Introduction and aim. Developing reliable biomarkers for hepatocellular carcinoma (HCC) patients who are at a high risk of recurrence after curative hepatic resection is very important for determining subsequent therapeutic strategies. We investigated the role of the cell cycle factor NIMA-related kinase 2 (NEK2) in HCC progression in hepatoma cells and post-surgery patients.
Material and methods. The effects of NEK2 on proliferation, invasion and migration of hepatoma HuH7 and SK-Hep1 cells were evaluated. In a post-surgery HCC cohort (N = 97), the Nek2 induction levels in the tumors were examined with real-time RT-PCR analysis, and the results were analyzed for their correlations with recurrence.
Results. NEK2 promoted G1 to S phase cell cycle progression by causing increases in cyclin D1 and AKT phosphorylation and decreases in the cyclin-dependent kinase inhibitor p27, indicating that NEK2 plays an important role during interphase in addition to its previously identified role in M phase. NEK2 also enhanced the proliferation, migration and invasion of hepatoma cells and regulated the expression of E-cadherin and MMP9. The Nek2 mRNA levels in the tumors were highly correlated with recurrence rates in the post-surgery HCC patients. Combined evaluation of the tumor AJCC stage and the Nek2level can serve as a reliable method for predicting the relative risk of HCC recurrence in these patients. Conclusions. NEK2 plays a significant role in cell cycle progression in the inter- and M-phases. NEK2 enhances HCC metastasis and is correlated with recurrence and thus can potentially serve a promising high-risk biomarker for HCC.
Hepatocellular carcinoma (HCC) is among the leading causes of deaths by cancer worldwide, with over 500,000 people affected every year.1 Understanding the etiologies and developing effective preventive strategies for HCC are very important for lowering its incidence. The main etiological factors causing HCC include chronic viral hepatitis B/C, alcohol intoxication, and chronic metabolic syndromes such as non-alcoholic fatty liver disease (NASH).2 In countries with hepatitis B/C epidemics, most HCCs are related to chronic viral hepatitis, whereas in most American and European countries, in which viral hepatitis is not highly prevalent, non-viral factors, such as alcohol addiction and chronic metabolic syndromes, are the major causes of HCC.2,3 Most early cases of HCC are not diagnosed due to the lack of observable symptoms, which leads to high recurrence rates after primary therapies.4 Therefore, developing reliable biomarkers to screen HCC patients with a high risk of recurrence after primary treatment is very important for identifying those that should engage aggressive therapeutic approaches or intensive follow ups to closely monitor possible disease progression.
Thus far, curative surgical resection of the tumor is the primary therapeutic approach for HCC in the early stages, such as the Barcelona Clinic Liver Cancer (BCLC) stage A.4 For these HCC cases, the tumor genotype has been demonstrated to greatly affect the efficacy of post-surgery adjuvant therapies and the relative risk of recurrence.5 Many high-risk prognostic biomarkers have been identified for HCC.5 An important group of high-risk biomarkers includes cell cycle and mitosis factors.6 During the prophase of mitosis, centrosome duplication and separation occur and equip the cell for chromosome alignment and bipolar movement.7 The efficiency of centrosome duplication/separation has been demonstrated to be highly correlated with cell proliferation and growth.8 Never in mitosis-related kinase 2 (NEK2), a member of the NEK serine/threonine kinase family, is an essential factor for centrosome assembly/separation and has been found to correlate with recurrence in some types of cancer.9–11 NEK2 interacts with the kinetochore complex component NDC80 in the control of centrosome separation and bipolar spindle formation in mitotic cells by phosphorylating centrosomal proteins, such as centrosome-associated protein (CEP) 250 and ninein-like (NINL), which results in their displacement from the centrosomes.12,13 NEK2 also regulates kinetochore microtubule attachment stability and the mitotic checkpoint protein complex via the phosphorylation of NDC80, cell-division cycle (CDC) 20 and mitotic arrest deficient 2 like 1 (mAD2L1).14
Although the role of NEK2 in centrosome regulation in mitosis had been extensively elaborated, its role in interphase remains unclear. Recent studies have documented that the nuclear localization of NEK2 occurs in neoplastic cells and is correlated with worse prognoses for colorectal cancer and testicular germ cell tumors (TGCT), which indicates that the non-centrosomal pool of NEK2 plays a unique role in tumor progression.15,16 Therefore, in this study, we explored the molecular mechanism by which NEK2 regulates HCC progression through a non-centrosomal role. We found that NEK2 plays a regulatory role in the G1-S phase transition. We also found that the Nek2 expression level is highly correlated with HCC recurrence in patients who have received hepatectomy surgeries. Therefore, NEK2 could potentially serve as a biomarker for a high risk of recurrence in post-surgery HCC patients.
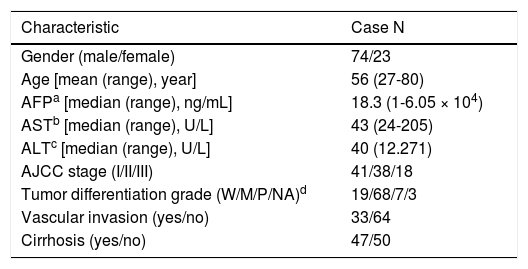
Material and MethodsHCC patients, cell lines and transgenic miceHuman liver tissue sections were collected from the HCC patients who were admitted to National Cheng Kung University Hospital (NCKUH) for hepatectomy surgery from 2008 to 2012. All the HCC was diagnosed by medical imaging evaluation and biopsy histopathologic reviews by two pathologists independently. The tumor staging and histologic grading followed the American Joint Committee on Cancer (AJCC) classification.31 Liver cirrhosis in the non-tumorous liver was observed with the stainings of the H&E and Masson’s trichrome methods. The patients were regularly followed-up at clinic visits every 1-3 months after a hepatic resection. HCC recurrence was detected by medical imaging and biopsy confirmed. All of the patients provided signed informed consent to use their surgical specimens for this research. And all of the protocols for this study were reviewed and approved by the NCKUH Institutional Review Board (IRB No.: ER-100-058 [04/25/2011]). A total of 109 HCC cases were recruited: 12 patients, including 6 HBV- and 6 HCV-related HCC cases, were analyzed with a cDNA microarray assay, and 97 HBV-related HCC cases (Table 1) were analyzed with a quantitative reverse transcription-polymerase chain reaction (RT-PCR) assay. The human hepatoma HuH7 and SK-Hep1 cell lines were used for in vitro cell culture studies. Nek2 and Plk1 (polo-like kinase 1) gene knockdown (KD) constructs were prepared using specific short hairpin RNAs (shRNAs) whose genes were cloned into the lentiviral vector pLKO.1, which was obtained from the National RNAi Core Facility (Academia Sinica, Taipei, Taiwan), according to a previously described protocol.17 An shRNA construct against GFP (shGFP) was used as a negative control. A HuH7 cell line with stable Nek2 overexpression was constructed using the Tet-off gene expression system (Clontech) for the Nek2 gene. HCC tumorous and peri-tumorous mouse liver tissue samples were dissected from 18-month-old C57BL/6 transgenic mice carrying HBx, pre-S2 mutant LHBS, and pre-S2/HBx double transgenes.18
Demographic characteristics of the HBV-related HCC cases (n = 97) in this study.
| Characteristic | Case N |
|---|---|
| Gender (male/female) | 74/23 |
| Age [mean (range), year] | 56 (27-80) |
| AFPa [median (range), ng/mL] | 18.3 (1-6.05 × 104) |
| ASTb [median (range), U/L] | 43 (24-205) |
| ALTc [median (range), U/L] | 40 (12.271) |
| AJCC stage (I/II/III) | 41/38/18 |
| Tumor differentiation grade (W/M/P/NA)d | 19/68/7/3 |
| Vascular invasion (yes/no) | 33/64 |
| Cirrhosis (yes/no) | 47/50 |
Cell proliferation and colony formation assays were employed to detect the effect of NEK2 on cell proliferation. Briefly, the Nek2 KD and vector control HuH7 cells were grown in 24-well cell culture plates at 1 × 105 cells/ well, and the cell numbers were determined using cell counting chambers after 24, 48 and 72 h of growth. For the colony formation assay, 1000 Nek2 knockdown, overexpression and control HuH7 cells were seeded in 10-cm cell culture dishes and then grown for 14 days before harvest. The numbers of cell colonies (diameter ≥ 0.5 mm) in the culture dishes were calculated according to a previously described protocol.18 Regarding the assays of cell sensitivity to the NEK2 inhibitor pelitinib,19 the Sk-Hep1 and HuH7 cells were treated with various doses of pelitinib (Cayman, Inc.) or else mock treated for 8 and 24 h and then subjected to the MTT [α3-(4,5-dimethylthia-zol-2-yl)-2,5-diphenyltetrazolium bromide] assay according to a previously described protocol.20
Cell cycle analysisThe Nek2 KD and control HuH7 cells were synchronized with nocodazole (330 nM) treatment for 16 h. The cells were then released into the regular growth medium and harvested after 4, 8, 12 and 24 h. After serial washing with phosphate-buffered saline (pH 7.4), the cells were fixed with 70% ethanol (v/v) overnight at -20 °C and then stained with a propidium iodide solution (20 µg/mL propidium iodide, 0.001% Triton X-100 and 200 µg/mL RNase) for 30 min at room temperature. The DNA contents of the cells were determined by flow cytometry| (BDTM Biosciences).
Cell migration and invasion assaysHuH7 cells overexpressing or with knocked down Nek2 were analyzed for migration activities following a previously described protocol. Briefly, 1 × 105 cells were seeded in each upper chamber of 24-well Transwell inserts (Corning, NY, USA) and grown in serum-free medium; the bottom chamber contained regular DMEM growth medium with 10% fetal bovine serum. After 24 and 48 h of incubation, the cells that adhered to the Transwell inserts were fixed with 4% formaldehyde and then stained with crystal violet. The cells on the upper surface of the Transwell membrane were wiped off using a cotton swab, and the cells that had migrated to the lower surface of the membrane were counted using Image J image processing software (NIH, USA). For the cell invasion assay, cells were grown in 24-well Transwell inserts that were precoated with 0.5% Matrigel (BDTM Biosciences) and grown for 24 and 48 h before harvest. The invading cells were counted according to the same protocol that was used in the cell migration assay. For the wound healing assay, 2 × 106/well cells were seeded in 6-well culture dishes and grown for 24 h to form a confluent monolayer. The wound was created by cutting a line on the cell monolayer using a pipette tip, and the detached cells were washed off with PBS (pH 7.4). The wound images were photographed immediately after the wound was created and after incubation for 24 h, and the wound healing rate was measured.
Quantitative reverse transcription polymerase chain reactionTotal RNA was extracted from the tumorous and adjacent non-tumorous HCC tissues using TRIzol reagent (Sigma-Aldrich) according to a previously described protocol and then reverse transcribed into cDNA.20 The expression levels of the Nek2, FoxM1, and Plk1 genes were examined by quantitative RT-PCR (Applied Biosystems, Inc.).18 Briefly, the liver cDNA was mixed with TaqMan™ Master Mix, the gene-specific PCR primers and TaqMan™ probes and then analyzed using an Applied Biosystems® 7500 Real-Time PCR System. The housekeeping gene Gapdh was used as endogenous control for gene expression. The real-time RT-PCR data were analyzed using the StepOne™ Software (ABI).
cDNA microarray analysisTwelve pairs of the tumorous and peri-tumorous liver tissues (6 HBV- and 6 HCV-related HCC) that were surgically resected from the HCC patients were subjected to cDNA microarray analysis (Agilent Human Gene Expression v2 4x44K Microarray Kit) to screen for genes whose expression levels were changed in the tumor. Moreover, the HCC tumorous and peri-tumorous mouse liver tissues from fourteen 18-month-old C57BL/6 transgenic mice carrying HBx, pre-S2 mutant LHBS, and pre-S2/HBx double transgenes were similarly analyzed using an Agilent Mouse Gene Expression v2 4x44K Microarray Kit.21 The experimental protocol and data analysis methods followed a previously described protocol.
Multivariate regression and Cox proportional hazards statistical analysesThe relative Nek2 mRNA levels, as determined by quantitative RT-PCR, were analyzed for their correlations with various clinicopathological factors. The association of the Nek2 level with HCC recurrence was analyzed using a Mann-Whitney test. Univariate logistic regression analysis was used to screen for the clinicopathologic factors that were correlated with HCC recurrence. Factors for which the p-value was < 0.1 (a value that is commonly used to screen for significant factors for uni- to multivariate analyses) were retained for multivariate regression analysis.22,23 The HCC recurrence probabilities for different combinations of Nek2 level and AJCC stage (i.e., well/ moderately and poorly differentiated stages) were then estimated based on Cox proportional hazards analyses. The relative risk scores of the various combinations in the groups with well/moderately and poorly differentiated tumors were computed, and Cox survival curves were developed.
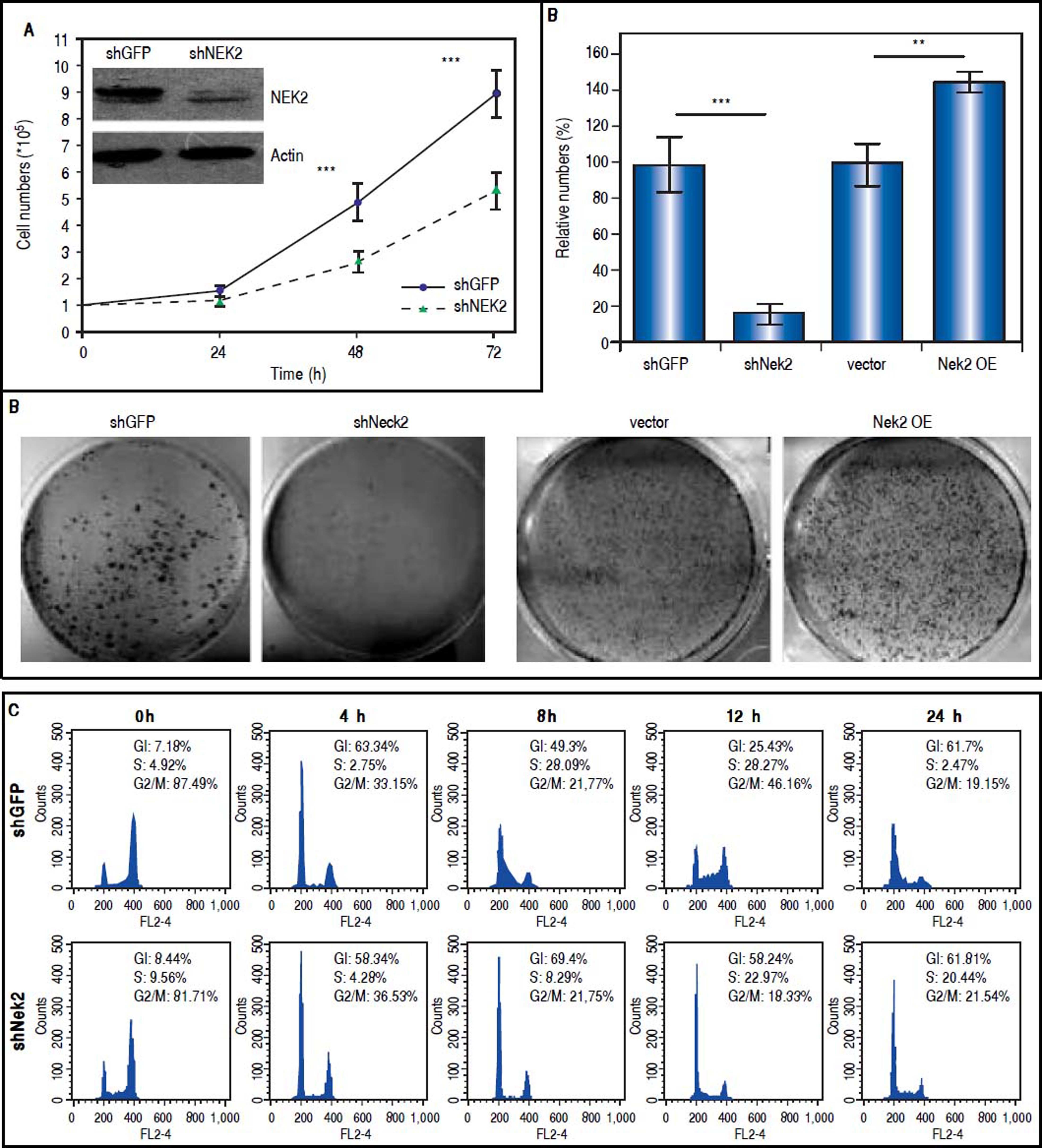
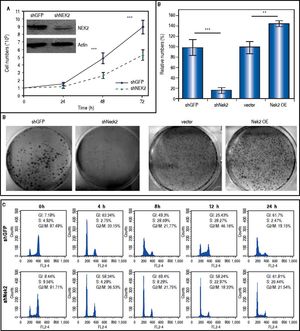
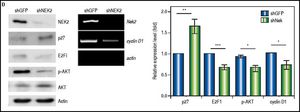
ResultsTo characterize the role of NEK2 in HCC progression, its effect on the proliferation of HCC cells was first analyzed. Based on the direct cell counting and colony formation assays, the Nek2 knockdown cells exhibited lower cell propagation and colony formation, whereas those that overexpressed Nek2 exhibited the opposite effects, which indicated that NEK2 promotes the proliferation of hepatoma HuH7 cells (Figure 1A and B). Recent studies have found that the nuclear localization of NEK2 is associated with worse prognoses for some cancers, implying that NEK2 plays a unique role in interphase. We found that, after the synchronization of cells in the M phase with nocodazole treatments, the Nek2 KD cells exhibited delayed G1 to S phase cell cycle progression, as 8 h after the release from arrest, 69% and 49% of the Nek2 KD and HuH7 control cells remained in G1 phase, respectively; 12 h after the release, the corresponding values were 58% and 25% (Figure 1C). These findings indicate that NEK2 promotes G1 to S phase cell cycle progression. Additionally, the knockdown of Nek2 caused decreases in cyclin D1 and E2F1 and AKT phosphorylation, as well as an increase in the cyclin-dependent kinase inhibitor p27kip1 (Figure 1D).24,25 Taken together, these results indicate that NEK2 regulates the expression of G1-S cell cycle factors and thereby enhances G1 to S progression.
Effects of NEK2 on cell growth and G1 to S phase cell cycle progression. A. Cell proliferation assay of human hepatoma HuH7 cells stably expressing shNek2 and shGFP constructs. The cell numbers after 24, 48 and 72 h of growth in regular medium were counted. The KD efficiency of Nek2 at the time of seeding was detected by Western blotting as shown in the top left region of the graph. B. Colony formation assay in the HuH7 cells stably expressing shNek2 and shGFP and overexpressing Nek2 or the vector only. Left: one set of representative images of the experimenta results; right: summary of the data from three independent experiments. C. Cell cycle profile analysis in the Nek2 KD and control HuH7 cells. After synchronization with nocodazole treatments, the cell cycle profiles were analyzed immediately after the treatment (0 h) and at various time points after release from nocodazole. The percentages of cells in the G1, S and G2/Mphases are indicated. The Nek2 KD cells exhibited G1 to S phase arrest. D. Expressions of the cell cycle regulatory factors that were affected by NEK2. Left: representative images of Western blotting and RT-PCR for the detection of the expression levels of p27, E2F1, phosphorylated AKT, and cyclin D1; right: summary of the data from three independent experiments. The p-AKT level in the bar chart indicates the ratio of the intensity of phosphorylated AKT to the total AKT. Actin served as the internal control. *: p < 0.05. **: p < 0.01. ***: p < 0.001.
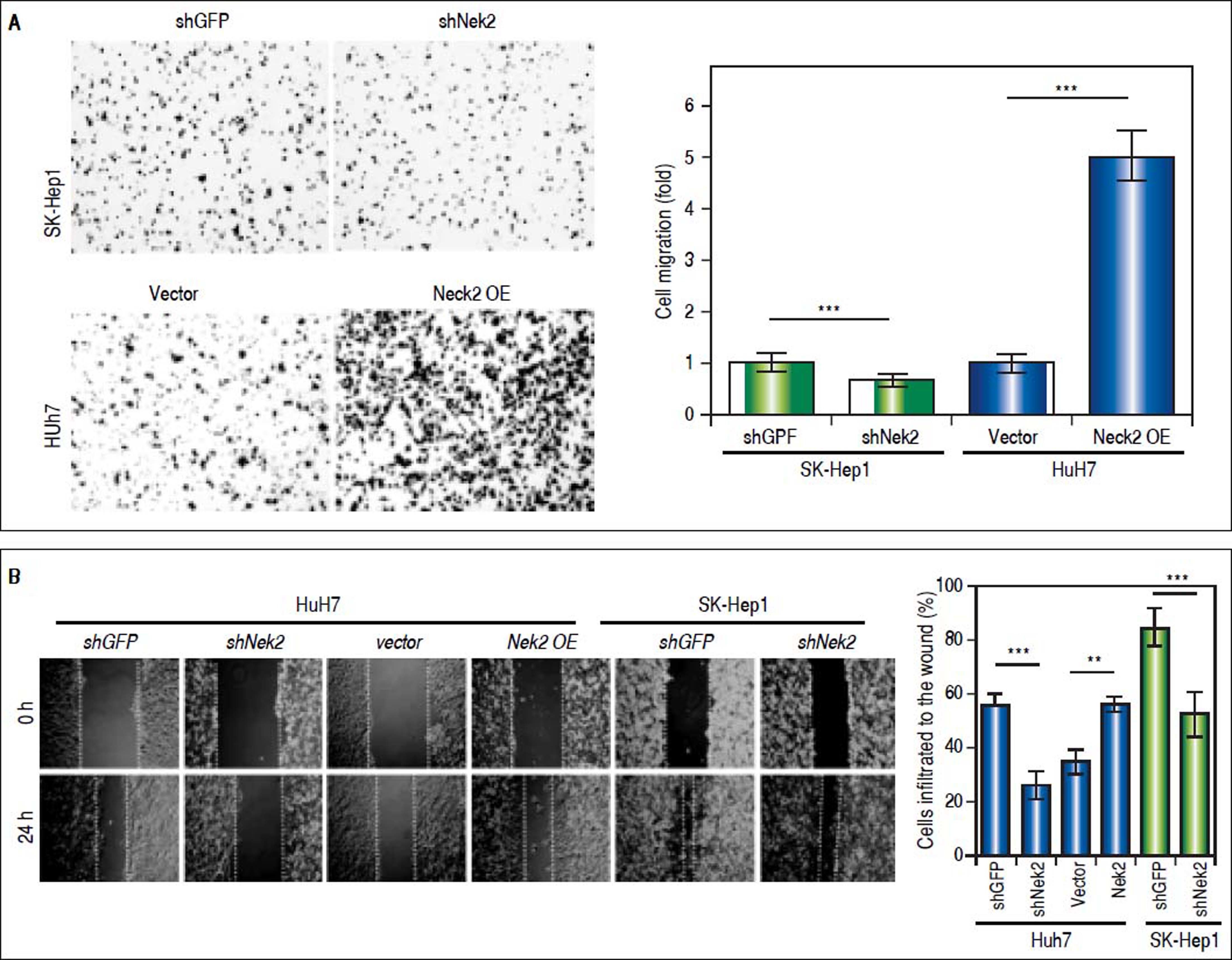
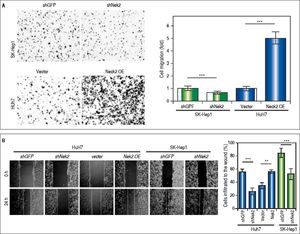
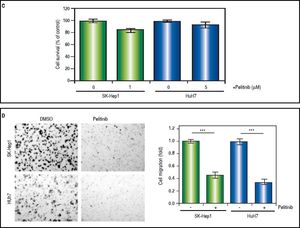
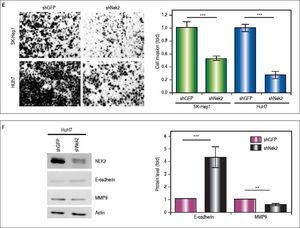
The effects of NEK2 on the invasion and migration of HuH7 and SK-Hep1 hepatoma cells were investigated. Analyses of the Transwell migration and wound healing assays revealed that the Nek2 expression level was positively correlated with the cell migration and wound healing activities (Figure 2A and B). Additionally, the small-molecule NEK2 inhibitor pelitinib (EKB-569), which binds to the NEK2 catalytic site with high affinity and blocks its kinase activity, was used to validate the effects of NEK2 on cell migration.19 The results revealed that while pelitinib at the concentrations used to treat the HuH7 (5 μM) and SK-Hep1 (1μM) cells did not cause significant cytotoxicity (Figure 2C), it greatly reduced the migration activities of the HuH7 and SK-Hep1 cells, which supports the notion that NEK2 is an essential factor for HCC cell migration (Figure 2D). Furthermore, analysis of the Matrigel cell invasion assays revealed that Nek2 KD significantly decreased the invasion activities (Figure 2E). In these cells, the level of NEK2 was closely correlated with the levels of invasion factors, including MMP9 and E-cadherin, which indicated that NEK2 promotes HCC invasion by regulating the expressions of these essential invasion factors (Figure 2F).26
Enhancement of cell migration and invasion by NEK2 in hepatoma cells. A. The Transwell migration assay to detect the effect of NEK2 on the migration abilities of the HuH7 and SK-Hep1 hepatoma cells. The Nek2 KD (shNek2) and control (shGFP) cells, as well as the cells overexpressing Nek2 (Nek2 OE) and the plasmid vector were analyzed. Left: representative images of the migrated cells among the various analyzed cell types; right: quantitation of the migrated cells summarized over three independent experiments. B. Wound healing assays. The Nek2 KD and overexpressing cells were analyzed for their migration activities toward a pre-cut wound in confluent cell cultures. Left: representative images of the data before (0 h) and after 24 h of incubation (24 h). The dotted lines indicate the edges of the wounds. Right: measurement of the percent of the areas of the cell wounds that were infiltrated by cell migration after 24 h of incubation summarized over the data from three independent experiments. C. Cytotoxicities of pelitinib to the HuH7 and SK-Hep1 cells as detected with MTT assays. Pelitinib was examined for its cytotoxicity at the concentrations used in the Transwell migration assays of the HuH7 (1 μM) and SK-Hep1 (5 μM) cells. The bar charts represent data summarized from three independent experiments. At the indicated dosages, pelitinib did not cause significant cell death. D. Inhibition of cell migration by the NEK2 inhibitor pelitinib as demonstrated in the Transwell migration assays with the HuH7 (1 μM) and SK-Hep1 (5 μM) cells. The left and right panels display representative images and quantitation of the data from three independent experiments, respectively. E. The Matrigel invasion assay to detect the cell invasion activities in the Nek2 KD (shNek2), control (shGFP) HuH7 and SK-Hep1 cells. Left, representative images of the cells that migrated through the Matrigel; right, quantitation of the invaded cells summarized from the data from three independent experiments. F. Western blotting to detect the levels of the cell invasion markers E-cadherin and MMP9, which are regulated by NEK2. Nek2 KD (shNek2) caused a significant decrease in MMP9 and an increase in E-cadherin. The left and right panels display representative images and quantitation of the data from three independent experiments (mean ± S.D.), respectively. **: p < 0.01. ***: p < 0.001.
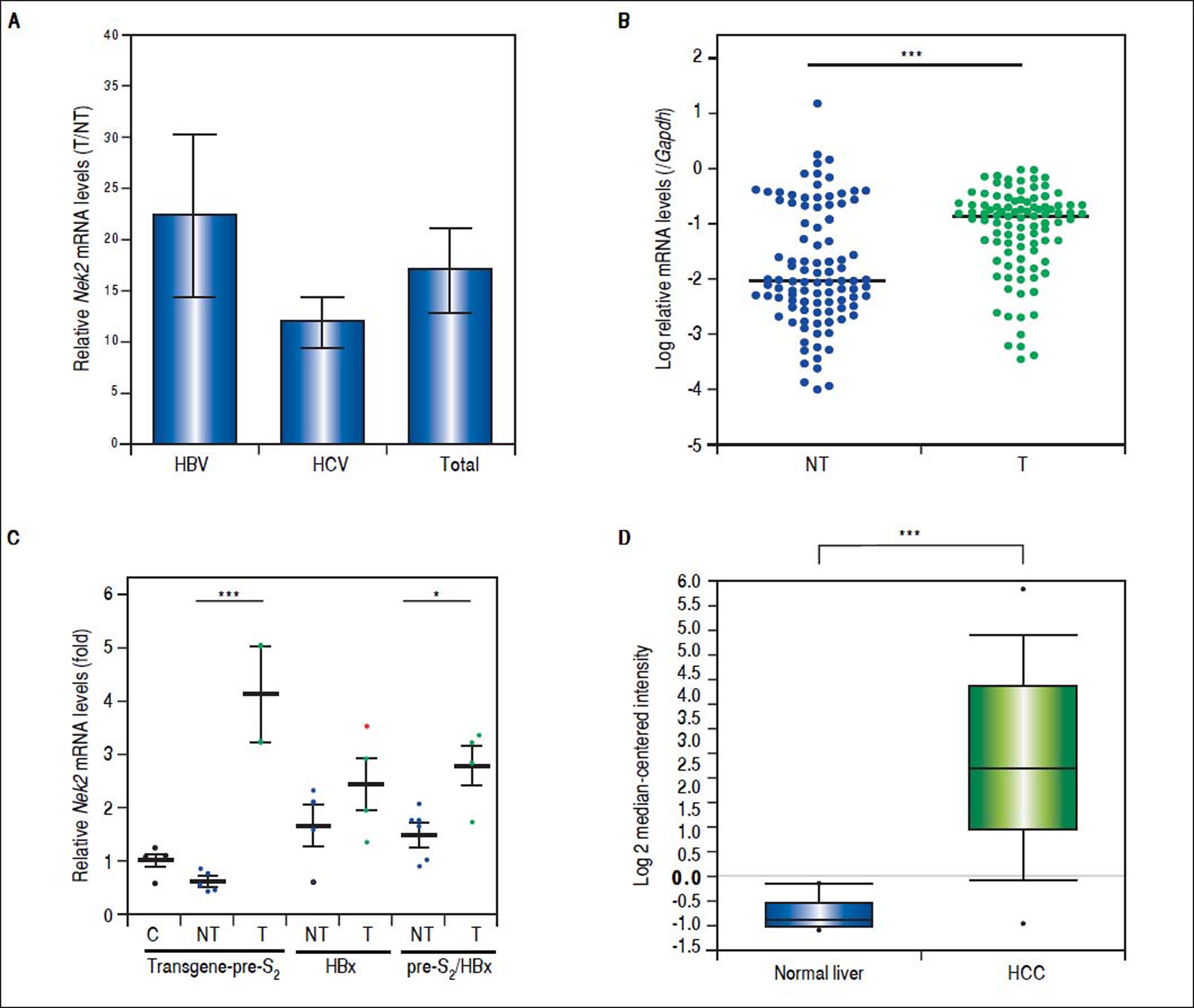
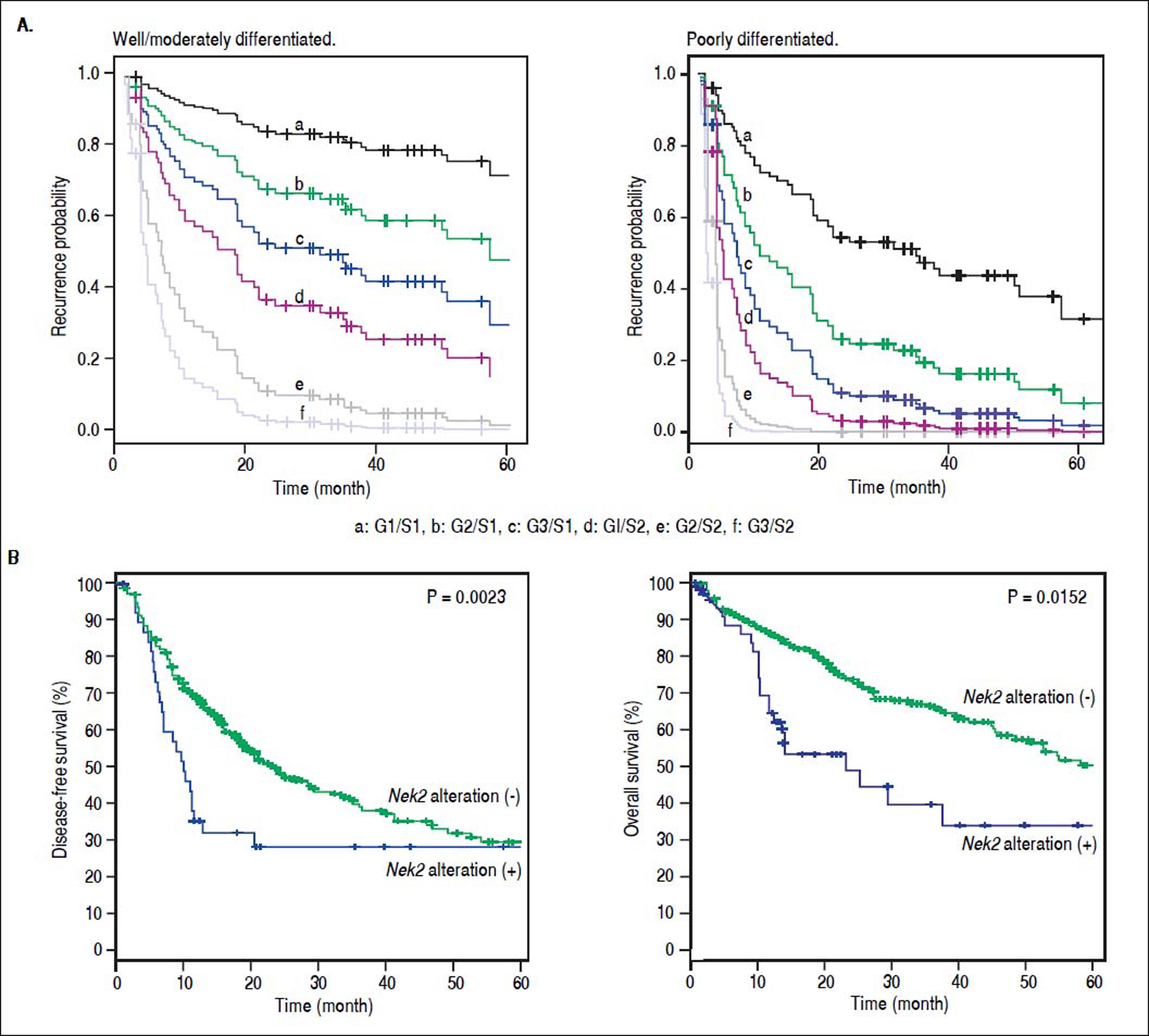
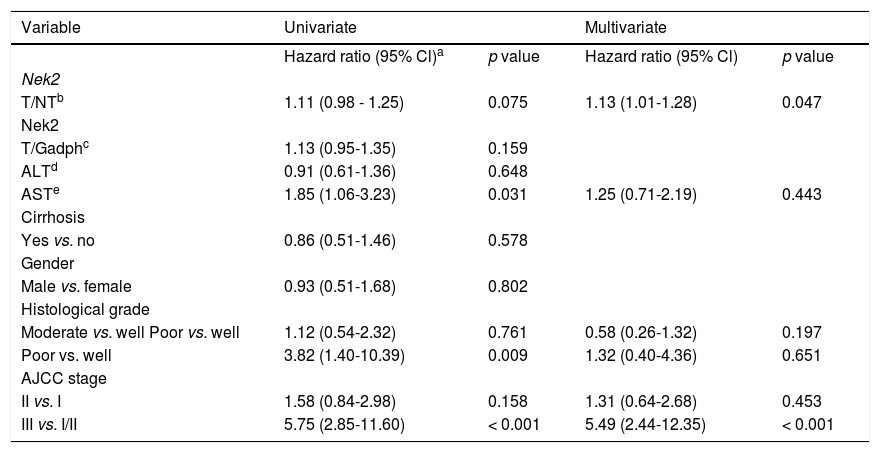
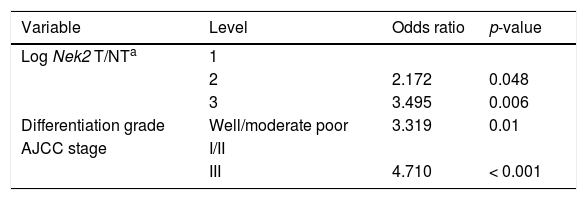
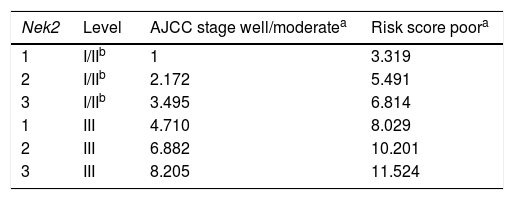
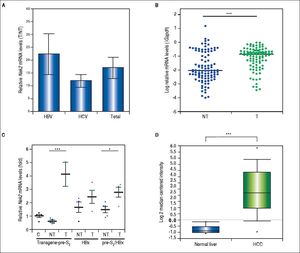
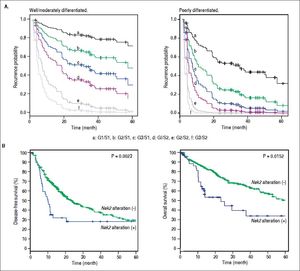
Given the findings that NEK2 contributes to the migration and invasion of HCC cells, an HCC cohort of patients who had undergone curative resection surgeries in NCKUH was recruited for the relevant clinical observations. Based on the cDNA microarray analysis (N = 12), both HBV- and HCV-related HCCs presented with increases in the Nek2 mRNA levels in the tumors compared with the levels in the peri-tumorous liver tissues (Figure 3A). Ninety-seven HBV-related HCC cases were further analyzed by real-time RT-PCR, which revealed that Nek2 expression in the tumorous regions was much higher than that in the peri-tumorous regions (Figure 3B). Additionally, the transgenic mice carrying the HBV oncogenes HBx and pre-S2 mutant LHBS and those carrying both genes all exhibited significant increases in Nek2 expression in the HCC tumors (Figure 3C). Similarly, in the Oncomine HCC database, Nek2 induction in primary liver cancer (N = 75) is clearly demonstrated (with a median increase value of 8-fold) compared with normal livers. The correlation of the Nek2 expression level with HCC recurrence in our cohort was further examined. Based on a multivariate regression statistical analysis, among the various clinicopathological factors analyzed, only the Nek2 induction level in the tumor (T) compared with the adjacent non-tumorous (NT) region and the AJCC stage (stage III vs. I/II) were found to be correlated with the HCC recurrence rate (Table 2). Moreover, among the patients with the same tumor AJCC stage and differentiation grade (i.e., poor or well/moderate), those with higher Nek2 levels exhibited higher odds ratios for recurrence than did the other patients (Table 3). The relative HCC recurrence risks in the post-hepatic resection patients were calculated based on a Cox proportional hazards analysis using the Nek2 level (level 1 to 3), the tumor AJCC stage (I/II vs. III) and the differentiation grade (well/moderate vs. poor) as covariables. This Cox analysis revealed that, for the patients with tumors at the same AJCC stage and differentiation grade, those with higher Nek2 levels had higher risk scores than did those with lower Nek2 levels. The tumor AJCC stage and differentiation grade also exhibited strong correlations with the HCC recurrence risk (Table 4). Cox survival curves were developed to examine the time-related HCC recurrence probabilities of the post-hepatic resection patients (Figure 4A). These curves can potentially serve as a convenient method for predicting the recurrence risk of HCC patients. In parallel, a similar finding was reported in the HCC database (N = 371) of the cBioPortal for Cancer Genomics, which reported positive correlations of alterations in Nek2 expression with disease-free and overall survival rates after hepatectomy surgeries (Figure 4B). Taken together, the findings of others and ours in various HCC cohorts indicate that the Nek2 level in HCC is a promising biomarker for a high risk of recurrence in patients following curative resection surgeries.
Overexpression of Nek2 in tumors in HCC patients and HBV HBx/pre-S2 mutant LHBS transgenic mice. Nek2 expressions were detected in the HCC patients (N = 12) by cDNA microarray and in the HBV-related HCC patients (N = 97) and HBx/pre-S2 mutant LHBS transgenic mice by real-time RT-PCR. A. Nek2 overexpression in human HCC as detected with the cDNA microarray analysis. Both the HBV- (N = 6) and HCV-related (N = 6) HCCs exhibited significant increases in Nek2 mRNA in the tumors. The data are indicated by the mean ± the S. E. M. B. Real-time RT-PCR to detect the Nek2 mRNA levels in the tumorous (T) and adjacent non-tumorous (NT) regions in the HCC cases (N = 97). The data are indicated as the relative mRNA levels of Nek2 to the Gapdh internal control gene in the same patient. The bar represents the median value for the set of samples. (C) Real-time RT-PCR to detect Nek2 expressions in the HCCs in the HBx (N = 4), pre-S2 mutant LHBS (N = 5), HBx/pre-S2 mutant LHBS double (N = 5) transgenic, and control C57BL/6 (N = 5) mice. D. Overexpression of Nek2 in the human primary HCCs and the corresponding normal liver tissues (N =75) as presented in the Oncomine™ Cancer Microarray Database. *: p < 0.05. ***: p < 0.001.
Multivariate regression analysis of the correlations of the various clinicopathologic factors with HCC recurrence.
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| Hazard ratio (95% Cl)a | p value | Hazard ratio (95% Cl) | p value | |
| Nek2 | ||||
| T/NTb | 1.11 (0.98 - 1.25) | 0.075 | 1.13 (1.01-1.28) | 0.047 |
| Nek2 | ||||
| T/Gadphc | 1.13 (0.95-1.35) | 0.159 | ||
| ALTd | 0.91 (0.61-1.36) | 0.648 | ||
| ASTe | 1.85 (1.06-3.23) | 0.031 | 1.25 (0.71-2.19) | 0.443 |
| Cirrhosis | ||||
| Yes vs. no | 0.86 (0.51-1.46) | 0.578 | ||
| Gender | ||||
| Male vs. female | 0.93 (0.51-1.68) | 0.802 | ||
| Histological grade | ||||
| Moderate vs. well Poor vs. well | 1.12 (0.54-2.32) | 0.761 | 0.58 (0.26-1.32) | 0.197 |
| Poor vs. well | 3.82 (1.40-10.39) | 0.009 | 1.32 (0.40-4.36) | 0.651 |
| AJCC stage | ||||
| II vs. I | 1.58 (0.84-2.98) | 0.158 | 1.31 (0.64-2.68) | 0.453 |
| III vs. I/II | 5.75 (2.85-11.60) | < 0.001 | 5.49 (2.44-12.35) | < 0.001 |
Odds ratios for HCC recurrence based on the AJCC stage, Nek2 level and differentiation grade (Fisher’s exact test).
| Variable | Level | Odds ratio | p-value |
|---|---|---|---|
| Log Nek2 T/NTa | 1 | ||
| 2 | 2.172 | 0.048 | |
| 3 | 3.495 | 0.006 | |
| Differentiation grade | Well/moderate poor | 3.319 | 0.01 |
| AJCC stage | I/II | ||
| III | 4.710 | < 0.001 |
Correlation of Nek2 expression with HCC recurrence. A. Cox survival curves for the HCC cases shown according to the risk scores of the AJCC stages (S) and Nek2 expression levels (G, also summarized in Table 4). In the well/moderately and poorly differentiated HCC group, both the AJCC stage and Nek2 expression are correlated with the recurrence probabilities after curative hepatic resections. B. Correlations of the alterations of Nek2 expression in the HCCs with the disease-free (left) and overal survivals as presented in the HCC database of cBioPortal for Cancer Genomics. The threshold for gene expression alteration (Z-score) in a tumor was a 1.8-fold increase or decrease compared with the level expressed in the peri-tumorous region. Both of HCC diseasefree (p value 0.0023) and overall (p value 0.0152) survival rates are correlated with alterations of Nek2 expression in the tumor.
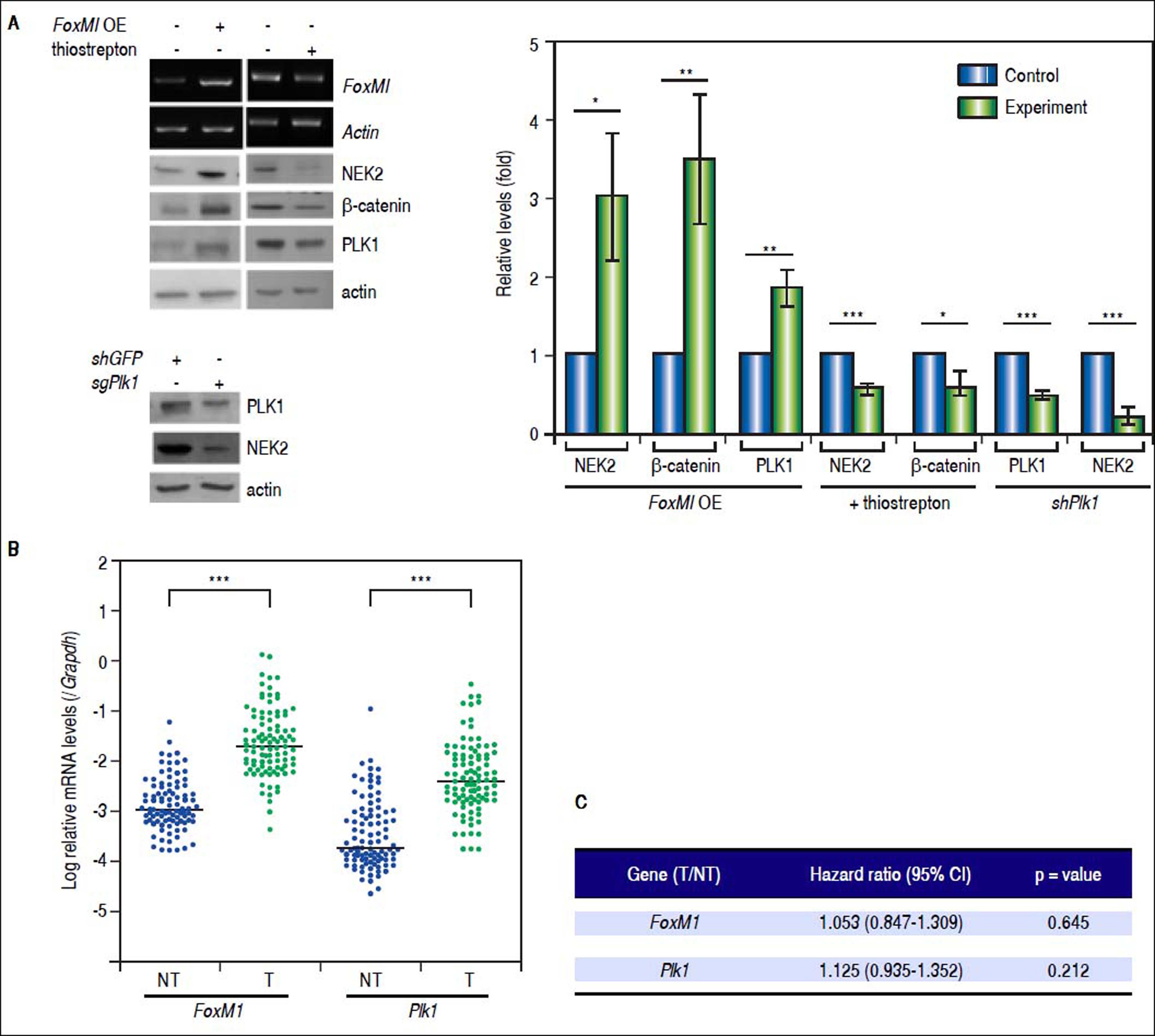
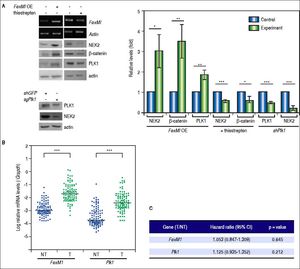
Recent studies have demonstrated that the cell cycle factors FOXM1 and PLK1 up-regulate the expression of Nek2, which enables Nek2 to execute its tasks in centrosome separation and maturation.27–30 In the FoxM1 -overexpressing cells, Nek2 as well as Plk1 and β-catenin were induced, and all of these increases were abolished by the FOXM1 inhibitor thiostrepton (5 mM).31 In the Plk1 KD cells, Nek2 was also greatly down-regulated (Figure 5A). These results supported the notion that FOXM1 and PLK1 up-regulated Nek2 expression and β-catenin-mediated invasion. Furthermore, the real-time RT-PCR analyses revealed that the expression levels of both the FoxM1 and Plk1 genes were greatly enhanced in the HCC tumors as was Nek2 (Figure 5B). However, in contrast to Nek2, neither FoxM1 nor Plk1 exhibited a positive correlation with HCC recurrence (Figure 5C). These findings indicate that, although the NEK2 activators FOXM1 and PLK1 play some roles in activating NEK2 in HCC, NEK2 behaves as a unique oncogenic factor that promotes HCC recurrence and can potentially serve as a biomarker for a high risk of recurrence.
The levels of the NEK2 activators FOXM1 and PLK1 are increased in HCC but are not correlated with recurrence. A. FoxM1 was over-expressed by plasmid transfection in HuH7 cells. Western blotting and RT-PCR assays revealed that FoxM1 overexpression (FoxM1 OE) increased the levels of NEK2, PLK1, and ß-catenin, whereas the FOXM1 inhibitor thiostrepton (5 mM) decreased these levels. Knockdown of Plk1 with shRNA aso caused a decrease in NEK2. Left, representative images of the experimental results. Right, quantitation of the data summarized from three independent experiments. Control, mock-treated cells; experiment, the cells transfected with exogenous gene or KD constructs or treated with thiostrepton. B. Expressions of FoxM1 and Plk1 in the tumorous (T) and adjacent non-tumorous (NT) regions of the HCCs (N = 97) as detected by real-time RT-PCR. The levels of the FoxM1 and Plk1 mRNAs were normalized to that of the housekeeping gene Gapdh. *:p < 0.05. **: p < 0.01. ***:p < 0.001. C. Neither the FoxM1 nor the Plk1 mRNA level in the tumor (T) compared with the level in the non-tumorous (NT) region was correlated with HCC recurrence after curative hepatic resections as indicated by Fisher’s exact statistical tests.
Currently, deep analysis and interpretation of biomarkers in cancer tissue is the most important approach for the execution of precision cancer medicine.32 Exploration of targetable biomarkers in cancer patients is the most highlighted field in the new generation of cancer therapy approaches.33 In this study, using whole-genome cDNA microarray analysis of surgically resected HCC tissues, we found that the cell cycle factor gene Nek2 was greatly overexpressed in tumors, and it was also highly correlated with the recurrence rate. In addition to its function in M phase progression, NEK2 was found to play an important role in G1 to S phase progression that is mediated by the regulation of the activities of some G1/S cell cycle checkpoints, which indicates that the function of NEK2 in interphase likely promotes cell proliferation and cancer metastasis. Interestingly, the upstream activators of NEK2, i.e., FOXM1 and PLK1, were not correlated with recurrence despite being overexpressed in most of the analyzed tumorous HCC tissues, which suggests that NEK2 is a unique factor in regulation of cancer progression.27–30 Several other studies in different populations have made similar findings that Nek2 expression level in HCC was correlated with high proliferation, invasion, and recurrence rates.34–36 Therefore, the current study in our HCC cohort in Southern Taiwan has revealed consistent findings with those in other regions, and together, clearly demonstrated that NEK2 is indeed an important high-risk factor for HCC progression.
The effect of NEK2 on cancer progression has been well documented mainly through its involvement in centrosome duplication and separation, which promotes mitosis progression.10–14 Thus, the expression of NEK2 has been reported to correlate with increased β-catenin relocalization and shortened cancer-related survival time.16 NEK2 has also been found to co-localize with nuclear splicing speckles through its interaction with and phosphorylation of the oncogenic splicing factors serine/arginine-rich splicing factor (SRSF) 1 and 2, which facilitate the splicing of the pyruvate kinase transcript and promotes aerobic glycolysis in multiple myeloma.37,38 Here, we also found that NEK2 enhanced AKT phosphorylation and p27Kip1 degradation, which led to the G1-to-S transition and thus demonstrated a new role of NEK2 in regulating cancer progression in interphase.
The expression of and associated centrosomal functions of NEK2 have been demonstrated to be regulated through oncoprotein FOXM1-mediated transactivation activity, which has been reported to enhance G1/S and G2/M cell cycle progression, tumor initiation and metastasis.29,30 In the current study, the expression level of FoxM1 was found to be greatly increased in the tumorous HCCs compared with the peri-tumorous regions. In hepatoma cells, the overexpression of FoxM1 also increased the level of NEK2. However, unlike Nek2, FoxM1 did not exhibit a significant correlation with HCC recurrence, suggesting that NEK2 mediates HCC progression through FOXM1-dependent and FOXM1-independent pathways and can serve as a unique promising biomarker for a high risk of HCC recurrence. Similarly, PLK1, which has been found to regulate NEK2 phosphorylation and thereby promote the stabilization of β-catenin and centrosome disjunction, has been found to increase the level of NEK2 in hepatoma cells.27,28 Moreover, similar to FoxM1, the mRNA levels of Plk1 were greatly increased in the HCC tumors; however, unlike Nek2, the Plk1 levels did not exhibit a correlation with HCC recurrence. Taken together, these results support the strong and unique positive association of NEK2 with HCC recurrence that is likely mediated by its integrated effects on the activation of β-catenin transactivation and the promotion of cell cycle progression in the M- and inter-phases.
In the current study, based on the Cox proportional hazard statistical analysis of the HCCs that were stratified by tumor differentiation stage, a predictive model indicating the recurrence probabilities in relation to time after surgery was developed using the AJCC tumor stage and the Nek2 induction level as covariables. This model could potentially serve as a convenient method for identifying the HCC patients who are at a relatively high risk for recurrence after surgery and those who ought to undertake intensive adjuvant therapies. In conclusion, the results of this study identified NEK2 as an important factor for the high risk of HCC recurrence after curative hepatic resection, and together with tumor AJCC stage and differentiation status, NEK2 could serve as a promising biomarker for precision medicine.
Abbreviations- •
BCLC: Barcelona Clinic Liver Cancer.
- •
CDC: cell division cycle.
- •
CEP: centrosome-associated protein.
- •
FoxMl: forkhead box protein M1.
- •
HCC: hepatocellular carcinoma.
- •
MAD2L1: mitotic arrest deficient 2 like 1.
- •
NASH: non-alcoholic fatty liver disease.
- •
NEK2: NIMA-related kinase 2.
- •
NINL: ninein-like.
- •
PLK1: polo-like kinase 1.
- •
RT-PCR: reverse transcription-polymerase chain reaction.
- •
shRNAs: short hairpin RNAs.
- •
SRSF: serine/arginine-rich splicing factor.
- •
TGCT: testicular gem cell tumors.
This study was supported by the Taiwan Ministry of Science and Technology (grant nos. 106-2622-B-006-003-CC2 and 106-2320-B-006-048-MY3 to WH), the Taiwan Ministry of Health and Welfare (grant no. MOHW106-TDU-B-211-113003 to CJY and WH), and the National Cheng Kung University Center of Infectious Disease and Signaling Research (grant no. D105-22004 to WH).
Author ContributionsYYC, YWC, and CYB analyzed the growth and cell cycle progression of hepatoma cells and drafted the manuscript; CJY recruited the main HCC study participants, did the clinical follow-up, and drafted the manuscript; SHC and YPL did the statistical analyses; CJH performed the analyses of the clinicopathological factors in HCC patients; and WH designed the study. All authors read and approved the final manuscript.