Both phytic acid (PA) and catechin (CA) are well known antioxidants of natural origin. They were frequently tried on experimental level as hepatoprotectants, relying only on their antioxidant properties. The present study was conducted mainly to outline the other biochemical pathways underlying the hepatotherapeutic potential of both drugs and to check a possible synergistic action if prescribed concomitantly. As both materials are frequently taken on a daily basis in food and drinks, it will be helpful to pursue their possible utility and/or to check if their value is really of medical importance. For this purpose, CCl4 was used as a hepatotoxin, we evaluated plasma total sialic acid (TSA), serum ascorbic acid (AA) levels, liver tissue thiobarbituric acid reactive species (TBARS) as a marker for lipid peroxidation and total protein (TP) content as a rough marker to measure hepatic synthetic capability in 80 male Wistar rats as experimental models. Animals were classified into 8 groups (10 rats each), the first as control, the second as PA treated (0.3 mg kg -1), orally, the third as CA treated (30 mg kg -1), intraperitoneally, the fourth given both drugs, as a single daily dose for 2 weeks. The same design was repeated 24 hours after CCl4-intoxication (1mL kg -1), intraperitoneally, as a single dose.
The results revealed that both PA and CA when used individually, significantly down-regulated TSA in both physiologic (no CCl4 treatment) and pathologic (CCl4-intoxication) states accompanied by significant decrease in lipoperoxidation. The therapeutic action against TSA and the antioxidant power were abolished by co-administration of both drugs. AA was only decreased by PA and the combination in the physiologic state. Both PA and CA showed significant therapeutic effect for protein synthesis against CCl4-intoxication, but the combination abolished this effect. We conclude that both drugs can be considered as a chemotherapeutic against hepatopathies and we for the first time contraindicate the concomitant use of both drugs.
Carbon tetrachloride (CCl4)- induced hepatotoxicity involves 2 phases. The initial phase involves CCl4 metabolism by cytochrome P450 which leads to the formation of free radicals and lipid peroxides.1-2 The second step is the activation of Kuppfer cells, mostly by free radicals, leading to production of pro-inflammatory mediators.3,1 Reactive oxygen species (ROS) have been implicated as a causative factor in the etiology of several degenerative diseases including cancer.4
Lipid peroxides were reported to result in a wide range of carbonyl products, some of which are extremely reactive towards biomolecules.5 In addition, CCl4 treatment leads to a significant decrease in liver content of TP, parallel to an increase in total lipids, total cholesterol and triglycerides which might be responsible for increased liver tissue weight in experimental models.6 Thus, hepatotoxicity in relation to CCl4 can be principally attributed to generation of free radicals leading to membrane lipid peroxidation, in conjunction with severe cellular protein and enzyme disturbances, which could be recovered by specific antioxidants.7
Sialic acid (N-acetyl neuraminic acid) is an acetyl substituent of the amino group of neuraminic acid. It plays a role in cellular functions as transporting positively charged compounds, cell to cell repulsion, conformation of glycoproteins on cell membranes and masking of the antigenic determinants on receptor molecules. It enters into the circulation bound to glycoproteins and glycolipids after shedding or cell lysis8. About 98% of serum sialic acid is bound to glycoproteins with only small amounts bound to lipids.9 Serum levels of sialic acid were documented to increase in both mice bearing mammary carcinomas and human carcinoma, where it was down-regulated in both cases after excision of tumors.10
Moreover, hepatocellular surface enzymes, isoenzymes and sialic acid were considered as useful tools in liver metastasis and higher levels of serum sialic acid were observed in comparison to normal subjects.11
Deficiency of circulating ascorbic acid (AA) was noticed in patients with liver diseases and biliary cirrhosis as well as, drug metabolism by the liver was delayed in AA-deficient patients.12 The necessity of AA as a hepato-protectant against drug-induced hepatotoxicity was potentiated by the hypothesis of healthy liver in subjects with normal AA levels.13
Both phytic acid (PA) and catechin (CA) were considered as antinutrients. This was observed by the depressive action of these substances on protein digestibility in vivo, however, lipid digestibility was reduced by PA only.14
PA is a hexaphosphorylated myo-inositol, known as inositol hexaphosphate (IP6). It is abundant constituent in plants, comprising 1-3% (W/W) of whole grains, cereals, legumes, nuts and oil seeds.15 In addition to being an antinutrient, depressing protein digestibility, in vitro,16 it also forms insoluble complexes with polycations decreasing its intestinal absorption,17,18 mostly due to the reactive phosphate groups attached to the inositol ring.
Dietary PA reduced hepatic lipids and depressed lipogenic enzyme activity.19 It also decreased starch digestibility in an in vitro study, inversely, CA had no effect on liver lipids.20 Catechins are polyphenolic antioxidant flavonoids found largely in human drinks and food, specially green tea (camellia sinensis). It constitutes about 25% of the dry weight of the fresh tea leaves.21 Catechins were reported to reduce atherosclerotic plaques22 and carcinogenesis.23 Both PA and CA exhibited chemopreventive activity due to their antioxidant potential.24
The present work was conducted mainly to explore the different pathways through which both PA and CA, individually or concomitantly can protect against CCl4 - induced hepatotoxicity, studying plasma TSA as early predictor for hepatocellular carcinoma, serum AA as a potent antioxidant, hepatic tissue lipid peroxidation and TP as representative for hepatic synthetic power.
Materials and methodsAnimals and experimental design: Eighty male Wistar albino rats (Rattus norvegicus), weighing 150 – 200 g were purchased from National Research Centre, El-Dokky, Giza, Egypt. The animals were housed in polyethylene cages at a temperature of 18 – 25 °C, moderately humid room in 12 hours light/12 hours dark adjusted condition. The animals were fed standard rat chow, allowed free access to normal tap water and left for 2 weeks to accommodate. Rats were classified into 2 major groups, CCl4 – non-treated groups (physiologic) and CCl4 – treated groups (pathologic), each major one was subdivided into 4 sub-groups. In CCl4 – non-treated group, the first sub-group served as control, given no medication, the second were given sodium phytate (Sigma), in a dose of 0.3 mg kg -1 as a single oral daily dose.25 The third sub-group was given a single daily dose of CA (as epicatechin, Sigma), (30 mg kg -1), intraperitoneally,26 the fourth was given both drugs, all for a period of 2 weeks.
In CCl4 – treated groups, the same sub-classification was followed after intraperitoneal injection of a single CCl4 dose of 1 mL kg -1 for all the animals.27 The first subgroup served as intoxicated control, the other three subgroups were given both PA and CA exactly as groups 2, 3 and 4, two days after CCl4 – injection. Animals were then killed, fasting blood samples were withdrawn, liver tissues were blotted between filter papers, all samples were kept frozen (at - 80°C) right the time of investigations.
Methods: Plasma TSA was determined in heparinized blood samples by the thiobarbituric acid method of Warren28. In this method, plasma was incubated for 1 hour at 80°C in 0.1 M sulfuric acid in order to liberate the bound sialic acid. A calibration curve was obtained from different concentrations of sialic acid (Sigma). Serum AA level was determined according to the method of Roe and Kuether,29 based on the oxidation of AA into dehy-droascorbic acid which is transformed to 2,3-diketogluconic acid. This stable end product is coupled with 2,4 dinitrophenyl hydrazine, then treated by sulfuric acid to produce a color that could be read colorimetrically.
Liver content of malondialdehyde (a TBARS) was also colorimetrically determined.30 Liver content of TP was extracted with cold 20% trichloroacetic acid solution, containing 0.2% triton-X (detergent), the sediment was dried off water, shaken with diethylether/ethanol (3:1 V/V) for washing off lipid impurities, the organic layer was discarded, while the sediment (protein) was dissolved in one molar NaOH,31 in which TP was colorimetrically determined.32
Statistical analysisThe data were analyzed by one-way ANOVA, depending on Duncan’s new multiple range test.33 The level of significance was considered significant when p was < 0.05. All values were expressed as mean ± SEM.
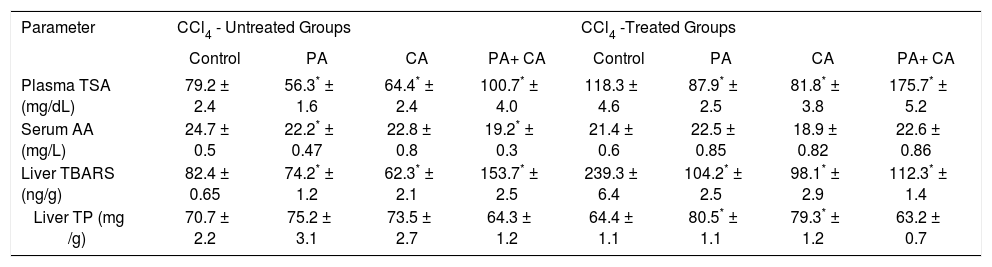
ResultsIn the physiologic (CCl4 – non treated) groups, both PA and CA treatments significantly decreased TSA, while a combination of both, significantly increased TSA level (p < 0.01). CCl4 intoxication significantly elevated TSA, which was significantly decreased by both PA and CA treatments, while a combination of both, significantly increased TSA level (p < 0.01). Serum AA in normal groups was significantly decreased by PA or the combination (p < 0.01), but not affected by CA. In the pathologic groups, neither CA nor PA nor the combination could restore AA level to normal physiologic level. TBARS was significantly down-regulated by CA and PA, but the combination significantly up-regulated this variable. CCl4 significantly up-regulated TBARS which was significantly down-regulated by PA, CA and their combination. TP was non-significantly elevated by individual PA or CA, but non-significantly decreased by the combination in the physiologic groups. CCl4 depressed TP synthesis which was significantly restored better than normal by PA and CA, but was not treated by the combination (Table I):
Effect of PA (0.3 mg kg orally) and CA (30 mg kg intraperitoneally), given individually or in combination on plasma TSA, serum AA, liver tissue contents of TBARS and TP of CCl4 - treated rats after single dose daily administration for two weeks.
| Parameter | CCl4 - Untreated Groups | CCl4 -Treated Groups | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | PA | CA | PA+ CA | Control | PA | CA | PA+ CA | |
| Plasma TSA (mg/dL) | 79.2 ± 2.4 | 56.3* ± 1.6 | 64.4* ± 2.4 | 100.7* ± 4.0 | 118.3 ± 4.6 | 87.9* ± 2.5 | 81.8* ± 3.8 | 175.7* ± 5.2 |
| Serum AA (mg/L) | 24.7 ± 0.5 | 22.2* ± 0.47 | 22.8 ± 0.8 | 19.2* ± 0.3 | 21.4 ± 0.6 | 22.5 ± 0.85 | 18.9 ± 0.82 | 22.6 ± 0.86 |
| Liver TBARS (ng/g) | 82.4 ± 0.65 | 74.2* ± 1.2 | 62.3* ± 2.1 | 153.7* ± 2.5 | 239.3 ± 6.4 | 104.2* ± 2.5 | 98.1* ± 2.9 | 112.3* ± 1.4 |
| Liver TP (mg /g) | 70.7 ± 2.2 | 75.2 ± 3.1 | 73.5 ± 2.7 | 64.3 ± 1.2 | 64.4 ± 1.1 | 80.5* ± 1.1 | 79.3* ± 1.2 | 63.2 ± 0.7 |
The antinutritional action of PA was observed in rats fed with different percentages in high sucrose diets. It drastically depressed growth, food intake, serum triglycerides and cholesterol levels.19 In addition to PA, CA and tannic acid were considered also as antinutritional factors. This character was mainly explored through studying the effect of these substances on digestibility of many nutrients including starch, protein, lipids and fiber degradation.14 Thus, the antinutritional values of both PA and CA were speculated to have conflicting activities on animal models of liver diseases. Individually, PA and CA significantly depressed plasma level of TSA, while a combination of both drugs significantly up-regulated plasma TSA level.
The same action was observed in CCl4 - treated rats. Our finding coincides with the work of Cowieson et al,34 who noticed that high dose of PA in diets increased the urinary excretion of TSA in broiler chickens. This action could - in part - be interpreted as a chemopreventive potential of both drugs if used solely.10
It could be recently confirmed by the published data of Vucenik and Shamsuddin,35 who observed that PA showed anticancer activity on different cancer cells as colon, breast, cervix, prostate and liver. In addition, decreased blood TSA was linked directly to herbal hepato-protection against drug-induced hepatotoxicity.36
On the other hand, the down-regulatory action of CA on TSA can also be added to its cytoprotective potential. CA exhibited a cancer chemopreventive action by maintaining DNA integrity and antigenotoxic behavior against bleomycin-induced DNA damage in human leukocytes.37 Earlier studies on hepatoprotective and cytoprotective activities of CA were referred to its potent antioxidant action.38 TSA was considered to be more sensitive than free sialic acid as a marker for alcohol abuse which is known to be hepatotoxic.39
Our finding proves, probably for the first time, that a concomitant administration of both PA and CA may abolish both individual actions on serum TSA level. This is clear from the significant up-regulation of TSA after CA plus CA administration.
Serum AA is significantly depressed by PA individually or combined with CA, but not affected by CA alone in CCl4 – untreated rats. No significant changes were noticed by both drugs in CCl4 – treated rats. AA administration protected against drug-induced hepatotoxicity in rats.13 While CCl4 – treatment significantly down-regulated serum AA level, none of the drugs could significantly combat this action, nor restore the level to CCl4 – non-treated control. This effect mostly augments the suggestion of considering both PA and CA as antinutrients. PA prohibited the absorbance of some essential antioxidant elements, leading to hepatitis.40
In the physiological state, the decreased AA level (as an antioxidant), with the increased hepatic lipoperoxidation by PA and CA may be interpreted as a pro-oxidant potential of both drugs in the physiological state, which could be supported by the observation of Srinivasan et al24 in an in vitro investigation.
On the contrary of that, both drugs showed a potent antioxidant activity in the pathological state (CCl4 intoxication), exhibited by the significant hepatic down-regulation of TBARS content. In addition, CA protected the liver against increased lipoperoxidation induced by the anticancer, antibiotic doxorubicin treatment.41 In other instance, CA protected liver microsomes against CCl4-induced lipoperoxidation in vitro.42 Our hypothesis of the utility of CA as a protector against CC14-induced hepatic damage is not coinciding with the study reporting a failure of CA to restore hepatic enzymes to normal, nor, hepatic hydroxproline - a marker for liver fibrosis-to normal levels after CCl4-alcohol treatment.43
This conflicting protective action of CA was also observed in another study, shown by the little difference in lipoperoxidation inhibition in CA-treated rats compared to induced lipoperoxidation of isolated hepatocytes from treated rats after incubation with tertiary butyl hydroperoxide or copper as hepatotoxins.44
Moreover, PA showed antioxidant properties in vitro, while it had no significant effect on liver antioxidant status in in vivo studies on TBARS as a marker for lipoper-oxidation in rat liver.45
Thus hepatoprotective action of PA against lipid peroxidation mostly relies on different mechanisms, which means that the drug may protect against changes of some markers but other oxidative markers may not be affected as an example lipoperoxidation marker known as acrolein-modified protein.46
In our study, the effects of both PA and CA on liver protein seems to be insignificant on physiological level, although each drug when used individually showed protective action against CCl4 - induced protein depression, but again, this effect was abolished when both drugs were used together. So, each individual drug is considered as hepatoprotective against hepatopathies accompanied by depressed protein synthesis,47 although it was previously reported that inclusion of total protein is not a useful marker in liver function test profile.48
In conclusion, the most promising action of both PA and CA is the down-regulation of serum TSA level, which may be due to decreased tissue release or increased urinary excretion. Both drugs may be a useful antioxidant and chemotherapeutic against hepatopathies, whether in physiologic or pathologic states. Unfortunately, when both drugs were used concomitantly, this chemotherapeutic action was approximately abolished. Physiologically, both drugs if used solely or concomitantly, shows antioxidant potential, and in pathologic state, a promising antioxidant potential is more prominent. Both drugs didn’t show significant impact on AA nor the hepatic synthetic power of TP, although each drug improved the pathologically depressed TP. Here, we could recommend the use of each drug as a chemotherapeutic antioxidant medication with a condition to avoid co-administration although this action didn’t completely depend on inhibition of hepatic lipoperoxidation. We contraindicate for the first time the concomitant use of both drugs, trying to avoid tea drinking after green legumes and cereals containing PA in food as both PA and CA decrease the health benefit of each other and report that, different pathways should be studied to elucidate the possible pathways underlying drug effects on a cellular level and in vitro experiments will be more advisable for interpretation of the drug-drug interaction between PA and CA.