Treatment with polyethylene glycol-modified interferon alfa-2a (peginterferon) alone produces significantly higher sustained antiviral responses than treatment with interferon alfa-2a alone in patients with chronic hepatitis C virus (HCV) infection. We compared the efficacy and safety of peginterferon alfa-2a plus rib-avirin, interferon alfa-2b plus ribavirin, and peginterferon alfa-2a alone in the initial treatment of chronic hepatitis C. Thirty-two patients were randomly assigned to treatment, and received at least one dose of medication consisting of 180 μ of peginterferon alfa-2a once weekly plus daily ribavirin (1,000 or 1,200 mg, depending on body weight) (n = 14), weekly peginterferon alfa-2a plus daily placebo (n = 6), or three million units of interferon alfa-2b thrice weekly plus daily ribavirin for 48 weeks (n = 12). More patients who received peginterferon alfa-2a plus ribavirin had a sustained virologic response (defined as the absence of detectable HCV RNA 24 weeks after cessation of therapy) than patients who received interferon alfa-2b plus ribavirin (7/14 vs. 4/12) or peginterferon alfa-2a plus placebo (0/6). The overall safety profiles of the three treatment regimens were similar. In conclusion, for patients with chronic hepatitis C, once-weekly peginterferon alfa-2a plus ribavirin was tolerated as well as interferon alfa-2b plus ribavirin and produced significant improvements in the rate of sustained viral reduction compared with interferon alfa-2b plus ribavirin or peginterferon alfa-2a alone.
Although the mechanism of action of ribavirin remains speculative,1 the current standard of care for patients with chronic hepatitis C involves the addition of ribavirin to interferon-based therapies.2-7 Unfortunately, some patients, particularly those with more resistant hepatitis C virus (HCV) genotypes, do not respond to these agents. Two types of polyethylene glycol-modified (pegylated) interferon, which differ in their pharmacokinetic and chemical properties, have been developed. Both have demonstrated significantly superior efficacy to non-pegylated interferons in several controlled clinical trials.8-12 Polyethylene glycolmodified interferon alfa-2b (with a 12 kDa linear polyethylene glycol moiety) plus ribavirin produced significantly improved sustained virologic responses compared with interferon alfa-2b plus ribavirin.13 Peginterferon alfa-2a (with a 40-kDa branched polyethylene glycol moiety) has an extended serum half-life that provides constant viral suppression for seven days, thus allowing once-weekly dosing and enhanced clinical efficacy.8-10,14-17 We undertook the present study as members of the Pegasys International Study Group to determine whether peginterferon alfa-2a plus ribavirin is more effective than interferon alfa-2b plus ribavirin or peginterferon alfa-2a alone for the treatment of chronic hepatitis C.
Experimental proceduresPatient selectionThis study was conducted by the Pegasys International Study Group. Eligible subjects were adult patients who had never received interferon and who had at least 2,000 copies of HCV RNA per milliliter of serum according to a polymerase chain reaction (PCR) assay (Cobas Amplicor HCV Monitor Test, version 2.0; Roche Diagnostics, Pleasanton, CA, USA), serum alanine aminotransferase activity above the upper limit of normal within six months before entry into the study, and a liver biopsy result consistent with the diagnosis of chronic hepatitis C. Patients were excluded from participation if they had neutropenia (fewer than 1,500 neutrophils per cubic millimeter); thrombocytopenia (fewer than 90,000 platelets per cubic millimeter); anemia (less than 12 g of hemoglobin per deciliter in women and less than 13 g of hemoglobin per deciliter in men); human immunodeficiency virus (HIV) infection; decompensated liver disease; a serum creatinine level more than 1.5 times the upper limit of normal; poorly controlled psychiatric diseases; alcohol or drug dependence within one year before entry into the study; or substantial coexisting medical conditions.
Study designThis randomized, controlled clinical trial was conducted at 81 centers worldwide from February 1999 to April 2001. In our country were included patients of three centers in the cities of Mexico DF, Guadalajara and Monterrey. The patients were randomly assigned in a 2:1:2 ratio (with a block size of five) to receive subcutaneous, once-weekly injections of 180 μg of peginterferon alfa-2a, (Pegasys, Hoffmann-La Roche) plus daily ribavirin (Hoffmann-La Roche) or placebo, or subcutaneous, thrice-weekly injections of 3 million units of interferon alfa-2b plus ribavirin (Rebetron, Schering-Plough Corp., Kenilworth, NJ, USA) for 48 weeks. Ribavirin was given orally at a dose of 1,000 mg per day for patients weighing 75 kg or less or 1,200 mg per day for those weighing more than 75 kg. Randomization was stratified according to country and HCV genotype (HCV genotype 1 vs. other genotypes). Genotyping was performed by sequence analysis of a portion of the 5' untranslated region of the HCV genome.18 Participants were followed up for 24 weeks after cessation of therapy. The sponsor (Hoffmann-La Roche), investigators, and patients who received peginterferon alfa-2a were unaware of who received ribavirin or placebo. The institutional review boards of the participating centers approved the protocol, and all patients provided written informed consent. The study was designed by the sponsor in collaboration with expert hepatologists. Data were collected by the Pegasys alfa-2a International Study Group. The study was conducted according to the guidelines of the Declaration of Helsinki, the applicable provisions of Good Clinical Practice, or both.
Assessment of efficacyThe primary efficacy end point was sustained virologic response, defined as the absence of detectable HCV RNA at the end of follow-up according to a PCR assay (Cobas Amplicor HCV Test, version 2.0; lower limit of detection, 100 copies [50 IU] per milliliter). For patients with at least 20 weeks of follow-up, the last observed HCV RNA level was used in assessments of efficacy. All patients with follow-up of less than 20 weeks were considered to have had no response to treatment.
Assessment of safetySafety was assessed by laboratory tests and evaluation of adverse events at weeks 1, 2, 4, 6, and 8; monthly thereafter during treatment; and then at weeks 52, 60, and 72. Patients who discontinued therapy prematurely because of intolerance were encouraged to remain in the study. Stepwise reductions in the peginterferon alfa-2a dosages to 135, 90, or 45 μg per week and reductions in ribavirin dosages to 800 or 600 mg per day were allowed to manage adverse events or laboratory abnormalities that had reached predetermined thresholds of severity. If the adverse event resolved or improved, a return to initial dosing levels was permitted unless the patient had received the reduced dose for more than four weeks. Patients were withdrawn from treatment if they continued to have viremia at week 24, if they missed four consecutive doses, or at the discretion of the investigator.
Statistical analysisThe data are presented as means and standard deviations or relative frequency. Because the sample size in this subset of patients was so small (n = 32), we did not attempt statistical comparisons between the three treatments, or between global results and our experience to identify any differences in regard to efficacy and safety.
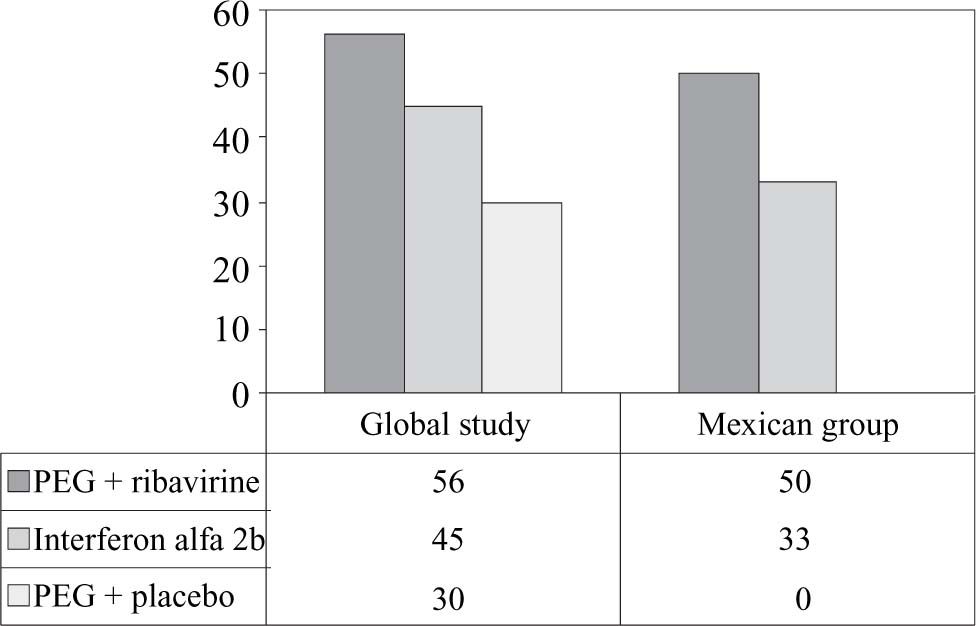
ResultsPatient demographicsIn the global study, of the 1,459 patients screened, 1,149 were randomly assigned to treatment and 1,121 were randomly assigned to treatment and received at least one dose of study medication. The patients who were excluded from the study did not have elevated alanine minotransferase levels, refused to participate, or failed to meet other inclusion criteria. In the Mexican group, we included 32 patients. The pretreatment characteristics of both global and Mexican groups are shown in tables IandII. We saw a higher proportion of cirrhosis in our sample of patients compared with the global group; however, the proportion between the three arms was similarly distributed. The body weights of the patients were similar between the groups treated with peginterferon alfa-2a plus ribavirin and interferon alfa-2b plus ribavirin. The weights of patients in the peginterferon alfa-2a plus placebo group were skewed because of the small sample size and because one patient weighed 122 kg.
Demographic data in the global group.
| PEG-IFN a-2a + RBV (n = 453) | IFN a-2b + RBV (n = 444) | PEG-IFN a-2a + Placebo (n = 224) | |
|---|---|---|---|
| Age (years) | 42.8 | 42.4 | 42.3 |
| Gender (male) | 71% | 73% | 68% |
| Weight (kg) | 79.6 | 78.1 | 78.9 |
| Genotype | |||
| 1 | 66% | 64% | 64% |
| no-1 | 31% | 33% | 31% |
| HCV RNA levels | |||
| (106 copies/mL) | 6.1 | 6.0 | 5.9 |
| Incidence of cirrhosis | 12% | 12% | 15% |
Demographic data in the Mexican group.
| PEG-IFN α-2a+ (n = 14) | IFN α-2b + RBV (n = 12) | PEG-IFN α-2a + Placebo (n = 6) | |
|---|---|---|---|
| Age (years) | 46 | 45 | 45 |
| Gender (female/male) | 6/8 | 5/7 | 2/4 |
| Weight (kg) | 75 | 68 | 81 |
| Genotype | |||
| 1 | 11 | 10 | 5 |
| no-1 | 3 | 2 | 1 |
| HCV RNA levels | |||
| (106 copies/mL) | 2.4 | 1.9 | 4.2 |
| Incidence of cirrhosis | 3 (21%) | 3 (25%) | 2 (33%) |
More patients treated with peginterferon alfa-2a plus ribavirin had end-of-treatment viral responses than patients treated with interferon alfa-2b plus ribavirin (7/14 patients vs. 4/12 patients) or peginterferon alfa-2a plus placebo (none of 6 patients) (Figure 1). In testing for predictive factors of bad response, we analyzed the roles of genotype and viral load. We found that 6/11 of patients with HCV genotype 1 who received peginterferon alfa-2a plus ribavirin had a sustained virologic response, compared with 2/10 of those who received interferon alfa-2b plus ribavirin and 0/5 of those who received peginterferon alfa-2a plus placebo (Figure 2).
Among patients with HCV genotype 1 and high base-line viral RNA levels (more than 2 million copies per milliliter), 2/6 of the patients receiving peginterferon alfa-2a plus ribavirin had a sustained virologic response, compared with 1/5 patients receiving interferon alfa-2b plus ribavirin.
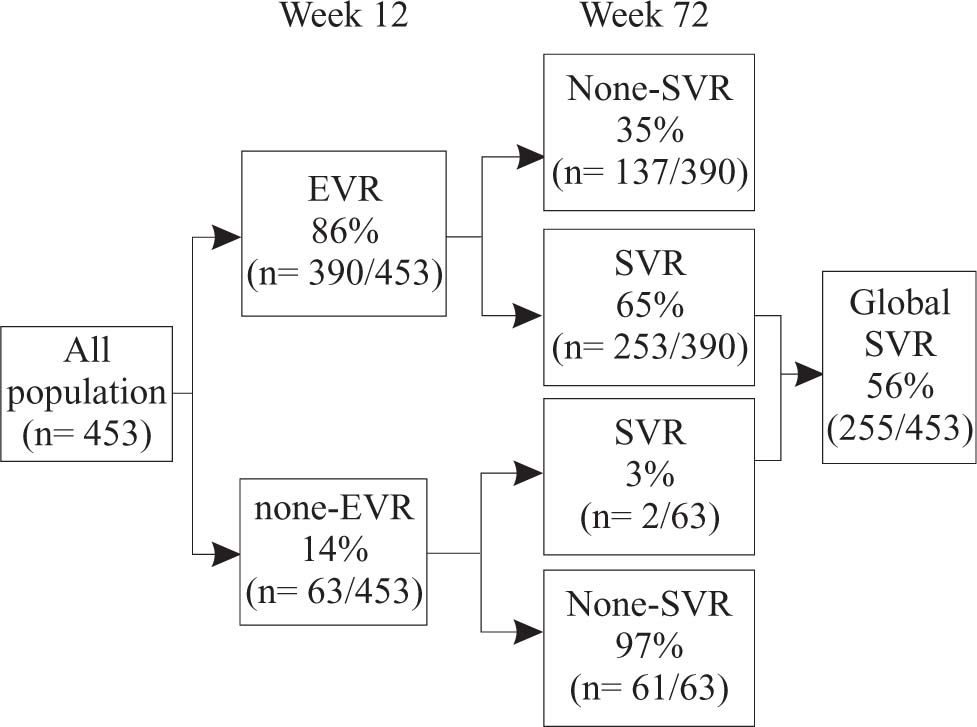
Predictive value of early viral load reductionIn the global group, by week 12,390 of 453 of patients (86%) treated with peginterferon alfa-2a plus ribavirin had achieved a significant viral reduction response, defined as a 2-log decrease from base-line HCV RNA levels (97 patients) or no detectable serum HCV RNA (293 patients) (Figure 3). Of those with early responses, 65% subsequently had a sustained virologic response. Those with no detectable HCV RNA by week 12 were more likely to have a sustained viral load reduction than those who had only a 2-log decrease in HCV RNA (221 of 293 vs. 32 of 97). In contrast, among the 63 patients who did not have an early viral load reduction, 61 (97%) did not have a sustained response.
Predictability of sustained virologic response. At week 12, 390/453 (86%) the patients treated with peginterferon alfa-2a plus ribavirin either had a 2-log drop in HCV RNA levels or had undetectable levels of HCV RNA early virologic response [EVR]). Of these patients, 253/390 (65%) went on to have sustained virologic response (SVR). Of the 63 patients who did not have a 2-log drop or undetectable levels of HCV RNA at week 12, 61 (97%) did not have a sustained virologic response.
Similar results were seen in our Mexican patients treated with peginterferon alfa-2a plus ribavirin. From a total of 14 patients in this group, we saw early significant viral load reductions in 10. Of these, six patients subsequently had a sustained virologic response (positive predictive value). Of the other four patients who did not have a 2-log drop or undetectable levels of HCV RNA at week 12, three did not show a sustained viral load reduction (negative predictive value) (Figure 4).
Predictability of sustained virologic response. At week 12, 10/14 of the patients treated with peginterferon alfa-2a plus ribavirin either had a 2-log drop in HCV RNA levels or had undetectable levels of HCV RNA. Of these patients, six went on to have a sustained virological response (positive predictive value). Of the four patients who did not have a 2-log drop or undetectable levels of HCV RNA at week 12, three did not have a sustained viral reduction (negative predictive value).
None of our patients were withdrawn from treatment because of laboratory abnormalities or other major adverse events. One patient in the interferon alfa-2b plus ribavirin group discontinued because of a psychiatric disorder (depression-related event with suicide ideation).
DiscussionPeginterferon alfa-2a plus ribavirin was more effective than interferon alfa-2b plus ribavirin or peginterferon alfa-2a plus placebo alone for the treatment of chronic hepatitis C. Overall, the rate of sustained virologic response was similar to that reported with the use of peginterferon alfa-2b plus ribavirin.13 In the present study, improved efficacy was seen in subgroups of patients with disease generally considered to have treatment-resistant characteristics.13 In particular, patients with all HCV genotypes and those with high base-line levels of HCV RNA (more than 2 million copies per milliliter) were more likely to have a sustained viral load reduction response when treated with peginterferon alfa-2a plus ribavirin than when treated with interferon alfa-2b plus ribavirin. Among patients considered to have the most treatment-resistant disease —that is, those with both HCV genotype 1 and high base-line viral levels— a higher proportion of those treated with peginterferon alfa-2a plus ribavirin had a sustained viral load reduction than of those treated with interferon alfa-2b plus ribavirin.
Early prediction of a viral load reduction response to interferon-based therapy can help identify patients who are unlikely to have a sustained response and allow clinicians the option to discontinue treatment, saving patients the side effects and cost of additional therapy.19 In the current sub-analysis, three of four patients who did not have an early viral load reduction response to peginterferon alfa-2a plus ribavirin by week 12 never developed a sustained viral load reduction. The incremental benefit of continuing therapy beyond 12 weeks for such patients must be considered for each patient individually.
Adverse events typically associated with the use of interferon (including influenza-like symptoms and depression) occurred in all three treatment groups. As is usual with interferon-based therapy, there were reductions in neutrophil and platelet counts with all treatments. Although these decreases were equivalent in patients treated with peginterferon alfa-2a plus ribavirin and in those treated with interferon alfa-2b plus ribavirin, they did not appear to be associated with serious consequences and were effectively managed by dosage modifications.
The results seen in this Mexican population suggest that the information from the global study is applicable in our patients in regard to overall efficacy and the predictability at 12 weeks. Peginterferon alfa-2a offered significantly enhanced sustained viral load reduction responses in all patients, regardless of HCV genotype and viral load, and a once-weekly dosing schedule. We believe that the ability to predict the absence of sustained viral load reduction response from HCV RNA levels at week 12 will be a useful clinical tool. This study indicates that combination therapy with peginterferon alfa-2a plus ribavirin provides a considerable clinical advantage over therapy with interferon alfa-2b plus ribavirin for the treatment of patients with HCV.











![Predictability of sustained virologic response. At week 12, 390/453 (86%) the patients treated with peginterferon alfa-2a plus ribavirin either had a 2-log drop in HCV RNA levels or had undetectable levels of HCV RNA early virologic response [EVR]). Of these patients, 253/390 (65%) went on to have sustained virologic response (SVR). Of the 63 patients who did not have a 2-log drop or undetectable levels of HCV RNA at week 12, 61 (97%) did not have a sustained virologic response. Predictability of sustained virologic response. At week 12, 390/453 (86%) the patients treated with peginterferon alfa-2a plus ribavirin either had a 2-log drop in HCV RNA levels or had undetectable levels of HCV RNA early virologic response [EVR]). Of these patients, 253/390 (65%) went on to have sustained virologic response (SVR). Of the 63 patients who did not have a 2-log drop or undetectable levels of HCV RNA at week 12, 61 (97%) did not have a sustained virologic response.](https://static.elsevier.es/multimedia/16652681/0000000200000003/v1_201906300821/S1665268119321398/v1_201906300821/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)





