Nucleic acid-amplification testing (NAT) is not routinely practiced in blood banks from most low-income countries. We did an exploratory comparison of the performance of the standard immunoassay-based screening tests for the hepatitis B (HBV) and C (HCV) viruses with that of NAT, in blood donors. From January 1999 to March 2005, 94,806 blood donors were screened for anti-HCV antibodies and for hepatitis B surface antigen (HBsAg). Also, an exploratory period of molecular screening was carried out on 100 consecutive blood donors to detect HBV DNA and HCV RNA by home-made PCR techniques without sera pooling. In the 75-month period of serologic screening, HBsAg was detected in 219 donors (0.23%; 95% CI, 0.20– 0.26%) and anti-HCV antibodies in 922 (0.97%; 95% CI, 0.90–1.03%). The annual trend for HBsAg prevalence had a decreasing pattern over the years (p < 0.001), whereas that for anti-HCV did not (p = 0.19). In the molecular screening cohort, HBV DNA was detected in one donor (1%; 95% CI, 0–6%) and HCV RNA in another (1%; 95% CI, 0–6%). All these 100 donors tested negative to HBsAg and anti-HCV. Thus, the prevalence of positive results for HBV and HCV did not differ if considering immunoassays or NAT; nevertheless, these methods did not coincide in detecting HBV or HCV in the molecular screening cohort. In conclusion, NAT can detect cases of HBV and HCV infections that standard immunoassay techniques can not, even in a highly selected population at low risk, like blood donors. Large-scale studies are warranted for NAT to be considered as a systematic method for screening of HBV and HCV in Mexican blood banks.
Abbrivations
CI: confidence interval;
HBV: hepatitis B virus;
HBsAg, hepatitis B surface antigen;
HCV: hepatitis C virus;
NAT: nucleic acid-amplification testing;
PCR: polymerase chain reaction;
S/CO: immunoassay signal strength of the sample to cut-off;
IntroductionHepatitis B (HBV) and C (HCV) viruses are the most important infectious agents causing non-alcoholic cirrhosis in Mexico.1 Parenteral exposure to blood is the most frequent mode of transmission, especially related to blood transfusion before the systematic practice of testing for HBV and HCV in blood donations, in 1986 and 1992, respectively.2 Since then, laboratory screening of potential blood donors has relied on the use of evolving immunoassays to detect viral antibodies or antigens. Non-remunerated blood donation and screening of potential donors by using a structured questionnaire about risk factors, as well as by anti-HCV and hepatitis B surface antigen (HBsAg) testing have been practiced altogether by law (among other laboratory tests) in Mexican blood banks since 1993.2,3 This is the last revision to the regulations of these practices in blood banks from Mexico. The advent of nucleic acid-amplification testing (NAT) has not changed this practice, possibly because is not considered cost-effective, in the context of a low-income population with other health priorities. However, HCV infection is now one of the two leading causes of cirrhosis in Mexico1 and it is predicted that chronic liver disease will be a major, still-growing cause of both morbidity and mortality in the following years.4 Moreover, if other risk practices related to HBV and HCV infections as the abuse of illicit intravenous drugs become more frequent, Mexico would be facing an increasing problem, and blood banks would need to change their conduct to minimize the possibility of transfusion-associated viral infections.
We aimed to describe the seroprevalence of HBV and HCV infections in a Mexican transfusion center and to do an exploratory comparison of the performance of the standard immunoassay-based screening tests for both viruses with that of NAT, in blood donors.
MethodsDesign, data source and sampleThis study was done at the Department of Transfusion Medicine and the Department of Molecular Biology in Medicine of the Hospital Civil de Guadalajara “Fray Antonio Alcalde”, which serves to urban and rural lowincome populations from the West of Mexico, usually lacking of health-care insurance. From January 1999 to March 2005, a total of 94,806 blood donors (aged 18 to 65 years) were screened for anti-HCV antibodies and for HBsAg, among other serologic markers for blood-borne infectious agents. Also, a period of molecular screening in March 2001 was conducted on 100 consecutive blood donors to detect HCV RNA and HBV DNA by homemade PCR techniques. Molecular screening (i.e., NAT) was done on approved candidates for blood donation, on the basis of a structured questionnaire regarding risk factors, as well as self-exclusion strategies. Researchers who performed NAT were not aware of the serologic status or any personal or clinical information of the blood donors, until completion of the molecular analysis of all samples. Also, no sera pooling was done for NAT.
The internal Committee of Ethics of our hospital approved the present work. Informed consent was obtained from the donors in all cases.
Detection of anti-HCV antibodies and of HBsAgAn automated third-generation microparticle enzyme immunoassay (MEIA, AxSYM HCV Version 3.0 Abbott Diagnostics, Chicago, IL, USA) was used to detect anti-HCV antibodies in all sera samples. The signal strength of immunoassay (i.e., fluorescence) of the sample is divided by that of the internal cut-off rate (S/CO) of each sample. This is a semi-quantitative assay in which S/CO value is directly proportional to the quantity of antibodies directed to HCV.5-7 S/CO ratio >1 is considered a positive result, according to the manufacturer’s instructions. Similarly, detection of HBsAg was done by using an automated third-generation microparticle enzyme immu-noassay (MEIA, AxSYM HBsAg Version 3.0 Abbott Diagnostics, Chicago, IL, USA). This is also a semi-quantitative assay in which S/CO value is calculated. S/CO is directly proportional to the quantity of HBsAg.8 For HBsAg, S/CO ratio >2 is considered a positive result, according to the manufacturer.
Detection of HBV DNA in serumFor HBV DNA assessment, a home-made qualitative nested PCR was performed. An aliquot of 200 μL of sera samples were obtained to isolate viral DNA using the QIAmp Blood Kit (QIAGEN, Chatsworth, CA). HBV DNA was amplified by standardized first-round and nested PCR of S-gene fragment using the primers and conditions described previously.10 These primers have a molecular specificity of 100% for all strains of HBV, as assessed by DNA sequencing and alignment.10 PCR assay was practiced per duplicate systematically in all sera samples. Cases with detectable HBV DNA in serum were confirmed in a third PCR assay, in order to adjudicate a confirmed diagnosis. Appropriate measures were taken to minimize the risk of sample cross-contamination. These measures comprised the inclusion of serum samples from normal subjects and aliquots of water as negative controls. A positive control of a HBV genotype A strain was always included from the extraction of nucleic acids. This home-made PCR has a detection limit of 10 copies per mL, as determined by in-house dilution experiments, from nucleic acids extraction to amplification (data not published).
Detection of HCV RNA in serumA home-made qualitative nested reverse-transcription polymerase chain reaction (RT-PCR) was used to detect HCV RNA in sera samples of the 100 donors of the molecular screening cohort. Total RNA was extracted from each serum without pooling, using QIAamp Viral RNA Mini Kit (QIAGEN, Chatsworth, CA) as indicated the manufacturer. Afterwards, RT was carried out to obtain complementary DNA (cDNA) using M-MLV RT kit (MMLV, GIBCO/BRL). PCR amplification of cDNA and later a nested-PCR were performed with two pairs of primers that hybridize in a segment of the 5' non-coding region of HCV genome, as is described elsewhere.9 Positive sera controls obtained with this technique and confirmed to be specific for HCV by nucleic acids sequencing and alignment of amplified products were always introduced from RNA extraction to cDNA amplification. This qualitative nested RT-PCR was performed per duplicate systematically in all samples and the products of the reaction were analyzed in gel electrophoresis. This home-made RT-PCR has a detection limit of <100 copies per mL, as assessed by in-house experiments (data not published). The criterion for adjudication of a confirmed diagnosis and the measures taken to minimize the risk of cross-contamination were the same as in the assessment of the presence of HBV DNA.
Data analysisThe frequency of positive results in serologic and molecular assessments is provided with the respective 95% confidence interval (CI, calculated with the modified Wald method). Pearson chi-square test was used to compare the frequency of seroreactive results obtained by immunoassay and molecular methods and to assess the annual trend of the serologic frequency for HBsAg and anti-HCV antibodies, from January 1999 through December 2004. A p < 0.05 was considered statistically significant.
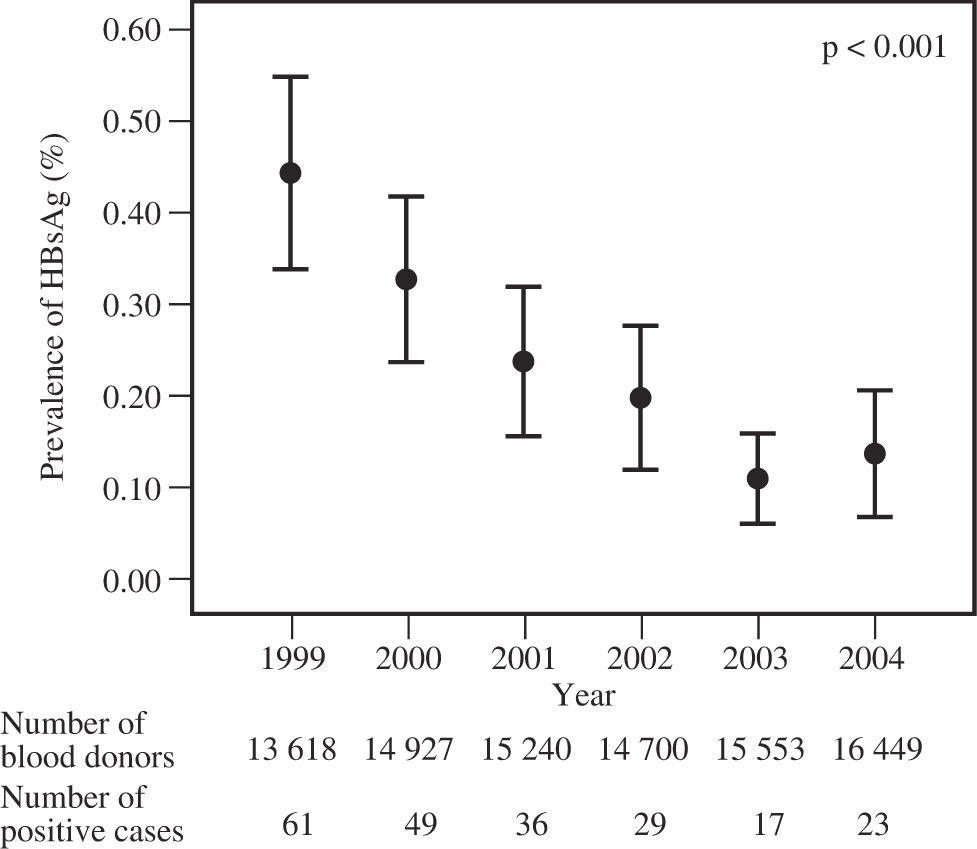
ResultsIn the 75-month period of serologic screening, a total of 94,806 blood donors were assessed for the presence of HBsAg and anti-HCV antibodies by the standard immunoassay techniques. A total of 219 persons tested positive to HBsAg (0.23%; 95% CI, 0.20 – 0.26%) and 922 to anti-HCV antibodies (0.97%; 95% CI, 0.90 – 1.03%). The annual frequency of the presence of HBsAg in blood donors clearly demonstrated a decreasing tendency (p for trend < 0.001) (Figure 1), whereas that for anti-HCV antibodies behaved relatively constant (p for trend = 0.19) (Figure 2).
In the period of molecular screening, a total of 100 consecutive blood donors were assessed for the presence of HBV DNA and HCV RNA by NAT in serum. None of these blood donors tested positive to HBsAg or anti-HCV antibodies. HBV DNA was detected in one person (1%; 95% CI, 0 – 6%) and HCV RNA in another case (1%; 95% CI, 0 – 6%). Therefore, the frequency of positive results for HBV and HCV infections did not differ when comparing the frequency of HBsAg and anti-HCV antibodies with that of HBV DNA and HCV RNA, respectively. However, and very important from the clinical and epidemiological perspective, NAT and immunoassay methods did not coincide in detecting neither HBV nor HCV infections in the persons who tested positive to viral nucleic acids. Hence, seronegative persons (i.e., with false-negative markers for infection) were detected by NAT, in the cohort of molecular screening. Since the two blood donors who tested positive to NAT came from rural areas, they could not be located to reassess their serologic and molecular status. Fortunately, the contaminated blood units were not transfused.
DiscussionWe found that seroprevalence of HBV infection (as assessed by the presence of HBsAg) among blood donors from our center is having a decreasing pattern. This may be due to the success in strategies aimed to minimize HBV acquisition in Mexican blood banks and in practice of sanitary personnel (i.e., dentists and surgeons) since the 1980’s. On the other hand, seroprevalence of HCV infection (as assessed by anti-HCV antibodies) have not changed in the last years, possibly reflecting the fact that most persons currently infected with HCV in Mexico acquired the infection by transfusion before the systematic screening in blood banks,7,9 strategy that was introduced in relatively recent years.3
Taking into account the limitation of a small cohort that received NAT assessment, we also found that serologic and molecular screenings do not always coincide in detecting HBV and HCV infections, and that NAT can detect cases that serologic approach can not, a very important issue that should change the way low-income countries are facing the problems of blood-borne infections. Furthermore, even when the risk for acquisition of HBV and HCV infections from a blood donation is markedly lower than previous years, today the possibility is not reduced to zero.11-13 Since the evolution of infectious diseases is a very dynamic process, the recent experience on HIV and HCV pandemics gave us the knowledge that transfusions need to be applied when undoubtedly indicated and that highly-effective preventive strategies should be applied, even when we apparently have safer blood units in the context of current blood-borne infectious diseases (but possibly not in that of future emerging infectious diseases).
There is no doubt regarding the superiority of NAT over the immunoassay techniques in detecting bloodborne viral hepatitis,5-7,12 but apparently the discussion is whether NAT is cost-effective in low-income countries with low prevalence of such infections. However, the best strategy among several approaches should be evaluated. For instance, minipooling of sera samples reduces the number of NAT assays to be performed, and it has resulted in a very efficient way to detect viral genomes in serum. Moreover, available semi-automated techniques reduce the need of highly-specialized personnel and the time it takes to provide a definite test result. With time, less expensive chemicals and devices will arise to market, facilitating even more these processes.
Given the exploratory nature of this study, data reported here should be interpreted with caution. More studies sufficiently powered to demonstrate that NAT should complement the screening of blood donations for HBV and HCV in Mexican blood banks are necessary. Moreover, although very cautious measures were taken to avoid errors, we used a home-made NAT which are plenty of steps that can be susceptible of technical mistakes in a large-scale work in blood banks. Possibly an automated method of nucleic acids extraction and amplification is the best option for this objective.
In conclusion, NAT can detect cases of HBV and HCV infections that standard immunoassays can not, even in blood donors, a highly selected population at low risk. NAT should receive more study in order to be considered as a systematic method to detect prevalent viral bloodborne infectious agents in Mexican blood banks. Therefore, large-scale technical and cost-effective studies are warranted.
AcknowledgmentsWe are indebted to the personnel of Blood Bank and Transfusion Medicine for their friendship and commitment with an excellent work in caring for the suffering.
This work was supported by a grant from The National Council of Science and Technology, Mexico, to Dr. Arturo Panduro (Salud 2004-C01-025, CONACyT).