Among people with type 2 diabetes (T2D), non-alcoholic fatty liver disease (NAFLD) is very common and has an increased risk of clinically significant liver disease. The use of sodium-glucose co-transporter 2 (SGLT2i) inhibitors and glucagon-like peptide-1 (GLP-1a) receptor agonists is endorsed to reduce major cardiovascular events and/or progression of chronic kidney disease. Their prevalence of use in people with T2D and co-existent NAFLD remains unclear. We sought to determine the prevalence of use of these medications at two different time periods, and their association with prevalence of clinically significant liver disease.
Materials and MethodsConsecutive people with type 2 diabetes (T2D) and non-alcoholic fatty liver disease (NAFLD) were recruited from diabetes clinics between Jun-2021 and Jun-2022 (‘current’ cohort). Liver stiffness measurements (LSM) using FibroScan were performed. Medication data were collected prospectively at recruitment and verified with the dispensing pharmacy or general practitioner medical records. Data for a historical cohort with NAFLD and T2D recruited from the same clinics during 2015–2017 (‘historical’ cohort) were available. Logistic regression was used to evaluate factors associated with LSM <8.0 or ≥8 kPa (clinically significant fibrosis).
ResultsThere were 292 participants, 177 in the historical cohort and 115 in the current cohort. In the current cohort, 57.4% of patients with T2D and NAFLD were taking a GLP-1a and 42.6% were taking a SGLT2i; a 2.6 to 3.4-fold higher prevalence than in 2015–2017. A lower proportion of the current cohort (23.9% compared to 38.4%) had clinically significant fibrosis (LSM ≥8 kPa; p = 0.012). When the cohorts were pooled and differences adjusted for in multivariable logistic regression analysis, patients taking a GLP-1a or a SGLT2i were 2 times more likely to have a lower LSM (<8 kPa) compared to patients not taking these drugs (OR=2.05, 95%CI 1.07–3.94, p = 0.03 and OR 2.07 95%CI 1.04–4.11, p = 0.04, respectively).
ConclusionsThe observation of a lower LSM in people taking SGLT2i and/or GLP-1a following adjustment for other relevant clinico-demographic variables provides support for clinical trials to assess their efficacy in reducing the progression of NAFLD.
Non-alcoholic fatty liver disease (NAFLD) is closely associated with obesity and cardiometabolic disorders including type 2 diabetes (T2D), vascular disease, hypertension and dyslipidaemia. Although NAFLD is highly prevalent among people with T2D (global prevalence 55.5%) [1], it is largely asymptomatic and often under-recognised. The liver disease in this patient group is heterogeneous, ranging from steatosis alone, to non-alcoholic steatohepatitis (NASH) characterized by necroinflammation, to progressive fibrosis and cirrhosis. Among people with T2D and NAFLD, the prevalence of advanced fibrosis is 10 – 20% [2] and these patients are at risk of developing liver-related complications, including hepatocellular carcinoma (HCC). Guidelines advise staging the disease as soon as it is recognised using biochemistry and imaging to target management accordingly, with consideration of referral to a hepatologist if advanced fibrosis is detected [3–5]. To date, there is no approved pharmacotherapy for NAFLD, and treatment focuses on weight reduction, healthy lifestyle, and management of the metabolic comorbidities.
NAFLD has a bidirectional relationship with T2D [6] and is associated with an increased risk of adverse cardiovascular events [7,8] and incident chronic kidney disease [9]. Regardless of the risk of liver disease complications, the leading causes of death in patients with NAFLD are cardiovascular disease and extrahepatic malignancy [10,11]. Therefore, a comprehensive approach to the management of cardiometabolic risk needs to be a key component of NAFLD care. Importantly, over the last few years there has been a paradigm shift in the management of T2D, with a greater focus on comorbid conditions and reducing clinical events with wider availability of newer diabetes pharmacotherapy [12,13]. In particular, two drug classes – sodium-glucose co-transporter 2 inhibitors (SGLT2i) and glucagon-like peptide-1 receptor agonists (GLP-1a) – have been shown to reduce major cardiovascular events and/or progression of chronic kidney disease [14,15]. Recent clinical practice recommendations for T2D now advise that pharmacotherapy should be based not only on a patient's glycated hemoglobin (HbA1C), but also on the presence of comorbidities and clinical characteristics . In view of shared cardiometabolic risk factors, SGLT2 inhibitors and GLP-1 receptor agonists may also be of benefit in people with NAFLD and T2D [16], and these agents are currently under evaluation for the treatment of NASH.
Despite endorsement by professional societies, the clinical uptake of pharmacotherapy for evidence-based indications often lags behind guideline recommendations [17]. There may be multiple barriers to adoption of pharmacotherapy with accepted benefit into patient care [18], and at present, the prevalence of use of new diabetes medications in people with coexistent NAFLD remains unclear. It is possible that people with T2D receiving cardio-protective pharmacotherapy may show a lower prevalence of NAFLD with advanced fibrosis, if these agents are also effective in reducing liver disease progression. Conversely, these patients may have more advanced liver disease, due to the presence of multiple metabolic comorbidities that together increase the risk of progressive fibrosis [19]. In this study, we sought to determine the prevalence of use of new diabetes medications (SGLT2i and/or GLP-1a) at two different time periods and their association with the prevalence of clinically significant liver disease.
2Materials and Methods2.1Study population and settingConsecutive people with T2D and NAFLD were recruited from specialist-led diabetes clinics between Jun-2021 and Jun-2022 (‘current’ cohort). Data for a historical cohort with NAFLD and T2D recruited from the same clinics during 2015–2017 (‘historical’ cohort) were available [20]. In both studies, consecutive patients were recruited prospectively and all patients attending the clinic when the study took place were offered participation. The diagnosis of NAFLD was based on a FibroScan CAP score ≥248 dB [21] or ultrasound confirmed steatosis in the presence of metabolic risk factors and the exclusion of significant alcohol consumption (≥20 g/d) and other chronic liver diseases. Only patients with a diagnosis of NAFLD based on the above definition were included in the analysis.
To classify NAFLD severity noninvasively, transient elastography was performed after a 3-hour fast by using FibroScan technology (Echosens, Paris, France) with the standard M or XL probes in line with the manufacturer's instructions. Examinations were performed by a trained clinician with adherence to recommended standard FibroScan operating procedures and criteria for the definition of reliable LSMs (minimum of 10 valid measurements with a success rate of ≥60% and interquartile range (IQR) ≤30% of the final result). The M or XL probe was selected using the software's automatic probe selection tool. For the purposes of this study, we used LSM cut-off values of ≥8.0 kPa for clinically significant fibrosis; the same cut-off value was used for both probes. At a cut-off <8 kPa, FibroScan has a 94%–100% negative predictive value for advanced fibrosis and cirrhosis [22–25].
Medication data were collected at recruitment and verified with the dispensing pharmacy or general practitioner medical records. Medical history was obtained during consultation using a structured questionnaire, including sociodemographic characteristics, previously diagnosed liver disease and medical conditions. Alcohol intake was assessed using the condensed Alcohol Use Disorders Identification Test (AUDIT-C) [26] and patients who were identified as having excessive alcohol intake (defined as ≥20 g/d) were excluded from this analysis. Anthropometric data were collected at consultation and the definition of obesity based on body mass index (BMI) was adjusted according to ethnicity for patients from South Asia (BMI 23–27.4 kg/m2 = over-weight, BMI 27.5–30.0 kg/m2 = Class 1 obese) [27] The most recent biochemical and haematologic data were used to calculate the Fibrosis-4 and NAFLD Fibrosis Score using readily available online calculators hosted by MDCalc (https://www.mdcalc.com/fibrosis-4-fib-4-index-liver-fibrosis; https://www.mdcalc.com/nafld-non-alcoholic-fatty-liver-disease-fibrosis-score).
2.2Data analysisData analyses were conducted using STATA SE (version 17; Stata Corporation, College Station, TX). Descriptive analyses were presented as mean with standard deviation (SD), median and interquartile range (IQR), and frequency and proportion of occurrences (%) depending on data distribution. P-values with 95% confidence level were calculated using the Chi-square test to compare categorical variables and the Mann-Whitney U test and two sample t-test where appropriate for continuous variables.
A logistic regression model was used to examine factors that were independently associated with a low risk of clinically significant liver disease, as represented by liver stiffness <8.0 kPa. The primary outcome was liver stiffness <8.0 kPa and results were presented as odds ratios (OR) with 95% confidence interval (CI). Reverse and stepwise logistic regression with the α-value set at 0.20 were used to select variables for inclusion in the multivariable model. The final logistic multivariable model also took into account our understanding of the relationships and dependencies among variables. All p-values were 2-sided (p<0.05 was considered statistically significant).
2.3Ethical statementInformed written consent was obtained from patients, and the protocol was approved by the Metro South Health and University of Queensland human research ethics committees (HREC/15/QPAH/301; UQ2015001047; HREC/2021/QMS/72,731).
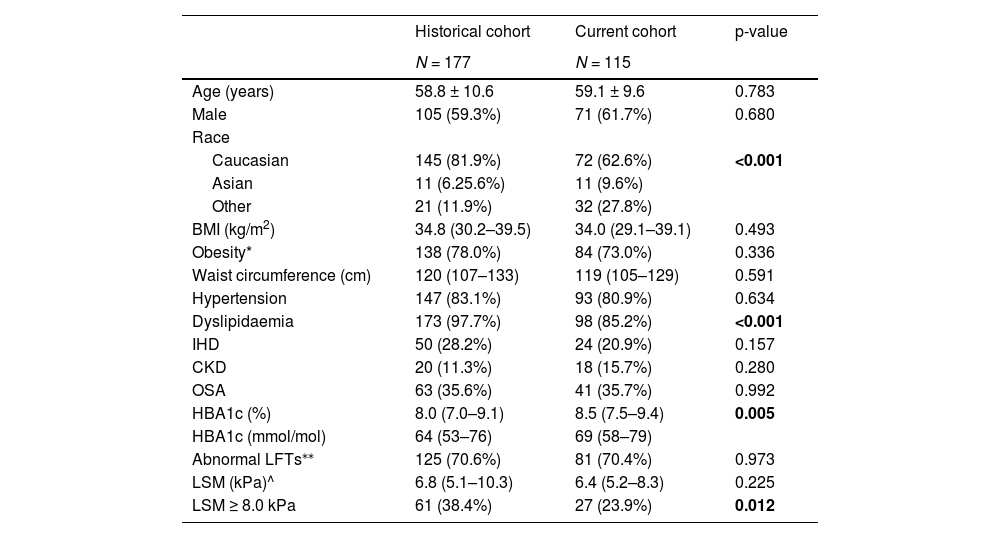
3Results3.1Patient characteristicsOne hundred and fifteen consecutive people with T2D and NAFLD were recruited from specialist-led diabetes clinics between Jun-2021 and Jun-2022. The historical cohort comprised 177 patients with NAFLD and T2D recruited from the same clinics during 2015–2017 [20]. The demographic and clinical characteristics of subjects in the current and historical cohorts are summarized in Table 1.
A higher mean HbA1c was seen in the current cohort compared to the historical cohort (p = 0.005). The prevalence of cardiometabolic comorbidities did not differ between groups, apart from a lower proportion of patients with dyslipidaemia in the current cohort (p<0.001). There was a difference in ethnicity between the 2 groups, with a lower proportion of people with Caucasian ethnicity and a higher proportion of people of Asian or “other” ethnicity in the current cohort (p<0.001). There was no difference in the prevalence of obesity or BMI between the groups.
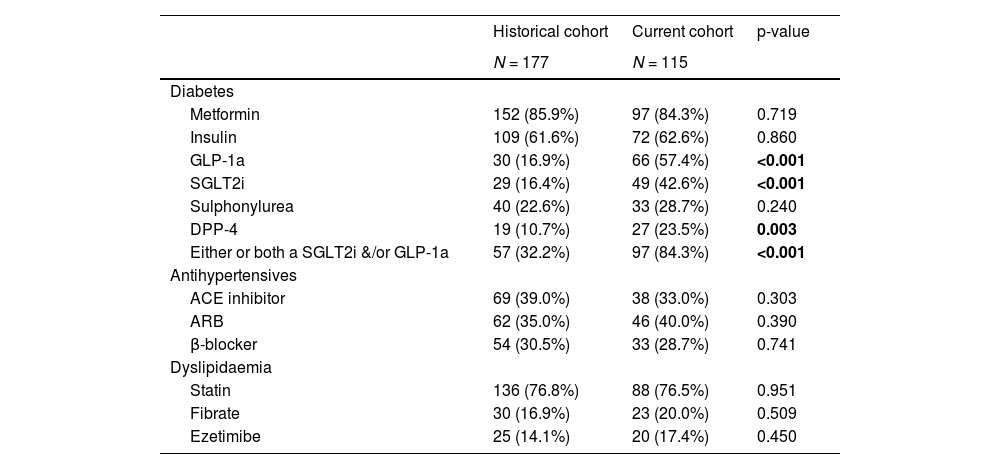
3.2Medication useThe use of metformin, sulphonylureas, statins and insulin therapy did not differ between the two cohorts (Table 2). In contrast, there was a marked difference in the prevalence of use of newer diabetes pharmacotherapy. The use of GLP-1a and SLGT2i was 57.4% and 42.6% respectively in the current cohort, compared to 16.9% and 16.4% in the historical cohort. This represents a 2.6 to 3.4-fold increase in the use of these medications from 2015 to 2017. The use of dipeptidyl peptidase-4 (DPP-4) inhibitors was also 2.2-fold higher in the current cohort. The prevalence of use of other commonly prescribed and/or cardiometabolic medications did not differ between the two groups.
3.3Assessment of NAFLD severityLSM were considered acceptable/good quality in 159 of 177 (89.8%) patients in the historical cohort and 113 of 115 (98.3%) patients in the current cohort and required use of the XL probe in 82.4% and 56.5% of patients, respectively. A lower proportion (23.9%) of patients in the current cohort had LSM ≥8 kPa compared with the historical cohort (38.4%; p = 0.012) (Table 1).
Clinical characteristics of participants with T2D and NAFLD in the historical and current cohorts.
| Historical cohort | Current cohort | p-value | |
|---|---|---|---|
| N = 177 | N = 115 | ||
| Age (years) | 58.8 ± 10.6 | 59.1 ± 9.6 | 0.783 |
| Male | 105 (59.3%) | 71 (61.7%) | 0.680 |
| Race | |||
| Caucasian | 145 (81.9%) | 72 (62.6%) | <0.001 |
| Asian | 11 (6.25.6%) | 11 (9.6%) | |
| Other | 21 (11.9%) | 32 (27.8%) | |
| BMI (kg/m2) | 34.8 (30.2–39.5) | 34.0 (29.1–39.1) | 0.493 |
| Obesity* | 138 (78.0%) | 84 (73.0%) | 0.336 |
| Waist circumference (cm) | 120 (107–133) | 119 (105–129) | 0.591 |
| Hypertension | 147 (83.1%) | 93 (80.9%) | 0.634 |
| Dyslipidaemia | 173 (97.7%) | 98 (85.2%) | <0.001 |
| IHD | 50 (28.2%) | 24 (20.9%) | 0.157 |
| CKD | 20 (11.3%) | 18 (15.7%) | 0.280 |
| OSA | 63 (35.6%) | 41 (35.7%) | 0.992 |
| HBA1c (%) | 8.0 (7.0–9.1) | 8.5 (7.5–9.4) | 0.005 |
| HBA1c (mmol/mol) | 64 (53–76) | 69 (58–79) | |
| Abnormal LFTs⁎⁎ | 125 (70.6%) | 81 (70.4%) | 0.973 |
| LSM (kPa)^ | 6.8 (5.1–10.3) | 6.4 (5.2–8.3) | 0.225 |
| LSM ≥ 8.0 kPa | 61 (38.4%) | 27 (23.9%) | 0.012 |
Data are presented as mean ± SD or median (IQR) for continuous measures, and n (%) for categorical measures.
* Obesity defined as BMI ≥30 kg/m2 and ≥27.5 kg/m2 for Asians.
⁎⁎ Healthy reference ranges (ALT males ≤30 U/L, females ≤19 U/L, AST both sexes ≤30 U/L, GGT both sexes ≤50 U/L).
^ LSM is shown only for scans that met quality criteria (≥10 valid measurements, success rate of ≥60%, IQR ≤30%)
BMI body mass index; IHD ischaemic heart disease; CKD chronic kidney disease; OSA obstructive sleep apnea; LFTs liver function tests; LSM liver stiffness measurement.
Prevalence of use of specific medications in participants with T2D and NAFLD from the historical or current cohort.
| Historical cohort | Current cohort | p-value | |
|---|---|---|---|
| N = 177 | N = 115 | ||
| Diabetes | |||
| Metformin | 152 (85.9%) | 97 (84.3%) | 0.719 |
| Insulin | 109 (61.6%) | 72 (62.6%) | 0.860 |
| GLP-1a | 30 (16.9%) | 66 (57.4%) | <0.001 |
| SGLT2i | 29 (16.4%) | 49 (42.6%) | <0.001 |
| Sulphonylurea | 40 (22.6%) | 33 (28.7%) | 0.240 |
| DPP-4 | 19 (10.7%) | 27 (23.5%) | 0.003 |
| Either or both a SGLT2i &/or GLP-1a | 57 (32.2%) | 97 (84.3%) | <0.001 |
| Antihypertensives | |||
| ACE inhibitor | 69 (39.0%) | 38 (33.0%) | 0.303 |
| ARB | 62 (35.0%) | 46 (40.0%) | 0.390 |
| β-blocker | 54 (30.5%) | 33 (28.7%) | 0.741 |
| Dyslipidaemia | |||
| Statin | 136 (76.8%) | 88 (76.5%) | 0.951 |
| Fibrate | 30 (16.9%) | 23 (20.0%) | 0.509 |
| Ezetimibe | 25 (14.1%) | 20 (17.4%) | 0.450 |
Data are presented n (%) for categorical measures.
GLP-1a glucagon-like peptide-1 receptor agonists; SGLT2i sodium-glucose co-transporter 2 inhibitors; DPP-4 Dipeptidyl peptidase 4 inhibitors; ACE inhibitor Angiotensin-converting-enzyme inhibitors; ARB angiotensin receptor blocker.
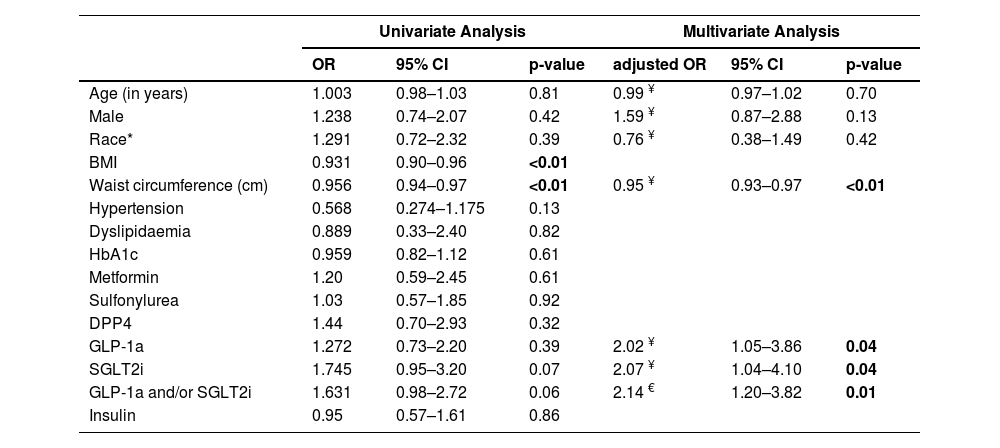
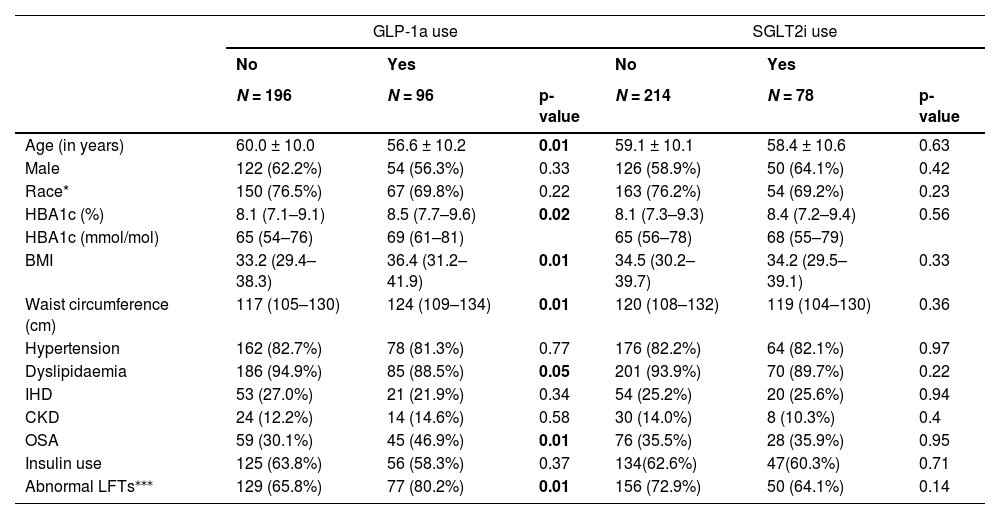
In univariable analysis of the pooled data, there was no statistically significant association between SGLT2i, GLP-1a or SGLT2i and/or GLP-1a use and LSM status (Table 3). Higher waist circumference and BMI were significantly associated with LSM ≥8 kPa. Those taking a GLP-1a were more likely to be younger, have a higher HbA1c, BMI, waist circumference and to have dyslipidaemia and obstructive sleep apnea (Table 4). There was no significant difference between the groups taking or not taking a SGLT2i.
Logistic regression analysis of factors associated with liver stiffness measurement <8 kPa among the pooled data (N = 272).
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | adjusted OR | 95% CI | p-value | |
| Age (in years) | 1.003 | 0.98–1.03 | 0.81 | 0.99 ¥ | 0.97–1.02 | 0.70 |
| Male | 1.238 | 0.74–2.07 | 0.42 | 1.59 ¥ | 0.87–2.88 | 0.13 |
| Race* | 1.291 | 0.72–2.32 | 0.39 | 0.76 ¥ | 0.38–1.49 | 0.42 |
| BMI | 0.931 | 0.90–0.96 | <0.01 | |||
| Waist circumference (cm) | 0.956 | 0.94–0.97 | <0.01 | 0.95 ¥ | 0.93–0.97 | <0.01 |
| Hypertension | 0.568 | 0.274–1.175 | 0.13 | |||
| Dyslipidaemia | 0.889 | 0.33–2.40 | 0.82 | |||
| HbA1c | 0.959 | 0.82–1.12 | 0.61 | |||
| Metformin | 1.20 | 0.59–2.45 | 0.61 | |||
| Sulfonylurea | 1.03 | 0.57–1.85 | 0.92 | |||
| DPP4 | 1.44 | 0.70–2.93 | 0.32 | |||
| GLP-1a | 1.272 | 0.73–2.20 | 0.39 | 2.02 ¥ | 1.05–3.86 | 0.04 |
| SGLT2i | 1.745 | 0.95–3.20 | 0.07 | 2.07 ¥ | 1.04–4.10 | 0.04 |
| GLP-1a and/or SGLT2i | 1.631 | 0.98–2.72 | 0.06 | 2.14 € | 1.20–3.82 | 0.01 |
| Insulin | 0.95 | 0.57–1.61 | 0.86 | |||
Note: The final multivariable model included 272 participants, 20 were excluded as their liver stiffness measurement did not meet quality criteria.
Clinical characteristics of participants using the new diabetes medications (GLP-1a and SGLT2i) compared to those not using these medications among the pooled data (N = 272).
| GLP-1a use | SGLT2i use | |||||
|---|---|---|---|---|---|---|
| No | Yes | No | Yes | |||
| N = 196 | N = 96 | p-value | N = 214 | N = 78 | p-value | |
| Age (in years) | 60.0 ± 10.0 | 56.6 ± 10.2 | 0.01 | 59.1 ± 10.1 | 58.4 ± 10.6 | 0.63 |
| Male | 122 (62.2%) | 54 (56.3%) | 0.33 | 126 (58.9%) | 50 (64.1%) | 0.42 |
| Race* | 150 (76.5%) | 67 (69.8%) | 0.22 | 163 (76.2%) | 54 (69.2%) | 0.23 |
| HBA1c (%) | 8.1 (7.1–9.1) | 8.5 (7.7–9.6) | 0.02 | 8.1 (7.3–9.3) | 8.4 (7.2–9.4) | 0.56 |
| HBA1c (mmol/mol) | 65 (54–76) | 69 (61–81) | 65 (56–78) | 68 (55–79) | ||
| BMI | 33.2 (29.4–38.3) | 36.4 (31.2–41.9) | 0.01 | 34.5 (30.2–39.7) | 34.2 (29.5–39.1) | 0.33 |
| Waist circumference (cm) | 117 (105–130) | 124 (109–134) | 0.01 | 120 (108–132) | 119 (104–130) | 0.36 |
| Hypertension | 162 (82.7%) | 78 (81.3%) | 0.77 | 176 (82.2%) | 64 (82.1%) | 0.97 |
| Dyslipidaemia | 186 (94.9%) | 85 (88.5%) | 0.05 | 201 (93.9%) | 70 (89.7%) | 0.22 |
| IHD | 53 (27.0%) | 21 (21.9%) | 0.34 | 54 (25.2%) | 20 (25.6%) | 0.94 |
| CKD | 24 (12.2%) | 14 (14.6%) | 0.58 | 30 (14.0%) | 8 (10.3%) | 0.4 |
| OSA | 59 (30.1%) | 45 (46.9%) | 0.01 | 76 (35.5%) | 28 (35.9%) | 0.95 |
| Insulin use | 125 (63.8%) | 56 (58.3%) | 0.37 | 134(62.6%) | 47(60.3%) | 0.71 |
| Abnormal LFTs⁎⁎⁎ | 129 (65.8%) | 77 (80.2%) | 0.01 | 156 (72.9%) | 50 (64.1%) | 0.14 |
Data are presented as mean ± SD or median (IQR) for continuous measures, and n (%) for categorical measures.
In the study, waist circumference was found to be highly correlated with BMI (R = 0.85, p<0.001). There was no correlation between the other variables examined (data not shown). In multivariable analysis, following adjustment for waist circumference, both SGLT2i and GLP-1a and/or SGLT2i use were independently associated with LSM status (adj OR=2.00, 95%CI 1.02–3.94 and adj OR=2.12, 95%CI 1.20–3.75, respectively), but not GLP-1a (adj OR=1.78, 95%CI 0.94–3.27). Following adjustment for BMI, SGLT2i and/or GLP-1a use was independently associated with LSM status (adj OR=1.82, 95%CI 1.06–3.12), but not GLP-1a (adj OR=1.62, 95%CI 0.90–2.91) or SGLT2i (adj OR=1.62, 95%CI 0.87–3.01) separately. In the final model including age, sex, race and waist circumference (BMI was not included in the final model), SGLT2i and/or GLP-1a use was independently associated with LSM status (Table 3). Patients taking a GLP-1a or a SGLT2i had twice the odds of having a lower LSM (<8 kPa) compared to patients not taking these drugs (OR=2.02, 95%CI 1.05–3.86, p = 0.04 and OR 2.07 95%CI 1.04–4.10, p = 0.04, respectively). Lower waist circumference remained significantly associated a lower LSM (<8 kPa; OR=0.95 95%CI, 0.93–0.97 p<0.01). When a variable combining the use of GLP-1 and/or SGLT2 was included in the model, instead of each of these drugs separately, the adjusted odds of having a lower LSM (<8 kPa) compared to patients not taking these drugs was 2.14 (95%CI, 1.20–3.82, p = 0.010).
4DiscussionWe observed a high prevalence (84.3%) of use of SGLT2i and GLP-1a use in a recent cohort of patients with T2D and NAFLD compared to a similar cohort (32.2%) recruited 5 years earlier. This finding likely reflects increased provider knowledge, experience, and ease of prescribing of these medications, due to expanded indications for subsidised access under Australia's Pharmaceutical Benefits Scheme (PBS). A GLP-1a (Exenatide) and SGLT2i (Canagliflozin) were first listed on the PBS in 2010 and 2013 respectively, with additional agents and combination therapies added over subsequent years. In contrast to our experience in Australia, centres in the US and Canada have reported a slower uptake in use of these classes of medications, largely attributed to cost and access barriers (reported by endocrinology providers and primary care physicians) or knowledge gaps (perceived by cardiology providers) [28,29]. The expanded use of GLP-1a and SGLT2i in our patients with NAFLD may have an important role in reducing their increased risk of adverse cardiovascular events and chronic kidney disease. Almost one-quarter (23.9%) of our patients with T2D and NAFLD had clinically significant fibrosis with an elevated LSM ≥8 kPa. These data are consistent with many recent studies demonstrating a high prevalence (15 – 23.8%) of moderate to advanced fibrosis among patients with T2D assessed using elastography [30–32]. Interestingly, our data show that patients taking a GLP-1a or SGLT2i were more likely to have a lower LSM (<8 kPa), suggesting that in addition to cardiometabolic benefit, these medications may also reduce the risk of progressive NAFLD. Our findings support two recent “real-world” studies using large patient cohorts from the United Kingdom and Italy [33,34]. In 2 large active comparator cohorts (30,291 and 225,320 new users of GLP-1a and DPP-4 inhibitors, respectively and 41,184 and 148,421 new users of SGLT-2i and DPP-4 inhibitors, respectively) from the U.K. Clinical Practice Research Datalink, GLP-1a and SGLT-2i were associated with a lower incidence of NAFLD (recorded using Read codes or SNOMED-CT classification) HR 0.86, 95% CI 0.73–1.01 and HR 0.78, 95% CI 0.68–0.89 respectively and a decreased risk of hepatic transaminase elevation (HR 0.89, 95% CI 0.83–0.95, and HR 0.66, 95% CI 0.61–0.71) [34]. Similarly, a study of 637 consecutive Italian patients with T2D showed that, compared to patients maintained on usual treatment, GLP-1a and SGLT-2i, but not DPP-4 inhibitors, significantly improved non-invasive biomarkers of steatosis and fibrosis (assessed using fatty liver index and FIB4 score respectively) [33]. In contrast to the UK study, our data did not demonstrate a reduction in abnormal liver function tests for people using GLP-1a. We hypothesize that this higher prevalence of abnormal liver function tests in the cohort taking GLP-1a may be due to a higher prevalence of more severe steatosis, associated with their higher BMI and waist circumference. As our cohort did not have longitudinal follow up, we could not ascertain whether these medication result in a relative decrease in transaminases.
There is increasing interest in the efficacy of GLP-1a and SGLT-2i in improving histological features of NAFLD. In comparison with placebo, SGLT2i and GLP-1a have been associated with reduced steatosis [35] and a greater improvement in liver cell injury and inflammation [36,37] in several randomized controlled trials. In the latter 2 studies, although there was no histological improvement in fibrosis stage, GLP-1a-treated patients had a reduced rate of fibrosis progression, and showed an improvement in non-invasive tests of fibrosis, assessed by transient elastography [37] and/or serum enhanced liver fibrosis test [36,37]. The authors hypothesize that as results for the non-invasive fibrosis tests are continuous variables, they may show earlier changes that are not detected using the categorial scores obtained by histological examination [37]. However, the sensitivity to change of these biomarkers and the exact connection between a change in biomarker score and change in fibrosis stage or longer-term clinical outcome remains unclear [38]. The variability in outcome measures utilised by randomized controlled trials have limited the conclusions that can be inferred in a meta-analysis of these studies [39]. Although the beneficial effects of GLP-1a and SGLT2i on steatosis and features of steatohepatitis may be largely mediated by weight loss and improved glycemic control, other indirect mechanisms of action may include anti-inflammatory effects, alterations in hepatic substrate supply and improvement in gut dysbiosis [40]. Combinations of GLP-1a with other enteropancreatic hormones, to enhance their metabolic effect, are now under evaluation [38].
Our study provides useful “real-world” data demonstrating that many patients with T2D are now taking SGLT2i and/or GLP-1a therapy irrespective of the presence or severity of co-morbid NAFLD [41]. The prospective recruitment of patients, history of medication use verified with the dispensing pharmacy or GP medical records, and assessment of alcohol intake using a validated tool, alongside the “real world” aspect of the data are study strengths. The historical and current patient cohorts differed with respect to ethnicity, dyslipidemia and control of diabetes (HBA1c), and these factors may have influenced the use of particular medications. It is possible that a GLP-1a or SGLT2i was preferentially started in the highest weight categories, and that they have had a beneficial effect on weight. However, without longitudinal data we cannot confirm this hypothesis. In addition, we could not ascertain the length of time patients had diabetes or were taking these new diabetes medications. The results of this study need to be interpreted with some caution as the study design cannot demonstrate causality, and further evidence of the antifibrotic efficacy of these agents needs to be produced in clinical trials.
5ConclusionsOur data, showing that patients taking a GLP-1a or SGLT2i were more likely to have a lower LSM (<8 kPa), support ongoing evaluation of the role of these medications to reduce the risk of progressive NAFLD. Given the increased risk of cardiovascular mortality associated with NAFLD and the evidence for benefit of GLP-1a and SGLT2i in patients with T2D and cardiovascular or renal disease, these medications may have wider benefit for NAFLD-associated comorbidities.
FundingLG and MA were supported by the Metro South Health Research Support Scheme Grant (#RSS_2022_012). KLH was supported by a Health Innovation, Investment and Research Office (HIIRO) Clinical Research Fellowship. All funding was external and the funding body had no role in the design of the study, collection, analysis and interpretation of data and in writing the manuscript.
Author contributionsEE Powell, Lucy Gracen and Withma Muthukumara contributed to the conception and design of the study. Lucy Gracen, Withma Muthukumara and Melanie Aikebuse assisted with the ascertainment and identification of patients for the study and Lucy Gracen and Melanie Aikebuse recruited participants. Lucy Gracen and Kelly Hayward performed the data analysis and take responsibility for the integrity and accuracy of data. Patricia Valery provided statistical advice. Lucy Gracen and Withma Muthukumara drafted the report. Anthony Russell, James O'Beirne, Katharine M Irvine, Suzanne Williams and Gaurav Puri, along with the other authors, provided advice regarding interpretation of data, critical review of the draft for important intellectual content, and approved the final version.
We thank the participants for their cooperation and assistance in performing the study.