Alveolar echinococcosis of the liver can be mistaken as a liver tumor. The occurrence of the fox tapeworm echinococcus multilocularis is increasing in formerly unaffected European regions. As a consequence, alveolar echinococcosis is becoming an important differential diagnosis in Eastern and Northern Europe.
Alveolar echinococcosis (AE) is a rare parasitic disease that is caused by the fox tapeworm Echinococcus multilocularis (EM). The zoonosis has intermediate (rodents and small lagomorphs) and final hosts (carnivores, especially foxes). The latter excrete the eggs of the tapeworm with the feaces. Because of their intrinsic mobility the eggs are able to contaminate the forest soil. Humans become infected when eating uncleaned forest mushrooms or fruits contaminated with the eggs. Humans are accidental hosts (Figure 1). Because two host species are needed for development of the larvae, transmission between humans is not possible.
Incubation time in humans is extremely long and can take 5 to 15 years. The infection remains asymptomatic for a long period and the mean age at diagnosis is 50 years. In humans the primary manifestation is located in the liver in almost all cases. The liver lesion is a solid mass consisting of alveolar structure, marked granulomatosis and reactive fibrosis. The infection resembles a slowly growing malignant tumor displaying invasion of the liver parenchyma, bile ducts and blood vessels. Hematogenous spreading to other organs including the lung and the brain is common.1
AE occurs only in the northern hemisphere.2 The areas of affected animals and humans are not identical. Endemic areas in Europe are the South-East of France, Northern Switzerland and the South of Germany with recent extension to the East and North of Europe.3
Case ReportA 50-year-old female patient presented with dyspnea on exertion that she had first noticed 2 years ago. She also reported suffering from a dull pressure in the right flank and from night sweats for six months. On examination, she had diminished breath sounds over the right lower chest. Besides that the physical examination was unremarkable. Blood tests showed slightly elevated GGT and total bilirubin levels. The midstrain urine and an ECG were normal. A chest X-ray did not reveal any pathological finding.
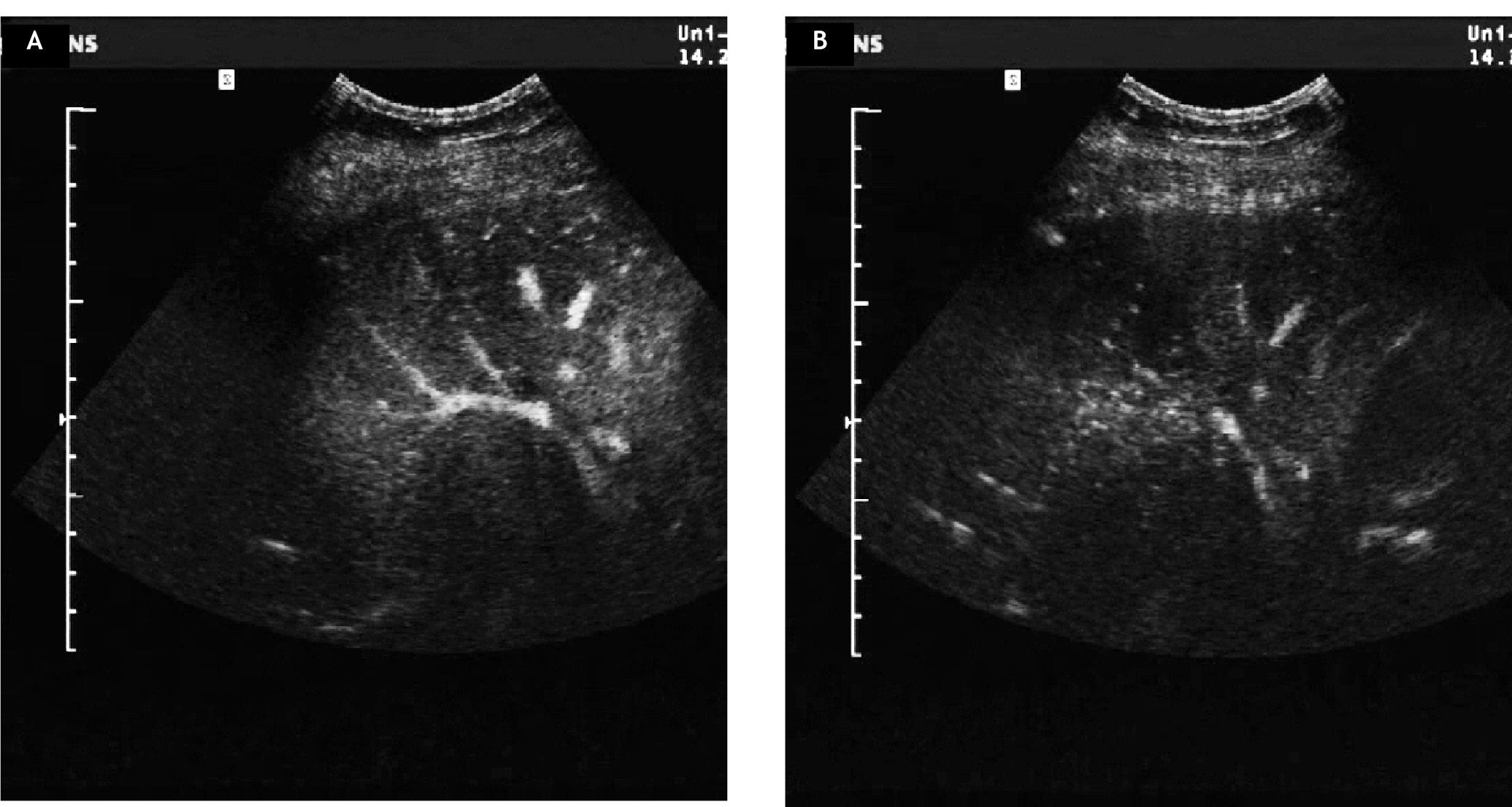
An abdominal ultrasound was performed and revealed a hyperdense mass next to the portal vein measuring 8 cm in diameter. The tumour encased the right liver vein and dislodged and compressed the middle liver vein; the left liver vein was not affected (Figure 2). Contrast-enhanced ultrasound could not detect any enhancement of the mass in the arterial phase and a faint enhancement in the late phase (Figure 3). CT-scan confirmed an inhomogeneous lesion in the liver that was deemed inoperable due to the encasement of the liver veins’ caval orifice and the proximity to the portal vein. Because metastasis of a malignant tumor was suspected a gastrointestinal and gynecological primary tumor was ruled out. Eventually, a biopsy was taken from the liver lesion. Histological examination showed only little liver tissue but a thin membrane structure with minimal germinal layer typical for AE (Figure 4). Furthermore, one side of the germinal layer was surrounded by a granulomatous inflammation and sclerosing connective tissue. Protoscolices, necrosis or calcification were not found. Indirect hemagglutination detected a high antibody titre for EM. The infection was confirmed by Western blot. To enable follow-up of disease activity a Fluordes-oxyglucose-PET (FDG-PET) scan of the liver was done and revealed elevated glucose metabolism in the area of the AE hydatide which is in accordance with active AE tissue. The remaining liver tissue showed an inhomogenous distribution of glucose metabolism with small areas of increased metabolic activity consistent with additional active AE areas (Figure 5).
A diagnostic biopsy was done because of a suspected metastasis of a carcinoma of unknown origin. Histological examination showed a rope ladder like germinal layer on the cyst like inner membrane-typical for AE. The surrounding connective tissue showed a granulomatous inflammation and sclerosing process.
A and B. Coronal, sagittal and transversal 18-F-FDG-PET slices. The images show a large photopenic lesion in approx. 5th segment of the liver with significant uptake of the radiotracer mostly at the ventrolateral margins (SUVmax = 6,71), corresponding most probably to a still active disease.
Therapy with albendazole at a dose of 400 mg twice a day was initiated. At follow-up 6 months after initiation of the therapy the clinical condition, blood count and liver enzymes were within normal limits. Ultrasonography of the abdomen demonstrated the known AE hydatide in unchanged size.
DiscussionAn infection with EM remains silent for many years. Once a liver lesion has developed, unspecific symptoms may still disguise the correct diagnosis. Due to the long latency period, the patient usually does not recollect an exposition to potentially contaminated food. Therefore, a liver lesion detected by a routine or diagnostic abdominal ultrasound is often the first sign of the disease. However, imaging procedures give unspecific results. A solid mass in the liver can be mistaken for a much more common secondary or primary liver malignancy. Diagnostic hints like a central necrosis, calcification or small peripheral cysts are unspecific. Vascular flow or contrast enhancement is usually not observed.4 Since AE shows an intensive uptake of glucose, an FDG-PET can be used for diagnosis and follow-up of AE patients during benzimidazole therapy. The activity of the disease directly correlates with the pathological storage of FDG.5
Taking a biopsy like in this case is not a routine diagnostic procedure. It is an option when other diagnostic tests are inconclusive and especially when a malignancy is suspected although there is a low risk of complications (anaphylaxis or iatrogenic spread of infection). The most important diagnostic method in combination with imaging studies is serology.6,7 Without doubt, serodiagnosis for EM should be primarily performed if the disease is suspected.
When considering the differential diagnosis of a liver mass it should be kept in mind that the geographic pattern of EM in Europe has shifted. Additional endemic areas have been identified and a spread to urban and suburban areas in central Europe has taken place.3 Genetic studies of the fox tapeworm in various European regions suggest that the central core of EM is located in Switzerland and Jura Swabe. The transmission of the parasite into distant regions can be described by a “mainland-island” system, in which the parasite can subsequently disseminate under spatial separation from the original focus. Moreover, considering the presence of similar genetic profiles between regions a founder event is likely.8 Trials to counteract the spread of EM have been performed by application of anthelminthic baits to foxes.9 However, the success of such interventions on a supraregional scale remains uncertain.
A surgical resection is the mainstay of treatment, whenever a curative resection with a safety margin is attainable. In the largest cohort published until now a curative resection was possible in 42%. In curatively resected patients the relapse rate after a median follow-up of more than 10 years was 11%.10 The majority of patients has unresectable disease, however, and the benefit of palliative surgery is uncertain due to the favourable long-term results of anthelminthic treatment alone. Liver transplantation is outdated because of a high rate of relapses under the necessary immunosuppressive therapy.11
More than 90% of untreated patients will die within the first ten years.12 Since the introduction of long term therapy with albendazole the poor prognosis improved significantly. Now the ten year survival rate is about 75 to 90%.1,13 Nevertheless, late lethality 13 to 28 years after diagnosis has been described in 28% of patients.10 It is important to monitor therapy by 3-monthly imaging procedures and at least for 10 years. Relapses are described as long as 15 years after remission. Serologic titers are also useful for monitoring.7
The present case highlights the importance of this rare but possibly increasing zoonotic disease in Europe. Due to imaging characteristics that can mimick malignant liver tumors it is important to take AE into account as a differential diagnosis in unexplained liver lesions. Radical liver resection should be performed when possible. The poor prognosis in the palliative situation has significantly improved with the introduction of long term albendazole therapy.
AcknowledgmentsWe thank T. Gradistanac for providing the histological figures.
No Financial Support Was ReceivedThe authors declare that they do not have anything to disclose regarding conflict of interest with respect to this manuscript.