sotalol | No | Yes |
| Drospirenone | Yes | No |
| Cisapride | Yes | Yes |
| St John’s Wort | Yes | Yes |
| Sildenafil (pulmonary arterial hypertension) | Yes | Yes |
| Tadalafil (pulmonary arterial hypertension) | Yes | Yes |
| Atorvastatin | No | Yes |
| Lovastatin | Yes | Yes |
| Simvastatin | Yes | Yes |
| Alfuzosin | Yes | Yes |
| Ergonovine | Yes | Yes |
Adapted: Back D. 2013.
The use of erythropoietin is important for maintaining adequate levels of RBV and should be optimized before starting the treatment. Patients should be followed up with weekly blood cell counts during the first month and every 2 weeks thereafter (rating 2B).
Information is scarce regarding the use of triple therapy with the first wave of PIsTVR and BOC. Small series show that their use is safe with close monitoring of anemia and renal function. Neither drug requires dose adjustments239–241(rating 1С).
When using SOF to treat or re-treat HCV infection in patients with appropriate GT, no dosage adjustment is required for patients with mild to moderate renal impairment (GFR > 30 mL/min) (rating 2B).242
Dose adjustment of PEG-interferon-a and ribavirin for patients with renal dysfunction.
| Renal dysfunction | CrCl | Peg-IFN | RBV |
|---|---|---|---|
| Mild 60-89 mL/min | 2a- 180 μg/w | Standard dose | |
| 2b- 1.5 μg/w | |||
| Moderate | 30-59 mL/min | 2a- 135 μg/w | 200-400 mg |
| 3 x s/week | |||
| 2b-1 μg/w | |||
| Severe | < 30 mL/min | 2a-135 μg/w | 200 mg/day |
| 2b-1 μg/w |
For SMV, no dosage adjustment is required for patients with mild, moderate or severe renal impairment, because renal clearance plays an insignificant role (< 1%) (rating 2B).242
16. Renal Failure with Hemodialysis and Indication for Kidney TransplantationEpidemiologyIt has been shown that the prevalence of HCV infection is invariably greater in patients on hemodialysis than in the general population but with very important variations in the incidence and prevalence in different geographical areas. Using third-generation anti-HCV antibody tests, the reported prevalence of HCV infection in hemodialysis patients varies from 5-10% in the USA243 to 49% in Syria.244
HCV infection has been found in 5-40% of patients after a kidney transplant, with a mean prevalence of 6.8%.245
In Brazil, the HCV infection prevalence varies from 4 to 14%, with a predominance of GT1.246 It has been reported to be 6.4% in Mexico247, 6.1% in Colombia248 and 25%-75% in Venezuela.249 The anti-HCV antibody prevalence in kidney transplant recipients was 20.6% in a report from Argentina,250 with no impact on mortality or morbidity.
HCV infection affectsthe survival of patients on hemodialysis. A meta-analysis including more than 2,000 patients showed an increased relative risk of mortality in infected patients of 1.57 (95% CI: 1.33-1.86) compared with uninfected patients.251 Even though infection by HCV has been shown also to have a negative impact on post-kidney-transplant survival,252,253 life expectancy is better in infected patients undergoing a transplant than in those not transplanted.254
Diagnosis and evaluationDiagnosis of HCV infection in patients on he-modialysis and after a renal transplant relies primarily on antibodies (third-generation ELISA). HCV viremia has been reported in anti-HCV antibody-negative patients (occult HCV infection), but this seems to be a more important phenomenon in high-prevalence areas and in patients with unexplained abnormal aminotransferase levels. HCV RNA tests, either qualitative PCR or quantitative assays, are considered to be the most sensitive diagnostic methods, but there are several reasons that may explain false positive and false negative results.255
HCV infection in hemodialysis patients is generally asymptomatic. There is no good correlation between aminotransferase levels and viral load or liver biopsy findings.236 Therefore, a liver biopsy has been suggested as the only reliable method for evaluating the severity of liver fibrosis.256 More recently, noninvasive methods for evaluating liver fibrosis such as transient elastography (Fibroscan®) have been shown to be reliable in the post-kidney-transplantation setting257 and will probably be preferred to liver biopsy in patients with renal dysfunction, who may have a higher risk of complications after a liver biopsy.
TreatmentGiven that conventional IFN-based treatments have low efficacy and low tolerance in patients on maintenance hemodialysis, it is generally recommended that therapy should be offered to patients who are at the highest risk of complications due to the infection, such as those with compensated cirrhosis or advanced fibrosis and those considered for a renal transplant.257–263 This means that evaluation of liver fibrosis in these patients is paramount for decision making. This indication is very likely to change when IFN-free therapies become the mainstay therapy for HCV infection. Patients with cirrhosis should be evaluated for double liver-kidney transplantation.
IFN and PEG-IFNIn a retrospective meta-analysis, regular IFN has been shown to be associated with a 41% and PEG-IFN with a 37% chance of SVR.264 Regular IFN-α2b at a dose of 3 million units 3 times per week for 6-12 months is usually recommended.256 The half-life of PEG-IFN is markedly increased in patients with ESRD: a recent study showed that PEG-IFN-α2a (135 μg/week) plus low-dose RBV (200 mg/day) for 48 weeks had a better SVR than monotherapy (64 vs. 33%) but with more side effects.238
RBVRBV clearance is markedly reduced in renal insufficiency and RBV,and its metabolites are not removed by hemodialysis.265 Thus, RBVuse in patients with creatinine clearance below 50 mL/min is not generally recommended, and if indicated, a low dose should be used (200 mg/day) with very close follow-up of hemoglobin level and titration of erythropoietin dose to treat anemia.
BOC and TVRThere are few data about the use of the first-generation PIsBOC and TVR in ESRD. Both BOC and TVR are metabolized primarily by the liver to inactive metabolites, so theoretically, no dosage adjustments are necessary in patients with ESRD on dialysis. A small case report shows promising results of TVR use in four patients undergoing hemodialysis.266
SOF and SMVSOF, a nucleotide analog HCV polymerase inhibitor, is metabolized in the liver to its active form (GS-461203), and its inactive metabolites are eliminated by the kidney by glomerular filtration and active tubular secretion. No dose modification is required for mild to moderate renal insufficiency, but its safety has not been established in patients with severe renal impairment or ESRD. There are studies being currently conducted of SOF in this patient population.
SMV is a second-generation PIthat is almost exclusively metabolized in the liver by CYP3A4. Renal elimination of SMV and its metabolites is negligible, but to date, there is insufficient information for treatment in patients with creatinine clearance below 30 mL/min or on maintenance dialysis.
PreventionFortunately, HCV infection in hemodialysis patients seems to be declining. Several risk factors have been associated with an increased risk of infection, including the number of blood transfusions,267 the duration of renal insufficiency,268 the mode of dialysis (greater in hemodialysis than in peritoneal dialysis)269 and strikingly, the prevalence of HCV infection in the dialysis unit.270,271 The available information shows that nosocomial transmission is the most common method of spread of the virus. Needlestick injury,272 physical proximity to an infected patient271 and using the same dialysis machine273 have been linked to an increased risk of HCV transmission, but there is good evidence that breakdown in standard infection-control practices (e.g., failure to change gloves or using multidose heparin vials) is the most common route of HCV transmission in outbreaks.274–276 All this evidence suggests that the best way of preventing HCV infection in dialysis units is the strict enforcement of universal precautions,277 with the use of dedicated dialysis machines for HCV-infected patients being more controversial and not mandatory.
Recommendations- 1.
In patients with ESRD and on dialysis, advanced liver fibrosis and candidacy for kidney transplantation are strong indications for antiviral treatment (Class 1, Level B).
- 2.
Patients on dialysis should be treated with regular IFN (3 MU 3 times per week) and low-dose RBV for 48 weeks (Class 1, Level C).
- 3.
PEG-IFN-α2a at an adjusted dose can also be used (Class 2, Level A).
- 4.
TVR or BOC could be added with caution to treatment of GT1 patients (Class 2, Level C).
- 5.
Strict adherence to universal precautions of infection control is the main action required in hemodialysis units to prevent transmission of HCV infection (Class 1, Level B).
- 6.
Patients with ESRD should be tested with a sensitive antibody assay for anti-HCV antibodies and infection confirmed by a sensitive HCV RNA test (Class 1, Level A).
- 7.
Patients with ESRD and unexplained abnormal aminotransferase levels should be tested for HCV RNA even in the absence of detectable anti-HCV antibodies (Class 2, Level C).
The advent of TVRand BOC has meant that knowledge of drug-drug interactions, a common and important aspect in the evaluation of patients starting and continuing on HCV therapy, has increased.
Drug-drug interactions are a difficult issue because only a relatively small number of drug-drug interaction studies can ever be performed during the drug development process, and subsequent postlicensing testing is an important method of detecting these.
BOC is given at 800 mg every 8 h with food. The area under the plasma concentration time curve (AUC) is increased up to 65% with food, although the bioavailability is similar whether taken with a high-fat or low-fat meal.278 BOC is metabolized by aldo-keto reductases (AKR1C2, AKR1C3) and CYP3A4.279 BOC is also a substrate for the efflux transporter P-glycoprotein (P-gp), which is present in many tissues, including the gastrointestinal tract, liver, blood-brain barrier and placenta.
TVR is given at 750 mg every 8 h. However, twice-daily dosing with 1,125 mg demonstrates an equivalent SVR to thrice-daily dosing.280,281 TVR needs to be taken with a high-fat (> 20 g) meal/ snack to give optional systemic availability.282 The primary route of metabolism of TVR is CYP3A4, and like many CYP3A4 substrates, it is also transported by P-gp.283
Both agents appear to be mechanism-based inhibitors of CYP3A; in addition to the inhibitory effect on CYP3A, both BOC and TVR are inhibitors of P-gp. TVR did not inhibit CYP1A2, CYP2C9, CYP2C19 or CYP2D6 and has a low potential to induce CYP2C, CYP3A or CYP1A.284 Similarly, BOC did not inhibit CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6 or CYP2E1, and there was no evidence of induction of CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19 or CYP2D6.278,285 Despite these clear effects, there are differences between the Euro-pean278,286 supplementary protection certificate (SPC) and the USA prescribing information regarding the cautions about drug-drug interactions.
The area of prolongation of the QT interval on electrocardiogram is an important issue when a patient is using BOC or TVR. TVR should not be coadministered with a Class Ia or III antiarrhythmic and should be used with caution with Class Ic antiarrhythmic drugs that are known to induce QT prolongation and that are CYP3A substrates, and drugs known to prolong the QT interval for which the metabolism is not mainly CYP3A dependent.286 BOC should not be coadministered with drugs that are dependent on CYP3A4 for clearance; this includes drugs such as pimozide, lumefantrine, and sunitinib, which have a tendency to prolong QT. Perhaps the most pragmatic approach is to identify those drugs that should be avoided when BOC or TVR is used (Table 10).
In the era of DAA agents, health care providers involved in the treatment of patients with HCV must consider potential drug interactions between DAAs and other drugs and supplements. Table 12 provides an algorithm for screening, adjusting and monitoring of potential drug interactions with DAA agents.287 Some specific and common examples of drug-drug interactions are given. The increase in cyclosporine levels is 2.7-fold with BOC287 and 4.6-fold with TVR.288 Similarly, for tacrolimus, the in- crease is 17-fold with BOC and 70-fold with TVR. Atorvastatin has a 7.9-fold increase in exposure with TVR and 2.3-fold with BOC.289–291 This suggests that during treatment with DAAs, statin treatment could be stopped temporarily.
Antiviral treatments for HIV are also an important concern: both BOC and TVR have bidirectional interactions with ritonavir-boosted HIV PIs, the magnitude and direction of which has been of concern in relation to antiviral efficacy. For TVR, darunavir/ritonavir, lopinavir/ritonavir and fosamprenavir/ritonavir are not recommended.286 For BOC, darunavir/ritonavir, atazanavir/ritonavir and lopinavir/ritonavir are not recommended.
Although TVR and BOC do not currently have regulatory approval for posttransplant patients, these individuals are arguably the patients in greatest need of treatment. For this reason, the protocol from the University of Colorado Denver, USA for using triple therapy in patients with recurrent HCV after LT could be considered (Figure 4).283
The available information for the most recent antivirals is limited and will be continuously updated.
However, despite the lack of availability, some information has been published286 suggesting several interactions with many commonly used drugs including anticoagulants, benzodiazepines and other antivirals (Table 11).
Drug-drug interactions with newer antivirals.
| Agent | Profile | Interactions reported |
|---|---|---|
| • Protease inhibitors | ||
| Faldaprevir | Moderate inhibitor of CYP3A. | Midazolam, omeprazole, wafarin, efavirenz, caffeine, dextromethorphan. |
| Simeprevir | Weak inhibitor of CYP3A and P-gp. | Methadone, midazolam, escitalopram, rilpivirine, raltegravir, tenofovir, cyclosporine, tacrolimus, ethinylestradiol/norethisterone, efavirenz. |
| Asunaprevir | Weak inhibitor of CYP2D6 and P-gp. Weakinducer of CYP3A4. | Midazolam, losartan, omeprazole, caffeine, dextromethorphan. |
| Danoprevir | CYP3A substrate. | Methadone, omeprazole, ranitidine, warfarin. |
| • Non-nucleoside polymerase inhibitors | ||
| Filibuvir | Weak inducer and inhibitor of CYP3A. | Midazolam, ketoconazole. |
| • NS5A inhibitors | ||
| Daclatasvir | P-gp inhibitor | Tenofovir, efavirenz, atazanavir, ethinlyoestradiol/norgestimate. |
| • Nucleoside polymerase inhibitors | ||
| Sofosbuvir | Not a CYP3A substrate. | |
| Renally excreted | Methadone, efavirenz, rilpivirine, raltegravir, tenofovir, emtricitabine, darunavir, cyclosporine, tacrolimus. | |
Adapted: Back D. 2013.
In conclusion, the study of drug-drug interactions is a very active field for gastroenterologists and hepatologists, and technology will help us to offer the best outcomes with fewer adverse events in patients treated with multiple drugs (Figures 3 and 4).
All patients under treatment with direct acting antivirals should be screened systematically for drug interactions, including herbal or over the counter drugs (Class 1, Level C).
18. Treatment of Special Populations: Treatment of Patients with Extrahepatic Manifestations (Cryoglobulinemia, Lichen, Overlap Syndromes, PCT)HCV is at the same time a hepatotropic and a lymphotropic virus and may cause hepatic and extrahepatic disease. Epidemiological data and biological plausibility support this association.292,293 The disappearance of these manifestations after viral clearance is a confirmation of the pathogenic role played by HCV in these situations.294,295 Associations between HCV infection and other clinical conditions, including dermatological, neurological, digestive, endocrinologic and pulmonary disorders, have been described previously.292,294,296–298
Among the numerous cutaneous manifestations, the most important are mixed cryoglobulinemia (MC), porphyria cutaneatarda (PCT) and lichen planus.292–294,296 PCT is characterized by deficient activity of the heme synthetic enzyme uroporphyrinogen decarboxylase. The main cutaneous manifestation is the presence of blisters in areas of sun exposure that lead to milia, dyspigmentation and scarring. HCV infection favors the clinical expression of the disease, and in many cases clearance of the virus will lead to disappearance of the manifestations of PCT. Thus, the best treatment will be the best antiviral drug available in each country.292,296,299 The initial management of PCT consists of phlebotomy to produce iron deficiency, and avoidance of sunlight, alcohol, estrogen and other chemicals/substances that can precipitate the disorder.
Lichen planus is an inflammatory pruritic disease of the skin and mucous membranes characterized by distinctive papules, with a predilection for the flexor surfaces and trunk. It can be associated with some commonly used drugs such as nonsteroidal anti-inflammatory drugs and hydrochlorothiazide, and in some geographic areas, it is associated with HCV with a higher incidence than the uninfected population. IFN treatment can exacerbate previous lichen lesions and in some cases can trigger the disease.296,300 It is a skin disease with exacerbations and remissions, and there are no reports of the outcome of the disease after elimination of HCV infection with an antiviral treatment. Therefore, careful consideration is required before starting IFN-based treatment, and these patients should await IFN-free treatment if possible (weak recommendation).301
Overlap syndrome is the occurrence of autoimmune hepatitis in patients with HCV infection. It is a rare association, and the diagnosis is made with a combination of clinical, laboratory and histological features. It is very important for it to be evaluated-correctly before treatment because IFN can exacerbate the autoimmune disease. Very high aminotrans-ferase levels in association with hypergamma-globulinemia and high titers of autoantibodies raise the suspicion of overlap disease that should be confirmed with histological assessment. Liver biopsy should reveal inflammation (piecemeal and acinar necrosis), with predominant presence of plasmocytes and/or confluent necrosis,which is an uncommon feature of hepatitis C infection.
The treatment in these typical cases of overlap should be initiated with immunosuppressive drugs, because in some cases, the disease is severe with rapid evolution to cirrhosis and hepatic failure (weak recommendation). However, we know that autoimmune hepatitis can be often be triggered by a viral infection. With the advent of DAAs and IFN-free treatments that cause a rapid decline in viral load and a high rate of disappearance of hepatitis C infection, an attempt to eradicate this infection could be the first step, with the use of immunosup-pressive treatment saved for those cases with permanent liver injury.302
The chronic antigenic stimulation of the humoral immune system in patients with chronic hepatitis C leads to an increase in titers of monoclonal and polyclonal antibodies.303 It has been postulated that a complex of anti-HCV-HCV lipoprotein could act as a B cell superantigen leading to the synthesis of non-HCV-reactive IgM with rheumatoid factor-like activity. These autoantibodies produce immune complexes that circulate in the body and are deposited in small-to-medium blood vessels, resulting in complement activation and extrahepatic injury.
The most common manifestation of MC is an asymptomatic cryoglobulin in the serum that can be shown in 20-40% of infected patients, but only 3-5% of HCV chronic hepatitis patients have symptomatic disease (cryoglobulinemic vasculitis). There are many organs that could be involved, but the most common clinical presentations are skin (leukoclastic vasculitis) followed by joint, neurological, renal and digestive involvement. In most cases (80%), the disease is mild or moderate and is characterized by recurrent vasculitis (purpura) in the legs and arthralgias, but severe disease with membranopro-liferative glomerulonephritis, cutaneous involvement with ulcers and ischemic neurosis, peripheral or central neuropathy, and mesenteric disease can lead to potentially severe complication and even death.292,303,304
The two possible treatments consist of the use of immunosuppressive drugs or antiviral drugs. After the discovery of HCV as the etiologic agent for most cases of MC, a new concern has risen about the use of a high dose of immunosuppressive drugs such as corticosteroids. Antiviral drugs such as PEG-IFN and RBV are the main options in HCV therapy and should be the first step/option in patients with mild or moderate MC. Although most of the reported results have come from case series, a meta-analysis of 10 clinical studies305 including 300 patients showed clinical improvement in 63% of cases and an SVR of 42%. The problem with this study is its heterogeneity. Patients with different GTsand different grades of liver fibrosis and severity of vasculitis were included. PEG-IFN was prescribed in only 66% of cases, and the number of included cases varied from 9 to 86. However, as could be expected, a small controlled study including 72 MC patients306 showed better results with PEG-IFN/RBV than with IFN/RBV. The rates of clinical remission and virological response were 67%/56.2% and 62.5%/53%, respectively.
So far, there has been no original study evaluating the new triple therapy for GT1 (PEG-IFN/RBV and NS3 PIssuch as TVR or BOC). Only one study307 reported partial results after 24 weeks of therapy with this combination in 23 patients with MC. Thirteen patients (56.5%) showed a complete clinical response, and 10 (43.5%) had a partial response. At week 24, 70% of the patients were negative for HCV. It is possible that the final result could be better than conventional therapy for patients with GT1.
An interesting new therapy in MC patients is the use of rituximab (RTX) (anti-CD20), which targets B-cells that are responsible for production of the cryoglobulin, immune complex deposition and finally vasculitis. The main indication for RTX therapy is the absence of response to previous therapies. It is the first-choice therapy for cases of severe vasculitis, which can be followed by IFN-based therapy. Most patients received consecutive 4-weekly IV infusions of 375 mg/m2 of RTX. The isolated use of RTX308 caused a rapid and complete clinical response in 73% patients with cutaneous involvement, 70% with glomerulonephritis and 36% with neuropathy. Relapse occurred in 36% of cases, pointing to the need for associated AVT.
There is an ongoing study that evaluates a lower dosage of RTX (250 mg/m2) and its association with clinical response.309 This drug is considered to be safe for HCV patients, and even those with liver cirrhosis had similar clinical results.310
Based on the limitations of each therapy, a combination of RTX with PEG-IFN/RBV seems plausible. Two recent controlled studies311,312 compared the efficacy and safety profile of PEG-IFN/RBV versus RTX with PEG-IFN/RBV therapy. In both studies, RTX with PEG-IFN/RBV-treated patients had a shorter time to clinical remission, better renal response rates and higher rates of cryoglobulin clearance. Some relapses occurred after the end of treatment, so it is very important to eradicate the viral infection.
Therapeutic guidelines for these situations are not considered in the international associations guidelines, but at the 16th International Vasculitis & ANCA Workshop,313 the following recommendations were made.
- •
Aggressive optimal therapy with PEG-IFN/RBV (plus PIs if HCV GT1 infection) should be considered to be the best treatment for HCV-MC patients with mild to moderate disease. Current treatment duration is 48 weeks for all HCV GTs (strong recommendation).301,313
- •
In patients presenting with more severe disease (worsening of renal function, mononeuritis multiplex, extensive skin disease with ulcers and distal necrosis), an induction phase of immunosuppression is often necessary while awaiting the generally slow response to antiviral treatment. RTX is the preferred drug for inducing an initial clinical response, followed by the best available antiviral treatment in each country. This drug combination is very important because it may attack both the B cell arm of autoimmunity and the viral trigger (strong recommendation).313,314
- •
For patients presenting with the fulminant form of vasculitis with any of the following events (peripheral necrosis of extremities, central nervous system vasculitis, mesenteric involvement, pulmonary complications, hyperviscosity), apheresis can have immediate results and should be combined with an immunosuppressive drug such as RTX to avoid rebound of MC. Antiviral treatment should be started after clinical improvement of the life-threatening complication.314
The prognosis of patients with HCV-positive MC is related to severity of fibrosis, serious infection, central nervous system vasculitis, renal function and/or cardiac involvement.315
Recommendations- 1.
The initial management of PCT consists of phlebotomy to produce iron deficiency, and avoidance of sunlight, alcohol, estrogen and other chemicals/substances that can precipitate the disorder.
- 2.
IFN treatment can exacerbate previous lichen lesions and in some cases can trigger the dis ease.
- 1.
Careful consideration should be taken before starting IFN-based treatment, and patients should await IFN-free treatment if possible.
- 2.
The treatment in these typical cases of overlap should be initiated with immunosuppressive drugs, because in some cases, the disease is severe with rapid evolution to cirrhosis and hepatic failure.
Therapeutic guidelines for these situations are not considered in the international associations’ guidelines, but in the 16th International Vasculitis & ANCA Workshop,313the following recommendations were made.
- 1.
Aggressive optimal therapy with PEG-IFN and RBV (plus PIs for HCV GT1 infection) should be considered to be the best treatment for HCV-MC patients with mild to moderate disease. Current treatment duration is 48 weeks for all HCV GTs (strong recommendation).307,313
- 2.
In patients presenting with more severe disease (worsening of renal function, mononeuritis multiplex, extensive skin disease with ulcers and distal necrosis), an induction phase of immunosuppression is often necessary while awaiting the generally slow response to antiviral treatment. RTX is the preferred drug for inducing an initial clinical response, followed by the best available antiviral treatment in each country. This combination is very important because it may attack both the B cell arm of autoimmunity and the viral trigger (strong recommendation).313,314
- 3.
For patients presenting with the fulminant form of vasculitis with any of the following events (peripheral necrosis of extremities, central nervous system vasculitis, mesenteric involvement, pulmonary complications, hyperviscosity), apheresis can have immediate results and should be combined with an immunosuppressive drug such as RTX to avoid rebound of MC. Antiviral treatment should be started after clinical improvement of the life-threatening complication.314
- •
AASLD: American Association for the Study of Liver Diseases.
- •
APRI: AST-to-platelet ratio index.
- •
AVT: antiviral therapy.
- •
BOC: boceprevir.
- •
CH: cholestatic hepatitis.
- •
CUPIC: Compassionate Use of Protease Inhibitors in Viral C Cirrhosis.
- •
DAAs: direct-acting antiviral.
- •
DCV: daclatasvir.
- •
EASL: European Association for the Study of the Liver.
- •
ESRD: end stage of renal disease.
- •
GRADE: Grading of Recommendations Assessment, Development and Evaluation.
- •
GT: genotype.
- •
HBV: hepatitis B virus.
- •
HCC: hepatocellular carcinoma.
- •
HCV: hepatitis C virus.
- •
HIV: human immunodeficiency virus.
- •
IFN: interferon.
- •
IVDU: intravenous drug use.
- •
LAASD: Latin American Association for the Study of the Liver.
- •
LT: liver transplantation.
- •
LDV: ledipasvir.
- •
NS: nonstructural.
- •
PCR: polymerase chain reaction.
- •
PCT: porphyria cutanea tarda.
- •
PEG-IFN: pegylated interferon.
- •
PEG-RBV: pegylated ribavirin. •PI: protease inhibitor.
- •
PK: drug pharmacokinetics.
- •
RAV: resistance-associated viral strain.
- •
RBV: ribavirin.
- •
RGT: response-guided therapy.
- •
RNA: ribonucleic acid.
- •
RVR: rapid virological response.
- •
SAE: serious adverse event.
- •
SMV: simeprevir.
- •
SOF: sofosbuvir.
- •
SVR: sustained virological response.
- •
SWE: shearwave elastography.
- •
TVR: telaprevir.
- •
WHO: World Health Organization.
Nahum Méndez-Sánchez
Grant and research support: BMS, AbbVie, Gilead, Janssen.
Adrian Gadano
Grant and research support: BMS, AbbVie, Gilead, Janssen.
Marcelo Silva
Grant and research support: AbbVie, BMS, Gilead, Janssen; MSD.
Maria L. Gomes-Ferraz
Grant and research support: MSD, Janssen, Gilead, Siemens, Roche, BMS.
Alejandro Soza
Grant and research support: Speaker for MSD, Roche, BMS. Consulting / Participation in Advisory Board Meetings for MSD, Abbvie, Gilead, Vertex, Roche, Janssen. Stocks: Gilead / Enanta.
M. Cassia Mendes-Correa
Grant and research support: Speaker for MSD, Roche, BMS.
Norberto C. Chávez-Tapia
Grant and research support: I receive research and educational grant from Medica Sur Clinic & Foundation.
Lucy Dagher
Grant and research support: Consultant speaker: MSD, Roche.
Martín Padilla
Grant and research support: Roche, Johnson & Johnson, MSD, Novartis, Bristol Myers Squibb.
Juan F. Sánchez-Avila
Grant and research support: Consultant and/or speaker in the last 12 months. Roche, Merck, Janssen, Abbvie.