Introduction and aim. Olfactory functions are altered to a variable degree by chronic liver disease. Few studies including only small populations of patients emphasized the possibility of hepatic encephalopathy (HE) influencing olfactory nervous tasks. So far, no study has explicitly focused on olfactory function depending on the severity of HE as assessed by objective diagnostic procedures. Thus we performed a study using the “Sniffin’ Sticks” test system, critical flicker-fusion frequency (CFF) and clinical West Haven criteria.
Material and methods. 54 cirrhotic patients with liver cirrhosis were included. Furthermore, 43 adult volunteers participating as a non-cirrhotic control group. Olfactory testing was performed using the “Sniffin’ Stick” test battery (Burghart Medizintechnik, Wedel, Germany) which renders a widely-used tool both in clinical and research settings for the assessment of olfactory threshold, odor identification and discrimination. Several complications of cirrhosis were diagnosed by reference methods. Statistical analysis of cirrhosis-associated complications and their relation to olfactory function was performed. Assessment of HE and classification of different stages were performed according to clinical criteria (West- Haven criteria) and according to CFF, which was determined using a portable analyzer.
Results. Olfactory function was significantly reduced in cirrhotic patients (in 61.1%) compared to controls (p < 0.001). Among cirrhotics patients, the prevalence of olfactory deficits (hyposmia, anosmia) increased with the severity of HE as assessed by CFF and clinical criteria (p = 0.008 and p = 0.097, respectively). No correlation was observed between olfactory deficits and severity of liver disease as assessed by Child-Pugh-Score, etiology of cirrhosis and complications of cirrhosis such as ascites and portal venous hypertension.
Conclusions. Olfactory testing serves as a screening tool for HE and may faciliate grading of HE-severity.
Hepatic encephalopathy (HE) comprises a complex of psycho-motoric symptoms and represents an important complication in cirrhotic patients. While relatively easy and successful therapeutic approaches exist, the valid diagnosis, especially in early stages, represents a major clinical problem. Therefore, there is a need for new objective diagnostic markers to detect HE in early stages.1
Olfactory deficits are frequently described in chronic liver diseases such as cirrhosis, in early studies, however, results are not conclusive. Interestingly, Bloomfeld, et al.2 demonstrated improvement of chemosensory function after orthotopic liver transplantation (OLT) by determining taste and smell detection and recognition thresholds before and after OLT in a small cohort of patients with cirrhosis. Since patients with cirrhosis often suffer from mixed deficiency of proteins, calories and micronutrients due to a progressive loss of fat and muscle mass, malnutrition might contribute to olfactory deficits in end-stage liver disease.3 Madden, et al. demonstrated an impaired gustatory function in cirrhotic patients with significantly higher thresholds for detection and recognition of several different tastes, together with a higher overall median gustatory score.4 They showed an association of impaired gustatory acuity with hypomagnesemia. Therefore, since malnutrition in cirrhosis is almost universal due to e.g. poor dietary intake or protein losses due to paracentesis and inappropriate long-term protein restrictions, sufficient enteral nutrition should be guaranteed if cirrhotic patients report about olfactory deficits. Burch, et al. analyzed the sensory modalities of taste and smell in few patients with cirrhosis by testing different concentrations of sucrose or sodium chloride to evaluate taste acuity showing a decreased acuity of taste and smell in in the cirrhotic subgroup.5 However, they did not find any association of lack of micronutrients with decreased acuity in perception of the individual test substances. Therefore, the reason for olfactory dysfunction in cirrhosis is far from being fully understood.
It is described in the literature that liver dysfunction affects the central nervous system.6 Two newer studies investigated olfactory dysfunction in liver diseases using the Sniffin’ Sticks. Temmel, et al. showed, that odor identification, but not odor threshold or odor discrimination is altered in cirrhotic patients.6 While Zucco, et al. demonstrated that odor threshold and odor identification is compromised in patients suffering from minimal HE, no study so far has focused on olfactory functions in relation to the severity of HE as assessed by objective diagnostic procedures.7
Apart from cirrhosis, different neurological diseases such as Parkinson, Alzheimer or Korsakoff are associated with olfactory alterations. In these disorders olfactory testing can be a useful screening tool at an early stage of the disease. HE and several neurological diseases share many similar clinical features, which supports the hypothesis that an impaired olfactory function might be related to the pathophysiology of HE. Surprisingly, there are only few studies investigating olfactory disturbances in cirrhotic patients and simultaneous HE.
The aim of the study was to examine if olfactory deficits are related to different grades of severity of HE. Therefore, we tested the olfactory function of cirrhotic patients and non-cirrhotics patients. The severity of the cirrhosis was analysed by measures of several cirrhosis-associated complications including different stages of HE (including sub-clinical stages), ascites and portal hypertension in relation to olfactory deficits.
Material and MethodsThe study was conducted at the Department of Gastroenterology, Klinikum Bogenhausen, Munich, Germany. The study protocol was in accordance with the declaration of Helsinki and approved by the local ethics board (http:// ethikkommission.blaek.de).
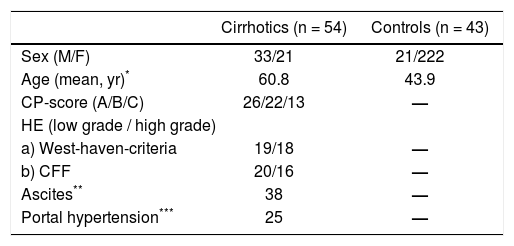
Patient Population54 patients with liver cirrhosis who were admitted to the Department of Gastroenterology, Hepatology and Gastrointestinal Oncology, Bogenhausen Hospital, were invited to enter the study between April 2011 and December 2012. Furthermore, 43 adult volunteers participated as a non-cirrhotic control group, who did not suffer from smell disorders in the present and past. The control group was recruited from the Department of Otorhinolaryngology, Head and Neck Surgery, Technical University of Munich, Munich, Germany and consisted of patients admitted to any kind of ENT surgery except surgery of the nose (mainly ear or neck surgery). The clinical characteristics of all patients (cirrhotic patients and controls) are presented in table 1.
Clinical characteristics of all patients (cirrhotics and controls).
Patients with preexisting smell or taste disorders, relevant nasal pathologies such as mucosal inflammation, significant septal deviation, nasal polyposis and excessive use of nose drops were excluded.
Cirrhosis was established either by histology or by clinical, laboratory, and sonographic or radiographic findings demonstrating impaired hepatic function and, morphological alterations of the liver typical of cirrhosis or signs of portal hypertension. For assessment of the severity of liver disease, Child-Pugh (CP) classification score was calculated for each individual patient (CP A, -7 points; CP B, 8-10 points; CP C, > 11 points) In the noncirrhotic control group, cirrhosis was excluded by the same criteria mentioned above.
Assessment of the severity of hepatic encephalopathyThe classification of HE followed the critical flickerfusion frequency (CFF) and clinical criteria (West-Haven criteria).8–9 CFF was measured with a portable analyzer, and HE was divided into low grade HE (stage 1 according to West-Haven criteria) or high grade HE (> stage 1 according to West-Haven criteria).
Determination of Critical Flicker-Fusion (CFF) frequency thresholdsThe CFF was measured in a quiet, semidarkened room without distracting noises. A portable, battery-powered analyzer was used (Hepatonorm TM Analyzer, Department of Gastroenterology, Hepatology, Infectiology, Heinrich Heine University Düsseldorf, Düsseldorf, Germany). The analyzer evokes an intrafoveal light stimulus with defined pulses of light at a wavelength of 650 nm, luminance of 270 cd/m2, and luminous intensity of 5.3 mcd. The frequency of the red light, which is initially generated as a high-frequency pulse (60 Hz) and which gives the patient the impression of a steady light, was reduced gradually until the patient had the impression that the steady light had changed to a flicker. The patient registered this change by pressing a hand-held switch. The process was repeated at least 5 times to ensure that the patient understood the procedure. Subsequently, the procedure was repeated 10 times, and from the resulting data, the mean values for each patient were calculated.
The appropriate cut off to identify abnormal CFF is still not consistently defined. In the pilot study by Kircheis, et al. the threshold of an abnormal CFF was 39 Hz when discriminating healthy subjects from patients with cirrhosis and HE.10 Grading of HE according to CFF was necessary to allow for an objective correlation of HE-severity with FCCs. Kircheis, et al. found an average CFF of 36.0 ± 1.4 Hz in subjects with HE I (according to West-Haven-criteria) while the average CFF in subjects with HE II (according to West-Haven-criteria) was 32.1 ± 2.7 Hz and HE III (according to West-Haven-criteria) was < 30 Hz.10,11 In our study, HE was either divided into low grade HE (average CFF ≤ 39 Hz ≥ 35 Hz) or high grade HE (average CFF ≤ 35 Hz) while an average CFF > 39 Hz excluded HE.10,11
Assessment of olfactory functionTo evaluate the ability to smell in patients with hepatic encephalopathy we employed the “Sniffin’ Sticks” test.12 The patients were blindfolded throughout the test. Odor threshold (T), odor discrimination (D) and odor identification (I) were tested. Odor thresholds for n-butanol were assessed using a single-staircase, triple-forced choice procedure. Three different pens were presented in a randomized order. Two of these pens contained nothing and the third one contained n-butanol at a certain dilution. The patient had to identify the smelling pen. The test started with the lowest concentration of n-butanol and concentration increased until the subject identified the pen containing n-butanol correctly two times. The correct identification triggered a reversal of the staircase, which means that now lower concentrations of n-butanol were presented until the subject did not identify the n-butanol pen. This again triggered a reversal of the staircase. The last four out of seven staircase reversal points were defined as threshold. The subjects’ scores ranged between 0 and 16.
The odor-discrimination test contained triplets of pens. These were presented to the patient in a randomized order. Two pens contained the same odorant and the third pen contained a different odorant. The patients had to identify the pen, smelling different compared to the two other pens. As a total of 16 triplets were tested. The subjects’ scores ranged from 0 to 16.
Odor identification was assessed by means of 16 different common odors. From a list of four descriptors an individual odorant had to be identified. The correct answers were added. The scores ranged from 0 to 16.
The sum of these 3 scores were calculated (TDI) to quantify olfactory function.12,13 The maximally achievable score was 48 points. This olfactory test is widely and internationally used and has been validated on large samples of healthy subjects and patients.12
Statistical analysisStatistical analysis was performed using SPSS 21.0 (SPSS Inc., Chicago, IL. USA). Absolute and relative frequencies of olfactory deficits were determined for relevant patient groups and healthy controls. Continuous data are described by amean and standard deviation or median and 1st and 3rd quartile. Group comparisons for categorical outcomes were performed using χ2 tests. Comparison of TDI was conducted by a Mann-Whitney U test as a skewed distribution of TDI values was observed. All statistical tests were performed two-sided on a significance level of 0.05.
ResultsCirrhotic groupIn total, 54 cirrhotic subjects entered this study [33 male, 21 female; mean age 60.8 (SD 10,73; range 33-80)]. The clinical characteristics of all patients (cirrhotic and controls) are presented in table 1.
The aetiology of cirrhosis was alcohol abuse in 74.1% (40/54), hepatitis C in 9.3% (5/54), hepatitis B in 3.7% (2/ 54), autoimmune hepatitis in 1.9% (1/54) and others in 11%(6/54). On the basis of CP-classification, 22.2% (12/54) of patients had stage A cirrhosis, 42.6% (23/54) had stage B and 35.2% (19/54) had stage C.
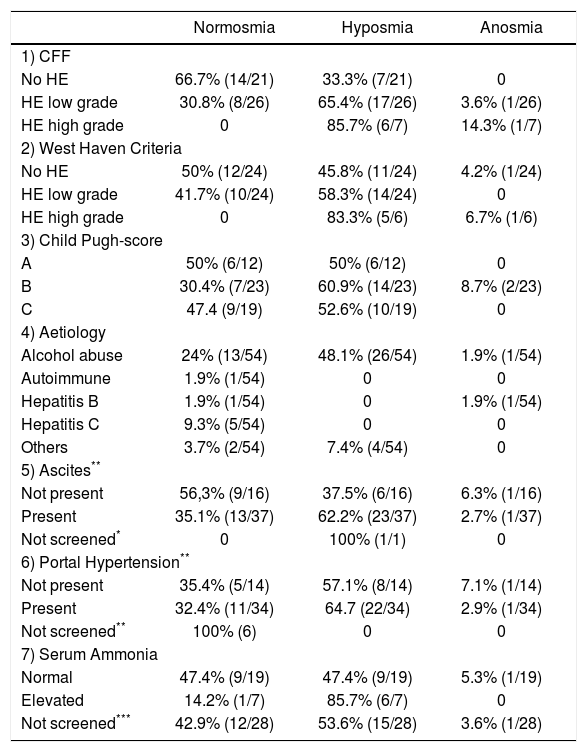
Olfactory function in controls and cirrhotic patientsOlfactory function was reduced in 61.1% of patients compared to controls. When comparing TDI values between both groups by means of a Mann-Whitney U-test, olfactory function was significantly higher in controls (median 35.5 [1st quartile: 34.3; 3rd quartile: 38.5]) compared with cirrhotic patients (29.0 [25.8; 33.5], p < 0.001). Association between cirrhosis-related complications and olfactory function was investigated. All results are summarized in table 2.
Results of olfactory testing using the Sniffin’ Sticks for the detection of complications of cirrhosis.
| Normosmia | Hyposmia | Anosmia | |
|---|---|---|---|
| 1) CFF | |||
| No HE | 66.7% (14/21) | 33.3% (7/21) | 0 |
| HE low grade | 30.8% (8/26) | 65.4% (17/26) | 3.6% (1/26) |
| HE high grade | 0 | 85.7% (6/7) | 14.3% (1/7) |
| 2) West Haven Criteria | |||
| No HE | 50% (12/24) | 45.8% (11/24) | 4.2% (1/24) |
| HE low grade | 41.7% (10/24) | 58.3% (14/24) | 0 |
| HE high grade | 0 | 83.3% (5/6) | 6.7% (1/6) |
| 3) Child Pugh-score | |||
| A | 50% (6/12) | 50% (6/12) | 0 |
| B | 30.4% (7/23) | 60.9% (14/23) | 8.7% (2/23) |
| C | 47.4 (9/19) | 52.6% (10/19) | 0 |
| 4) Aetiology | |||
| Alcohol abuse | 24% (13/54) | 48.1% (26/54) | 1.9% (1/54) |
| Autoimmune | 1.9% (1/54) | 0 | 0 |
| Hepatitis B | 1.9% (1/54) | 0 | 1.9% (1/54) |
| Hepatitis C | 9.3% (5/54) | 0 | 0 |
| Others | 3.7% (2/54) | 7.4% (4/54) | 0 |
| 5) Ascites** | |||
| Not present | 56,3% (9/16) | 37.5% (6/16) | 6.3% (1/16) |
| Present | 35.1% (13/37) | 62.2% (23/37) | 2.7% (1/37) |
| Not screened* | 0 | 100% (1/1) | 0 |
| 6) Portal Hypertension** | |||
| Not present | 35.4% (5/14) | 57.1% (8/14) | 7.1% (1/14) |
| Present | 32.4% (11/34) | 64.7 (22/34) | 2.9% (1/34) |
| Not screened** | 100% (6) | 0 | 0 |
| 7) Serum Ammonia | |||
| Normal | 47.4% (9/19) | 47.4% (9/19) | 5.3% (1/19) |
| Elevated | 14.2% (1/7) | 85.7% (6/7) | 0 |
| Not screened*** | 42.9% (12/28) | 53.6% (15/28) | 3.6% (1/28) |
Percentage of patients according to each subgroup suffering from cirrhosis (absolute number in brackets).
When assessing HE by using CFF, 46.3% of the cirrhotic patients (26/54) were classified as low grade and 7 (13%) as high grade HE while HE was ruled out in 38% (21/54) of all patients.
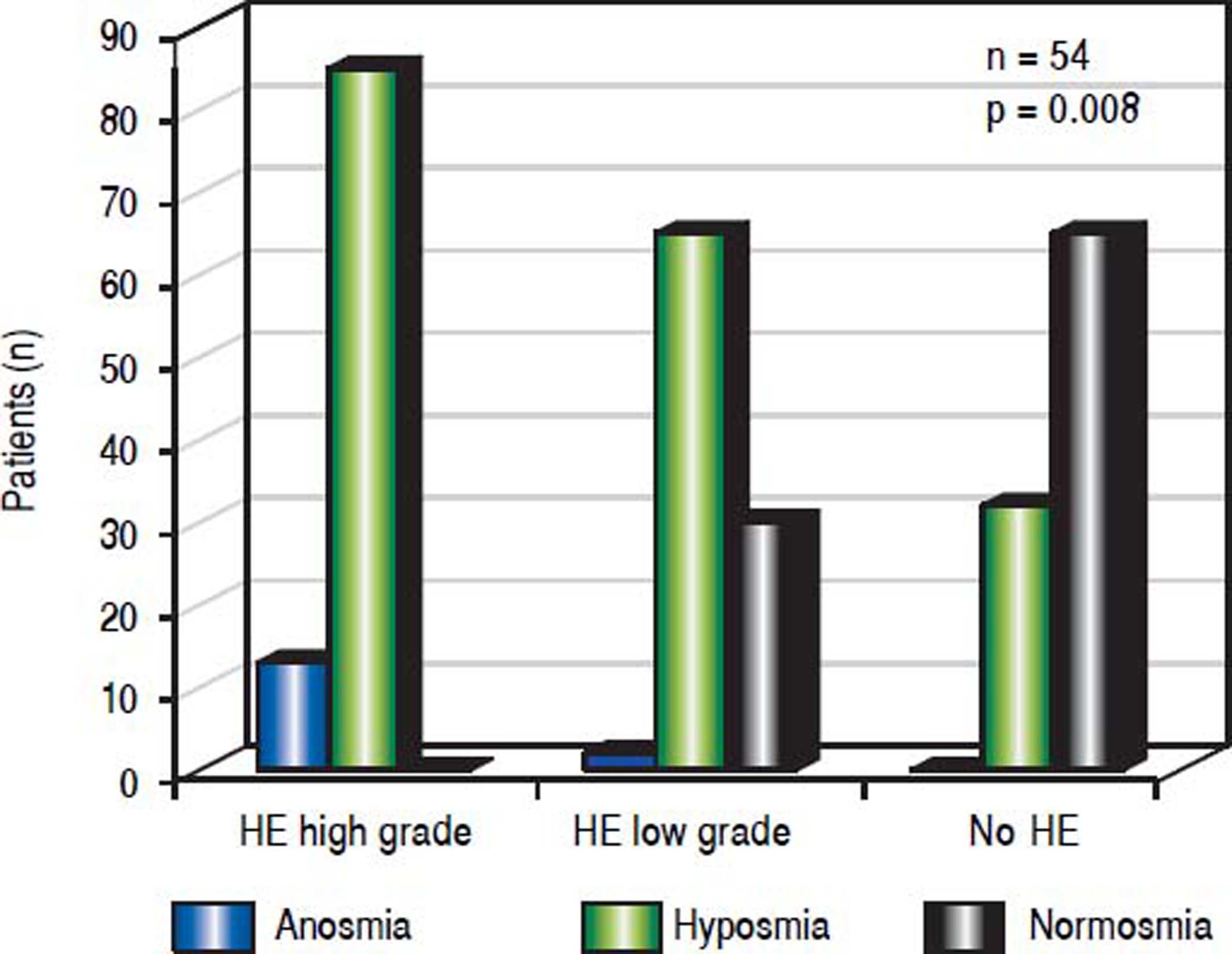
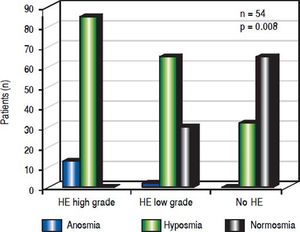
A significant association emerged between olfactory deficits and HE grading as measured by CFF (p < 0.01, Figures 1-2). While normosia was observed for 14 of the 21 patients without HE (66.7%), only 29.6% of the patients with low grade HE (8/27) and none of the 6 patients with high grade HE presented with normosia.
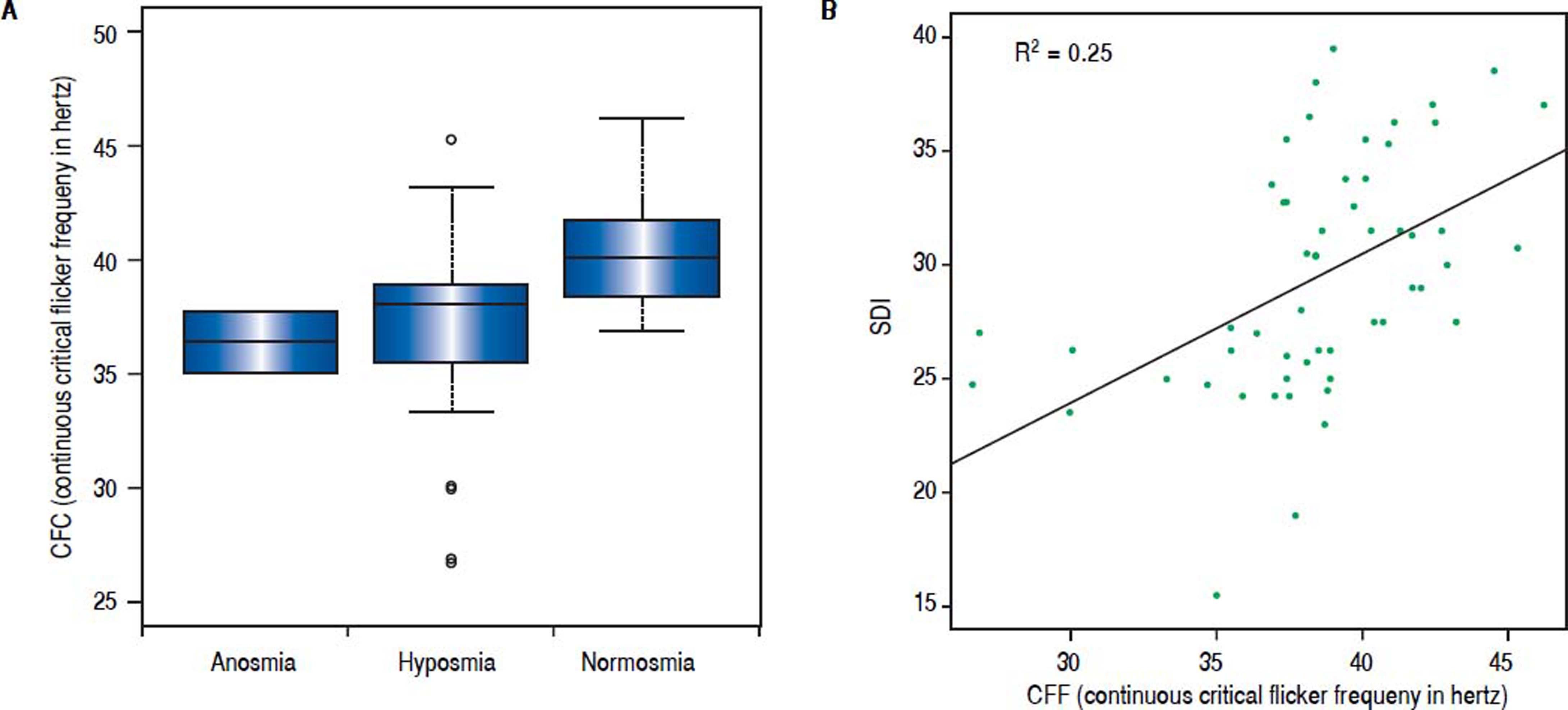
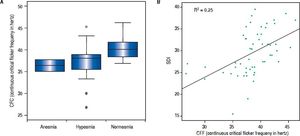
In addition, we performed an analysis using continuous critical ficker frequency (CFF) as a Boxplot-graph or using continuous CFF and standard deviation (SDI) demonstrating an association between olfactory deficits and the severity of hepatic encephalopathy, which was statistical significance (p = 0.008).
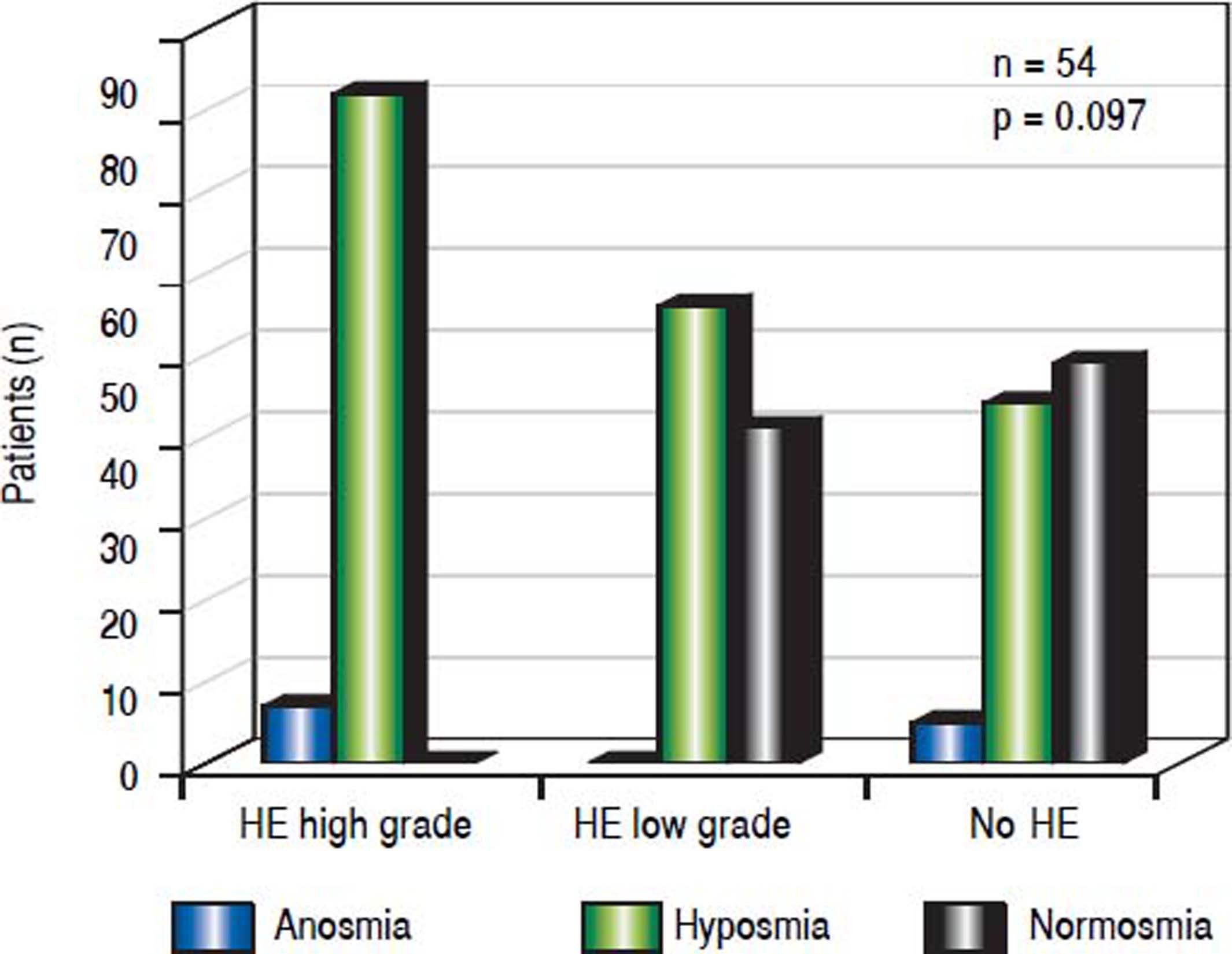
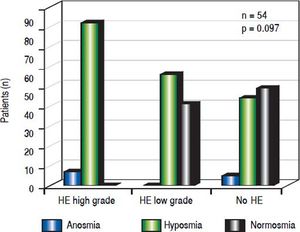
When assessing HE by using clinical criteria (West Haven-criteria, WHC), 43.6% (24/54) were classified as low grade-HE, 11.1% (6/54) as high grade-HE, while 43.6% (24/ 54) of all patients were classified as asymptomatic (Figure 2). In high-grade HE, all patients showed olfactory deficits (Figure 3).
According to those patients, in which WHC and CFF revealed the same grade of HE (70.3%; 38/54), in patients with high-grade HE anosmia was seen in 100% (1/1), and in those with low-grade HE hyposmia appeared in 65% (13/20) and normosia in 35% (7/20). In 28% of our patients (15/54) WHC and CFF did not show the same grade of HE. In those patients, in which CFF showed low-grade HE and WHC did not (46%; 7/15), anosmia occurred in 14% (1/7), hyposmia in 29% (2/7) and normosmia in 57% (4/7). Assessing high-grade HE diagnosed only by CFF (20%; 3/15), all three patients revealed anosmia (100%; 3/ 3). In those patients, in which WHC showed low-grade HE while CFF did not (13%; 2/15), 100% showed normosmia. Moreover, in those patients, in which only WHC revealed high-grade HE (20%; 3/15), hyposmia occured in 100%.
There was an association between olfactory deficits and the severity of HE assessed by West Haven Criteria. However, this was not statistically significant (p = 0.097, Figure 3).
Plasma ammonia levels (obtained by venous blood sampling) were obtained from 26 patients of our study population. 87.5% (7/8) of patients with elevated plasma levels showed deficits in olfactoric testing (all hyposmia), while 50.0% (9/18) of patients with normal plasma ammonia level presented with normosmia, 44.4% (8/18) with hyposmia and 1 patient (5.6%) with anosmia (p = 0.119).
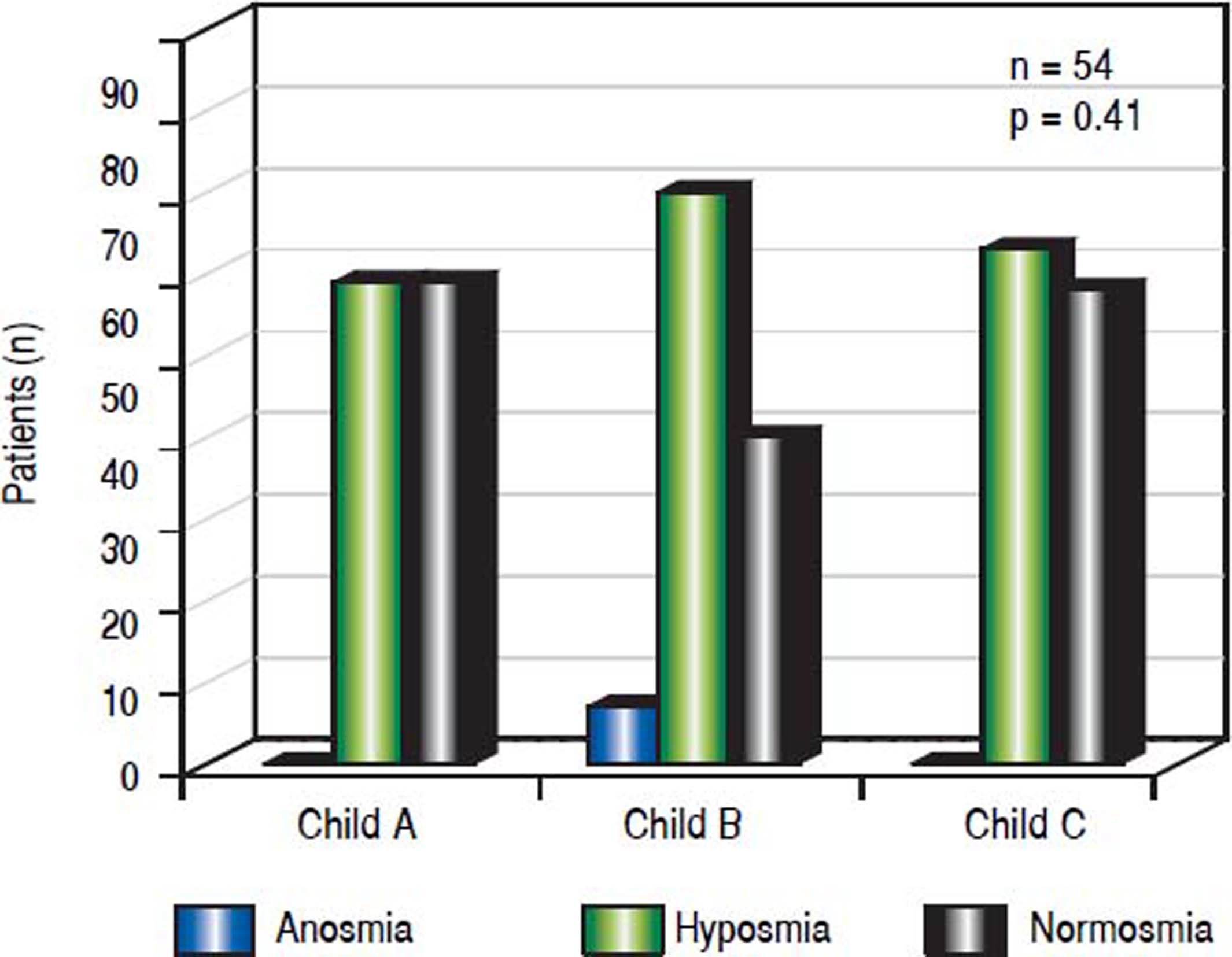
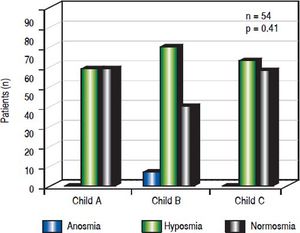
Association with severity of liver diseaseNormosmia and hyposmia occurred each in 50% (6/ 12) of patients with Child Pugh (CP) score A, respectively, while anosmia was not observed. In patients suffering from Child B cirrhosis anosmia was revealed in 8.7% (2/23), hyposmia in 60.9% (14/23) and normosmia in 30.4% (7/23), respectively. In patients suffering from Child C cirrhosis anosmia was detected in none of the patients, while hyposmia and normosmia were revealed in 52.6% (10/19) and 47.4% (9/19), respectively. (p = 0.41, Figure 4).
When comparing cirrhotic subjects with and without ascites, olfactory deficits occurred in 44.4% (24/54) when ascites was present. Patients with ascites showed anosmia in 2.7% (1/37), hyposmia in 62.2% (23/37) and normosmia in 35.1% (13/37). In patients without ascites anosmia was seen in 63% (1/16), hyposmia in 37.5% (6/16) and normosmia in 56.3% (9/16). However, these differences were not statistically significant (p = 0.35).
In cirrhotic patients with portal hypertension hypo- or anosmia was seen in 24.1% (13/54). 2.9% (1/34) of patients with portal hypertension showed an anosmia, 64.7% (22/ 34) hyposmia and 32.4% (11/34) a normosmia, while in patients without portal hypertension anosmia occurred in 7.1% (1/14), hyposmia in 57.1% (8/14) and normosmia in 35.7% (5/14). Similar to the cirrhotic patients, there was no significance in correlation between patients with or without portal hypertension (p = 0.1).
In addition, the patient’s ability to identify odors, but not odor thresholds or odor discrimination, was related to the severity of cirrhosis. Hepatic encephalopathy as assessed by psychometric testing correlated inversely with the ability to identify odors, but not with abilities to discriminate odors or with odor thresholds.
DiscussionIn this study we were able to show, that among cirrhotics patients, the prevalence of olfactory deficits (hyposmia, anosmia) increased with the severity of HE as assessed by CFF and clinical criteria. At the same time no correlation was seen between olfactory deficits and severity of liver disease (as assessed by Child-Pugh-Score), etiology of cirrhosis and complications of cirrhosis such as ascites and portal venous hypertension. Including all stages, HE is a frequent complication of liver cirrhosis with a high prevalence up to 80% of all cirrhotic patients.8 However, compared with e.g. ascites or oesophageal variceal bleeding, HE seems to represent an often overlooked complication of cirrhosis since the possibilities to diagnose this condition in everyday’s practice are limited to only few feasible methods. Several tools have been used to diagnose the severity of HE clinically (e.g. West-Haven criteria, Table 1) and technically using objective techniques such as CFF. Olfactory deficits were frequently described in liver cirrhosis. Some studies demonstrated that an alteration of olfactory function is not dependent on the severity of cirrhosis as assessed by CP score.6,7 Since HE and the neurological diseases share many clinical symptoms, olfactory deficits in cirrhosis might be related to the cognitive dysfunction in HE. However, so far no studies have been published focusing on the olfactory function in cirrhotic patients and its potential alteration by HE of different grades of severity as assessed by objective procedures such as CFF. The olfactory deficits increases with the severity of HE.
In the studies quoted above demonstrating olfactory deficits in patients with liver cirrhosis HE was assessed by using various methodological tests. However, many of those are not appropriate for clinical practice due to e.g. lack of normative data, time needed for administration and limited availability.12 Therefore, the “Sniffin’ Sticks” test kit has been represents a valid tool for the routine clinical assessment of olfactory function12–14 and may therefore serve as the diagnostic gold standard for analyzing olfactory deficits in cirrhotic patients with HE. Temmel et al investigated the relation between olfactory impairment and several laboratory and psychological parameters compatible with HE by using the “Sniffin’ Stick test kit”.6 In this study, the authors demonstrated an inverse correlation of odor identification with, HE. A major limitation of this study, however, was the diagnosis of HE by performing exclusively the single psychometric tests which is not a valid diagnostic method to rule out or confirm.7 Zucco performed a pilot study by evaluating olfactory deficits in a small group of patients with minimal HE.7 In this study, olfactory function was impaired when minimal HE was present as cirrhotic patients performed significantly worse than a control group for both odor identification and recognition tasks. However, inaccuracies in conducting paper-pencil tests and the subjectivity of interpretation require the application of objective diagnostic procedures such as CFF when evaluating a potential correlation of impaired olfactory function and HE. CFF is easy to perform, reliable and accurate and has the potential to distinguish reliably between the different severity grades of HE.10,11 To the best of our knowledge, the olfactory function as assessed by an objective diagnostic procedure such as CFF has not yet been studied to date in cirrhotic patients.
In accordance with previously published data, we found olfactory function to be reduced in the present study in the majority of cirrhotic patients (61.1%) when compared to healthy controls (p < 0.001). Among cirrhotics, prevalence of olfactory deficits (hyposmia, anosmia) was increased according to HE-severity as assessed by CFF and clinical criteria (p = 0.008 and p = 0.097, respectively). Regarding CFF, the sensitivity was 100% for high grade and 68% for low grade HE. Specificity was 98.7% for high grade and 66.7% for low grade HE. No significant correlation was observed between plasma ammonia values and olfactory deficits. As shown before, we could not observe a correlation between olfactory deficits and severity of cirrhosis (as assessed by Child-Pugh-Score) and underlying etiology suggesting that impaired olfactory function is not caused by the liver disease itself but rather by concurrent complications. In our study, complications of cirrhosis such as ascites and portal hypertension were not significantly correlated with olfactory deficits supporting the assumption that an impaired olfactory function in cirrhosis is related to the pathophysiology of HE.
There are common pathophysiological mechanisms suggesting a relationship between HE and olfactory dysfunction. Butterworth, et al. described neurotoxic substances (manganese, ammonia) that exert harmful effects on the brain.15,16 Glutamate and dopamine are involved as transmitters in the olfactory process.17 Manganese and ammonia can harm these neurotransmitter systems. Behar et al showed that the GABA-ergic systems are altered in HE patients.18 High concentrations of manganese can accumulate in the pallidum (globus pallidus) and trigger extrapyramidal symptoms.19 Glutamate is the neurotransmitter of the receptor cells in the olfactory system and dopamine is mainly found as neurotransmitter in the periglomerular cells.20,21
In conclusion, our study has demonstrated that olfactory function is significantly altered in cirrhotic patient’s dependent on the severity of HE. Assessing olfactory deficits may serve as a screening tool for HE also in sub-clinical stages. We observed a significant correlation between loss of olfactory function and increased severity grade of HE. Prospective studies are needed to analyse detection and in-course-control of chemosensibility in sub-clinical and manifest HE in patients suffering from cirrhosis before and after specific medical treatment.
Abbreviations- •
CFF: critical flicker frequency.
- •
CP: Child PughM.
- •
ENT: Ear Nose Throat.
- •
HE: hepatic encephalopathy.
- •
Hz: Herz.
- •
OLT: orthotopic liver transplantation.
- •
SD: standard deviation.
- •
WHC: West Haven Criteria.
No funding is to be disclosed. For this clinical trial no financial support was received.
Conflict of InterestThere is no conflict of interest.
Ethics StatementEthic committee of the Bayerische Landesärztekammer (Bavarian Medical Council) approved this study.
AcknowledgementsThe authors are indebted to Petra Blankenburg and Doris Decker for their technical assistance.