Introduction and Aim. EVBL is a procedure frequently performed in cirrhotic patients for primary prophylaxis of bleeding. Patients with cirrhosis display various degrees of alteration of common coagulation parameters, and it is not known whether these alterations may predict post-EVBL bleeding. To evaluate factors predictive of post-endoscopic variceal band ligation (EVBL) bleeding in cirrhotic patients with thrombocytopenia.
Material and methods. We included 109 patients with cirrhosis undergoing EVBL for primary prophylaxis of variceal bleeding. Common coagulation parameters (INR, fibrinogen levels) and complete haemogram were obtained in all patients and evaluated subdividing patients in bleeders and non bleeders following EVBL.
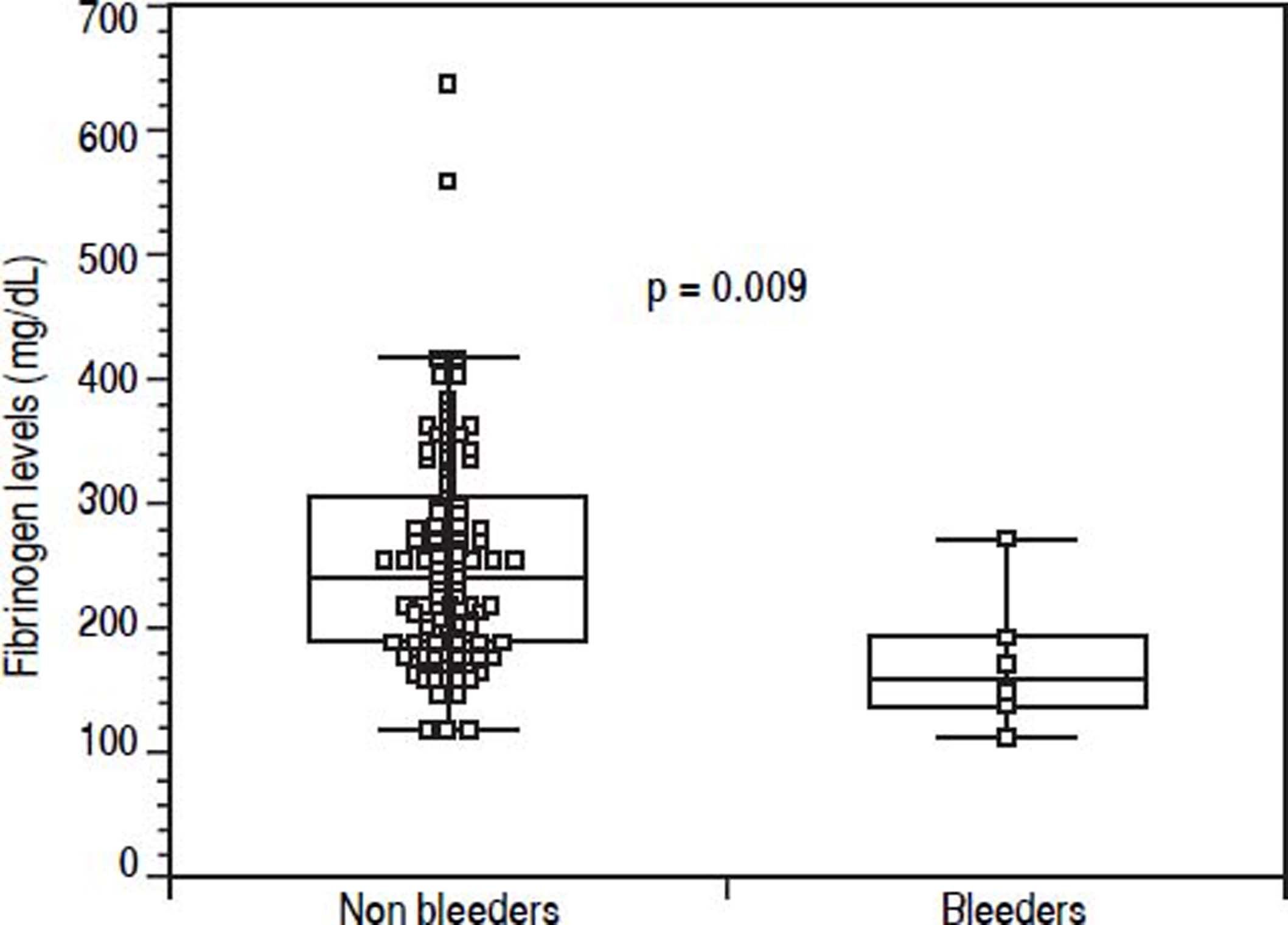
Results. The incidence of post-EVBL bleeding was 5.5% (6 patients). INR and platelet counts, considered as continuous or dichotomous variables according to common cut-offs (i.e., INR > 1.5, platelet count < 50 × 109/L) were not predictors of post-EVBL bleeding. Patients who bled had significantly lower fibrinogen levels [146 mg/dL (98 - 262) vs. 230 mg/dL (104 - 638), P = 0.009], and no other biochemical or clinical predictors of bleeding were identified. A fibrinogen cut-off of 179 mg/dL had 98.6% negative predictive value for bleeding.
Conclusion. Low fibrinogen levels are associated with an increased risk of bleeding following prophylactic EVBL in cirrhotic patients, and might be used to stratify patients’ risk. However, due to their preliminary nature, these findings need to be confirmed in larger populations.
Oesophageal varices can be found in approximately 60% of cirrhotic patients, with or without hepatocellular carcinoma, undergoing screening endoscopy, and their prevalence increases with increasing severity of liver disease.1–3 When varices at risk of bleeding are identified, patients should undergo prophylaxis with non-selective beta-blockers or endoscopic variceal band ligation (EVBL).4 Although beta-blockers are suggested as first choice for primary prophylaxis of variceal bleeding, also due to additional positive effects exerted by these drugs on other complications of portal hypertension, between 15 and 20% of cirrhotic patients with varices needing prophylaxis either have contraindications to beta-blockers or develop side effects that preclude their use.5–7 Furthermore, the use of beta-blockers has recently been a matter of a heated debate due to the possible deleterious effects on pa- tients’ survival when iterative paracenteses are performed in patients with refractory ascites.8–11 Thus, in everyday clinical practice, prophylactic EVBL is a frequent procedure in a large proportion of cirrhotic patients with at-risk varices.
Patients with cirrhosis often display various degrees of thrombocytopenia as well as alteration of common coagulation tests, although the relevance of these abnormalities to the actual risk of bleeding following invasive procedures has not been established.12–16 In a post hoc analysis of a study carried out in cirrhotic patients being evaluated for liver transplant we found that increased International Normalised Ratio (INR) values were not associated with an increased risk of bleeding following invasive procedures, while bleeding tended to occur more frequently in patients with severe thrombocytopenia; another recent study found that both conventional and expanded coagulation indices were not predictive of ulcer bleeding after EVBL in patients with cirrhosis of the liver.13,15 Although sophisticated, global haemostasis tests may be able to evaluate more comprehensively the complex coagulation balance of cirrhotic patients, and even assess its modification in response to blood or blood products transfusions, these assays are not commonly available in clinical practice.17,18 Therefore, in this prospective study we sought to assess whether standard coagulation parameters were predictive of bleeding in a series of cirrhotic patients undergoing EVBL. We focussed on thrombocytopenic patients in order to equalise patients for one possible confounding factor, and hypothesised that identification of simple parameters able to stratify patients’ risk of bleeding may guide clinicians decisions in everyday clinical practice.
Material and MethodsPatientsThis study included 109 consecutive cirrhotic patients with thrombocytopenia (i.e., platelet count < 150 × 109/L) who were referred to our Endoscopy Unit for performing prophylactic EVBL. Patients were referred due to the finding of at-risk varices, while patients with previous endoscopic treatment of oesophageal varices, acute variceal bleeding, and infection were not included.
MethodsEVBL was performed in all patients for primary prophylaxis of bleeding, and in 41 patients (37.6%) EVBL was selected due to beta-blocker intolerance or patients preference to EVBL. The endoscope used was an adult gastroscope type Olympus GIF 165 (Olympus Medical Systems, Hamburg, Germany). EVBL was carried out by an experienced endoscopist using the same procedure for each patient. The elastic bands (median 6, range 5-7) were placed on varices with the same device (Speedband Superview Super 7™ Multiple Band Ligator, Boston Scientific Corporation, Marlborough, MA, USA) in an ascending way through the oesophagus. Food intake was allowed 24 h following the endoscopic procedure, and pantoprazole 40 mg bid was administrated for at least 3 weeks in all patients. After EVBL, patients were followed and regularly monitored at our outpatients for up to 3 weeks, and emergency endoscopies were carried out at our Endoscopy Unit. Post-EVBL bleeding was defined as any clinically significant (at least a 2 g drop in haemoglobin, or any drop accompanied by systemic signs) episode of haematemesis, melena, or both and was confirmed by endoscopic evaluation. Blood samples for complete haemogram, biochemical tests and coagulation parameters were obtained on the same morning of the EVBL procedure. The Child-Pugh score and the Model for End-stage Liver Disease score were used to assess severity of liver disease.19,20
Statistical analysisContinuous variables are shown as median and range and were compared using Mann-Whitney U-test, while categorical variables are shown as absolute count and percentage and were compared using Fisher’s exact test. Receiver Operating Characteristic (ROC) curve was used to identify the fibrinogen level with the highest accuracy for identifying patients who bled, and sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios for this cut-of were assessed. All analyses were carried out using MedCalc version 8.0.1.0 (MedCalc Software BVBA, Ostend, Belgium).
EthicsThe study was carried according to the standards of Helsinki declaration and was approved by the local review board. Informed consent was waived as all the study procedures were carried out as standard of care of clinical practice.
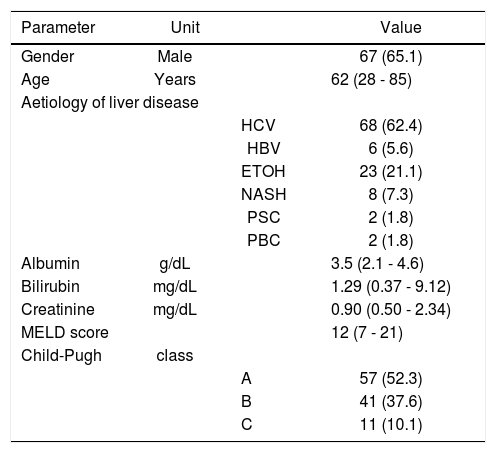
ResultsThe main demographic, biochemical, and clinical characteristics of the 109 patients who made up the study population are shown in table 1. Ascites and encephalopathy were present in 54 (49.5%) and 28 (25.6%) patients, respectively. Twenty-four patients had hepatocellular carcinoma (22.0%) and 4 patients had portal vein thrombosis (3.7%). Aetiology of liver disease was due to chronic infection with hepatitis virus in the majority of patients (n = 74, 68.0%).
Main demographic, biochemical, and clinical characteristics of the study population.
| Parameter | Unit | Value | |
|---|---|---|---|
| Gender | Male | 67 (65.1) | |
| Age | Years | 62 (28 - 85) | |
| Aetiology of liver disease | |||
| HCV | 68 (62.4) | ||
| HBV | 6 (5.6) | ||
| ETOH | 23 (21.1) | ||
| NASH | 8 (7.3) | ||
| PSC | 2 (1.8) | ||
| PBC | 2 (1.8) | ||
| Albumin | g/dL | 3.5 (2.1 - 4.6) | |
| Bilirubin | mg/dL | 1.29 (0.37 - 9.12) | |
| Creatinine | mg/dL | 0.90 (0.50 - 2.34) | |
| MELD score | 12 (7 - 21) | ||
| Child-Pugh | class | ||
| A | 57 (52.3) | ||
| B | 41 (37.6) | ||
| C | 11 (10.1) | ||
MELD: Model for End-stage Live Disease. HCV: hepatitis C virus. HBV: hepatitis B virus. ETOH: alcohol. NASH: nonalcoholic steatohepatitis. PSC: primary sclerosing cholangitis. PBC: primary biliary cholangitis.
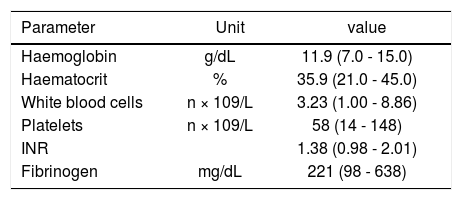
Table 2 shows the patients haematological and coagulation parameters. Twenty-nine patients (26.6%) had an INR > 1.5, and median platelet count was in the severe thrombocytopenia range (58 × 109/L; range, 14-148 × 109/ L), with 31 patients (28.4%) having a platelet count < 50 × 109/L.
Main haematological and coagulation parameters of the study population.
| Parameter | Unit | value |
|---|---|---|
| Haemoglobin | g/dL | 11.9 (7.0 - 15.0) |
| Haematocrit | % | 35.9 (21.0 - 45.0) |
| White blood cells | n × 109/L | 3.23 (1.00 - 8.86) |
| Platelets | n × 109/L | 58 (14 - 148) |
| INR | 1.38 (0.98 - 2.01) | |
| Fibrinogen | mg/dL | 221 (98 - 638) |
INR: International Normalised Ratio.
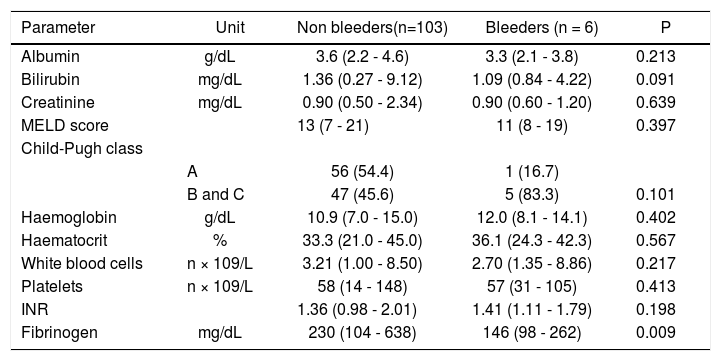
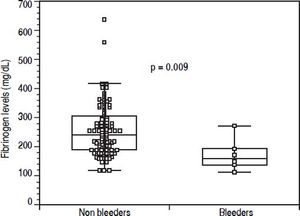
Before EVBL 22 patients (20.2%), all with platelet count < 50 × 109/L, received prophylactic platelet transfusions. Bleeding occurred in 6 patients (5.5%) a median of 12 days (range, 7-18 days) after EVBL, and post-EVBL bleeding was successfully managed by pharmacologic and endoscopic treatment in all patients. No patients received plasma or platelet transfusions, or coagulation factors transfusions at the time of bleeding, and no deaths were recorded due to bleeding. Table 3 reports the biochemical, haematological, and coagulation parameters of patients subdivided according to the occurrence of post-EVBL bleeding. Eleven patients (10.1%) had a creatinine level above the upper limit of normal (i.e., 1.3 mg/dL) and none of them bled. Moreover, among the 22 patients with hepatocellular carcinoma only one bled (4.5%) and none of the 4 patients with portal vein thrombosis bled. Patients who bled had significantly lower fibrinogen levels [146 mg/dL (98 - 262) vs. 230 mg/dL (104 - 638), P = 0.009, Figure 1], while there was no significant difference in median platelet counts [58 × 109/L (31 - 105) vs. 54 × 109/L (14 - 148), P = 0.413] and INR values [1.41 (1.11 - 1.79) vs. 1.36 (0.98 -2.01), P = 0.198] as well as in the proportion of patients with either an INR > 1.5 [2 (33.3%) vs. 27 (26.2%), P = 0.656] or a platelet count < 50 × 109/L [2 (33.3%) vs. 29 (28.2%), P = 1.0] between patients who bled and those who did not bleed. No association was found either between haematocrit, haemoglobin levels, or white blood cells count and bleeding.
Main biochemical, haematological, and coagulation parameters of the study population sub-divided according to post-variceal banding bleeding.
| Parameter | Unit | Non bleeders(n=103) | Bleeders (n = 6) | P |
|---|---|---|---|---|
| Albumin | g/dL | 3.6 (2.2 - 4.6) | 3.3 (2.1 - 3.8) | 0.213 |
| Bilirubin | mg/dL | 1.36 (0.27 - 9.12) | 1.09 (0.84 - 4.22) | 0.091 |
| Creatinine | mg/dL | 0.90 (0.50 - 2.34) | 0.90 (0.60 - 1.20) | 0.639 |
| MELD score | 13 (7 - 21) | 11 (8 - 19) | 0.397 | |
| Child-Pugh class | ||||
| A | 56 (54.4) | 1 (16.7) | ||
| B and C | 47 (45.6) | 5 (83.3) | 0.101 | |
| Haemoglobin | g/dL | 10.9 (7.0 - 15.0) | 12.0 (8.1 - 14.1) | 0.402 |
| Haematocrit | % | 33.3 (21.0 - 45.0) | 36.1 (24.3 - 42.3) | 0.567 |
| White blood cells | n × 109/L | 3.21 (1.00 - 8.50) | 2.70 (1.35 - 8.86) | 0.217 |
| Platelets | n × 109/L | 58 (14 - 148) | 57 (31 - 105) | 0.413 |
| INR | 1.36 (0.98 - 2.01) | 1.41 (1.11 - 1.79) | 0.198 | |
| Fibrinogen | mg/dL | 230 (104 - 638) | 146 (98 - 262) | 0.009 |
INR: International Normalised Ratio.
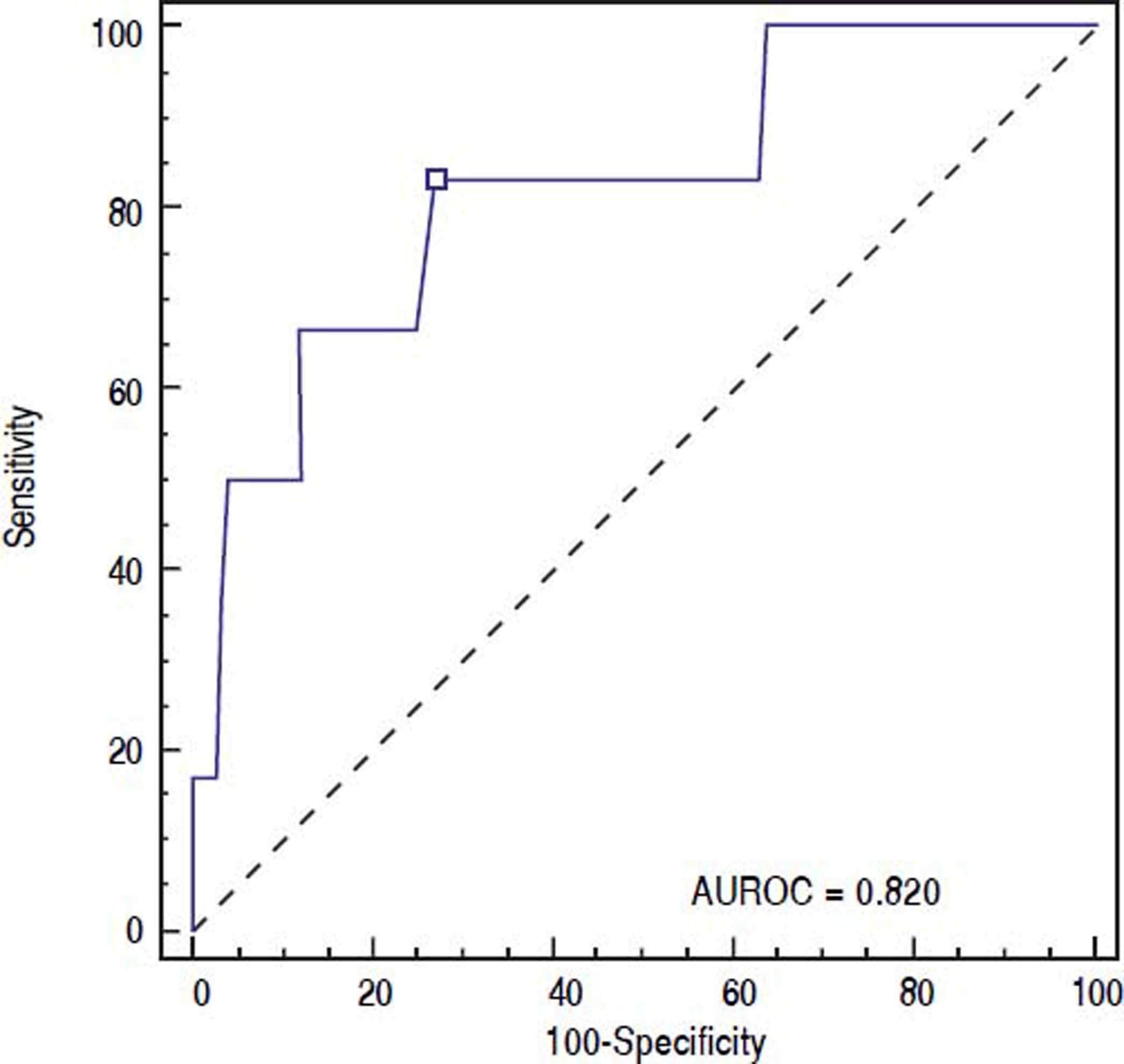
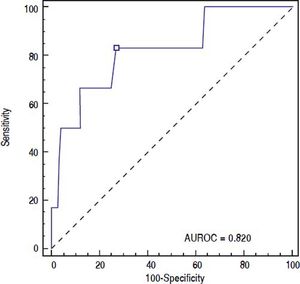
ROC curve was used to identify the fibrinogen level with the highest accuracy for predicting post-EVBL bleeding (Figure 2). We found that a fibrinogen level cut-off of 179 mg/dL had an 83.3% sensitivity, 73.0 specificity, 15.6% positive predictive value, 98.6% negative predictive value, and 0.820 accuracy (95% confidence interval = 0.733 to 0.888) for predicting bleeding.
DiscussionIn patients with cirrhosis, primary prophylaxis of variceal bleeding often relies on EVBL due to the relatively frequent intolerance to beta-blockers and to the lack of an adequate means to evaluate effectiveness of drug prophylaxis when hepatic venous gradient measurement is not available. Bleeding after EVBL reportedly occurs in 1.2 -7.3% of patients undergoing this procedure, and the predictive factors for bleeding have been investigated in 2 previous studies.15,21,22 In particular, Vanbiervliet, et al. reported that a low prothrombin activity was an independent predictor of post-EVBL bleeding in a case-control study, while Vieira da Rocha, et al. found that neither conventional nor expanded coagulation tests were predictive of bleeding after EVBL.15,22 However, the former study was not focused on coagulation parameters, included patients with prevalently alcoholic cirrhosis (71%) and a history of previous variceal bleeding in 59% of cases, and reported a mortality due to post-EVBL as high as 52%, while the latter study purposely assessed coagulation parameters as possible predictors of bleeding, but in doing so categorised patients according to pre-defined INR and platelet count cut-offs.15,22
In this study we aimed at evaluating whether platelet counts or conventional coagulation parameters were predictors of bleeding following EVBL. We included only patients with thrombocytopenia (i.e., platelet count < 150 × 109/L) in order to evaluate patients where an hypothetical risk factor for bleeding was present and to exclude a possible bias related to a skewed distribution for this characteristic. Moreover, we decided to avoid categorising patients a priori according to pre-defined coagulation and platelet count cut-offs, due to the absence of a clear evidence in the literature suggesting a definite threshold for increased risk of bleeding regarding these parameters.17,23 Lastly, we also included patients with renal failure and hepatocellular carcinoma as we deemed that the results of a study carried out in this population might more closely reflect everyday clinical practice. Overall, we found that both INR values and platelet counts were not associated with bleeding, and that this absence of association was confirmed also when patients were categorised according to hypothetical risk factors for bleeding such as an INR > 1.5 or a platelet count < 50 × 109/L. Although some patients underwent platelet transfusion before EVBL, it is unlikely that this might have influenced bleeding rate as in these patients single platelet transfusion reportedly have minimal efficacy in increasing platelet counts.18 As previous reports suggested that decreased haematocrit may increase the risk of bleeding in cirrhotic patients due to the rheological effect of erythrocytes on platelet adhesion, we also evaluated whether haematocrit and haemoglobin levels were related to bleeding, and found no association between these parameters and post-EVBL bleeding.24,25 Noteworthy, we also observed no association between bleeding and creatinine levels or the presence of renal failure, although we have to emphasize that all the patients included in this study were in stable conditions. The main result of our study was that patients who had post-EVLB bleeding had significantly lower fibrinogen levels, and that a fibrinogen level of 179 mg/dL or less had a negative predictive value for bleeding of 98.6%. As the negative predictive value of a variable depends on the disease prevalence, we also calculated that the negative predictive value of the identified fibrinogen cut-off with an incidence of bleeding ranging from 1.2 to 7.3% -as reported in the literature- ranged between 99.7 and 98.2%.15,22 Thus, although the positive predictive value was low, in clinical practice the use of fibrinogen with this cut-off may be able to identify a population of cirrhotic patients where post-EVBL bleeding is very unlikely, and therefore shifting the attention of the clinician on the follow-up of patients with a fibrinogen level below this cut-off.
As far as the possible role of fibrinogen on the risk of bleeding in patients with cirrhosis in concerned, it is well known that these patients may display abnormal fibrinogen, and some studies have advocated the presence of hyperfibrinolysis as one of the possible mechanisms for increased risk of bleeding in cirrhotic patients, identifying a correlation between decreased fibrinogen levels and increased bleeding time, despite there is still debate on the presence and role of this laboratory characteristic.26–29 As the role of fibrinogen is to facilitate platelet aggregation by binding glycoprotein IIb/IIIa, and this effects mainly depends upon fibrinogen concentration, the inclusion in our study of patients with thrombocytopenia might have equalised the study population for one possible confounding factor, thus emphasizing the role of fibrinogen, as decreased fibrinogen levels might further impair platelet-to-platelet bridge formation ad increase the likelihood of bleeding.30
One of the possible drawbacks of this study is that we recorded a small number of events, and this finding may have relevance for the predictive values of the identified fibrinogen cut-off. However, we also calculated the negative predictive values of the fibrinogen cut-off identified in this study using the bleeding incidence identified in previous studies, and we found that the predictive ability of the cut-off was not affected. Moreover, we must emphasize that the fibrinogen cut-off identified in our study (i.e., 179 mg/dL) is higher than the threshold suggested for prophylactic fibrinogen replacement in patients undergoing invasive procedures (i.e., 120-150 mg/dL), although we also have to take into account that in our study we did not push the envelope so far as to suggest that patients with a fibrinogen level below 179 mg/dL must receive some form of pre-procedure prophylaxis, and that the fibrinogen cut-offs suggested in the literature are mainly based on experts’ opinion.31 All in all, we found that use of this fibrinogen cut-off allowed to predict a moderate decrease in the likelihood of bleeding, although we feel that due to the small number of patients included in our study these results need to be confirmed in larger cohort of patients. Lastly, it might be that other bio-markers or more sophisticated tests may be able to better predict the risk of bleeding in these patients although, as far as we know, the only study that used both expanded coagulation tests and thromboelastography reported negative results.15
In conclusion, in this study we identified low fibrinogen levels as the coagulation parameter associated with an increased risk of bleeding following prophylactic EVBL in cirrhotic patients. Use of this simple and readily available parameter may help stratify patients’ risk in clinical practice, although these preliminary results need to be confirmed in larger series.
Abbreviations- •
ETOH: alcohol.
- •
EVBL: endoscopic variceal band ligation.
- •
HBV: hepatitis B virus.
- •
HCV: Hepatitis C virus.
- •
INR: International Normalised Ratio.
- •
MELD: Model for End-stage Live Disease.
- •
NASH: Nonalcoholic Steatohepatitis.
- •
PBC: Primary Biliary Cholangitis.
- •
PSC: Primary Sclerosing Cholangitis.
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Declaration of Funding SourceThis study was supported by a grant “Progetto di ricerca di Ateneo 2014” dell’Università degli Studi di Genova.
Authorship Statement- •
Guarantor of article: Edoardo G. Giannini.
- •
Specific author contributions: Edoardo G. Giannini, Elisa Giambruno, Matteo Brunacci, Maria Corina Plaz Torres, Manuele Furnari, and Giorgia Bodini performed the research, Edoardo G. Giannini, Elisa Giambruno, Matteo Brunacci, and Maria Corina Plaz Torres collected and analysed the data, Edoardo G. Giannini designed the research study and wrote the paper, and Patrizia Zentilin, Manuele Furnari, Giorgia Bodini, and Vincenzo Savarino contributed to the design of the study.
All authors approved the final version of the article, including the authorship list.