Background and aims. A subclassification system for intermediate hepatocellular carcinoma (HCC) was recently proposed to optimize treatment allocation. The aim of this study was to assess the prognostic ability of that substaging proposal.
Patients and methods. This is a retrospective multicenter cohort study including patients with intermediate HCC treated with transarterial chemoembolization (TACE). Predictors of survival were identified using the Cox proportional regression model.
Results. 289 Barcelona Clinic Liver Cancer (BCLC) B patients were included. Median overall survival of the whole cohort was 23 months (C.I. 95% 20.2– 25.8). Child A status (H.R. 1.35, C.I. 95% 1.02–1.78) and tumour burden beyond the up-to-seven criterion (H.R. 1.39, C.I. 95% 1.07– 1.80) were independent prognostic factors for overall survival on multivariate analysis. Analysis of the substages showed that median survival was 33.0 months for B1 stage (n = 81), 20.8 months for B2 stage (n = 106), 16.1 months for B3 stage (n = 24), 22.2 months for B4 stage (n = 42) and 15.0 months for quasi-C stage (n = 36). Regarding the discriminatory ability of the substaging proposal, the log rank test showed a significant survival difference for B1 vs. B4 (p = 0.003) and B1 vs. Quasi-C (p = 0.039) and a trend for B1 vs. B2 (p = 0.05) and B1 vs. B3 (p = 0.05).
Conclusions. Apart from substage B1, BCLC-B subclassification does not discriminate perfectly patients treated with TACE. Also some patients in substage B4 can benefit from TACE.
Globally, hepatocellular carcinoma (HCC) is the second most frequent cause of cancer mortality in men and the leading cause of death among patients with liver cirrhosis.1,2 The prognosis of HCC and treatment allocation are influenced not only by tumour burden, but also by the degree of liver function impairment.3 Currently, the Barcelona Clinic Liver Cancer (BCLC) staging system is the standard recommended by the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL) guidelines.4,5 The BCLC staging system defines the intermediate stage as multinodular tumour with relatively preserved liver function and absence of vascular invasion or cancer-related symptoms and proposed transarterial chemoembolization (TACE) as first-line treatment.6,7 In the real world, patients with HCC treated with TACE represent a prognostically heterogeneous population: median survival has been reported to range from 14 months to 48 months according to our own experience and that of others.8–11 Many studies have reported patient-, tumour- and treatment-related characteristics associated with better survival after TACE, but results from individual studies are sometimes conflicting.12 An important focus of research is the ability to distinguish patients who will benefit from TACE compared to other treatment options such as radioembolization, transplantation and sorafenib.13 In 2012, a panel of experts proposed a subclassification system for intermediate HCC in order to optimize treatment allocation.14 The proposed subclassification has been applied to Asian and European cohorts of treated patients with no definitive validation.15–17 Giannini, et al. confirmed the prognostic efficacy of this subclassification in untreated patients enrolled in the ITA.LI.CA. cohort.18 The aim of the current study was to assess the prognostic ability of BCLC-B subclassification in intermediate HCC patients from different tertiary centres who were treated with TACE.
Patients and MethodsThis is a retrospective multicenter cohort study performed in four teaching hospitals in Italy (Rome Gemelli, Rome Sapienza, Milan, Palermo). Recruiting period is included between January 1, 1997, and December 31, 2012. Follow-up ended on December 31, 2013. Eligibility criteria include patients with HCC in the intermediate stage treated with their first TACE procedure. HCC was diagnosed by pathology or by non-invasive criteria according to Barcelona criteria until 200519 and subsequently according to AASLD guidelines.20 Intermediate stage was defined as multinodular tumour with Child-Pugh score A or B21 and absence of vascular invasion or cancer-related symptoms according to BCLC staging system.4,5
Clinical charts were retrospectively reviewed in order to collected pre-treatment data. Follow up was carried out by telephone interview to determine date of death if more than 3 months had elapsed since the last follow-up visit and death did not occur in our hospitals or was not reported by the family.
The endpoint of the study is overall survival; according to Panel Experts in HCC,22 cancer-specific survival is considered a more subjective endpoint than overall survival, because it can be difficult to accurately assign the cause of death in HCC to tumor progression rather than liver failure or treatment-related toxicity.
Variables collected include demographic details, performance status (PS) according to Eastern Cooperative Oncology Group (ECOG),23 aetiology of liver disease, biochemical data, haematological data, assessment of hepatic function based on Child-Pugh score, Model for End-stage Liver Disease (MELD) score,24 and possible previous treatment. Hepatitis C and B were diagnosed by detecting antibodies to hepatitis C virus (HCV) and serum hepatitis B surface antigen (HbsAg) respectively, through standardized tests. Alcoholic liver disease was defined by alcohol consumption of more than 80 g/d in men and 60 g/d in women for > 10 years. The presence of underlying cirrhosis was assessed histologically or based on clinical and blood chemistry findings indicative of chronic liver disease together with evidence of portal hypertension (defined by the presence of at least one of the following: thrombocytopenia < 100,000/mm3, gastro-oesophageal varices at endoscopy or spleen diameter > 12 cm on ultrasound). Ascites was classified according to the European Association for the Study of the Liver (EASL) classification.25 Extra-hepatic disease was assessed using abdominal multiphasic Computed Tomography (CT) or Magnetic Resonance Imaging (MRI) and chest radiography. Portal vein thrombosis was classified as non-tumour on the basis of the lack of contrast enhancement in the arterial phase. Bone metastases were sought using scintigraphy, if clinically suspected. Patients were either not candidates for resection or ablation, or had failed/recurred after resection/ ablation, and were receiving conventional TACE or TACE with drug eluting beads (DEB) at the discretion of the clinical centre. Details of the TACE procedure for each centre are described elsewhere.9,26–28 About one month after TACE, patients underwent a multiphasic CT scan. TACE was repeated on demand, at least 2 months after the first procedure, in patients with evidence of viable tumour persistence, as defined by the amended RECIST criteria.19 TACE was discontinued whenever vascular contraindications, poor hepatic function, severe adverse effects, progressive disease with vascular involvement or extrahepatic metastases developed. None of this cohort was treated with sorafenib. Patients who underwent Orthotopic Liver Transplantation (OLT) after TACE were excluded from the analysis.
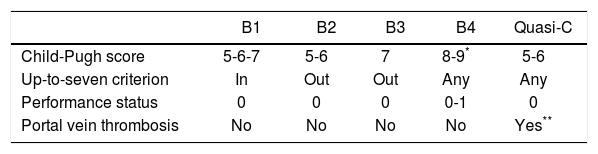
Patients were classified according to the BCLC-B subclassification proposal14 on the basis of the Child-Pugh score, tumour burden according to the up-to-seven criterion,29 ECOG PS and presence of portal vein thrombosis. B1 stage includes patients with tumor burdens within up-to-7 criterion and Child-Pugh A and B7. B2 stage includes patients with tumor burded exceeding up-to-7 criterion and Child-Pugh A only. B3 stage includes patients with tumor burdens exceeding up-to-7 criterion and ChildPugh B7. B4 stage comprises patients with decompensated liver cirrhosis (Child Pugh B8-9) or symptomatic disease. Quasi-C stage includes patients with Child Pugh A and segmentary or subsegmentary portal vein thrombosis (Table 1).
This subclassification proposal includes in the intermediate stage some patients classified in other stages in the original BCLC staging system30 such as patients with single nodule > 5 cm that should be classified as BCLC A while patients with performance status = 1 should be classified as BCLC C.
We reanalysed the whole cohort after excluding these cases of controversial allocation.
The sample size calculations were based on the following assumptions: sensitivity: 95%; power: 80%; overall survival: 40%; estimated Hazard Ratio for BCLC-B1 compared to BCLC-quasi C: 1.5. Based on these assumptions we estimated that we needed to enroll at least 275 patients.
The Kolmogorov Smirnov test was used to assess the distribution of all the continuous variables. Continuous data were expressed as the median [interquartile range (IQR)]. Overall survival was calculated using the Kaplan-Meier function and expressed as median and 95% Confidence Interval. Survival curves for each substage were calculated using the Kaplan-Meier method and compared using the log rank test. A univariate analysis using the Kaplan-Meier method of survival function was performed to identify baseline demographic, clinical, biochemical and radiological predictors of survival at the time of the first TACE procedure. For the multivariate analysis a Cox proportional regression model was used. We used the empirical rule of thumb that Cox models should be used with a minimum of 10 events per predictor variable (EPV), the so called Peduzzi Method.31,32 Continuous variables were transformed into dichotomous ones using median values as the cut-off values. Variables with an alpha less than 0.25 at the univariate analysis were included in a backward regression model to identify independent predictors of survival. The results of this regression analysis are presented as Hazard Ratio (HR) and 95% Confidence Intervals (95% CI). The proportional hazard assumption was checked graphically using -ln-ln curves. All statistical analyses were performed using SPSS version 19.0 (SPSS Inc., Chicago, IL).
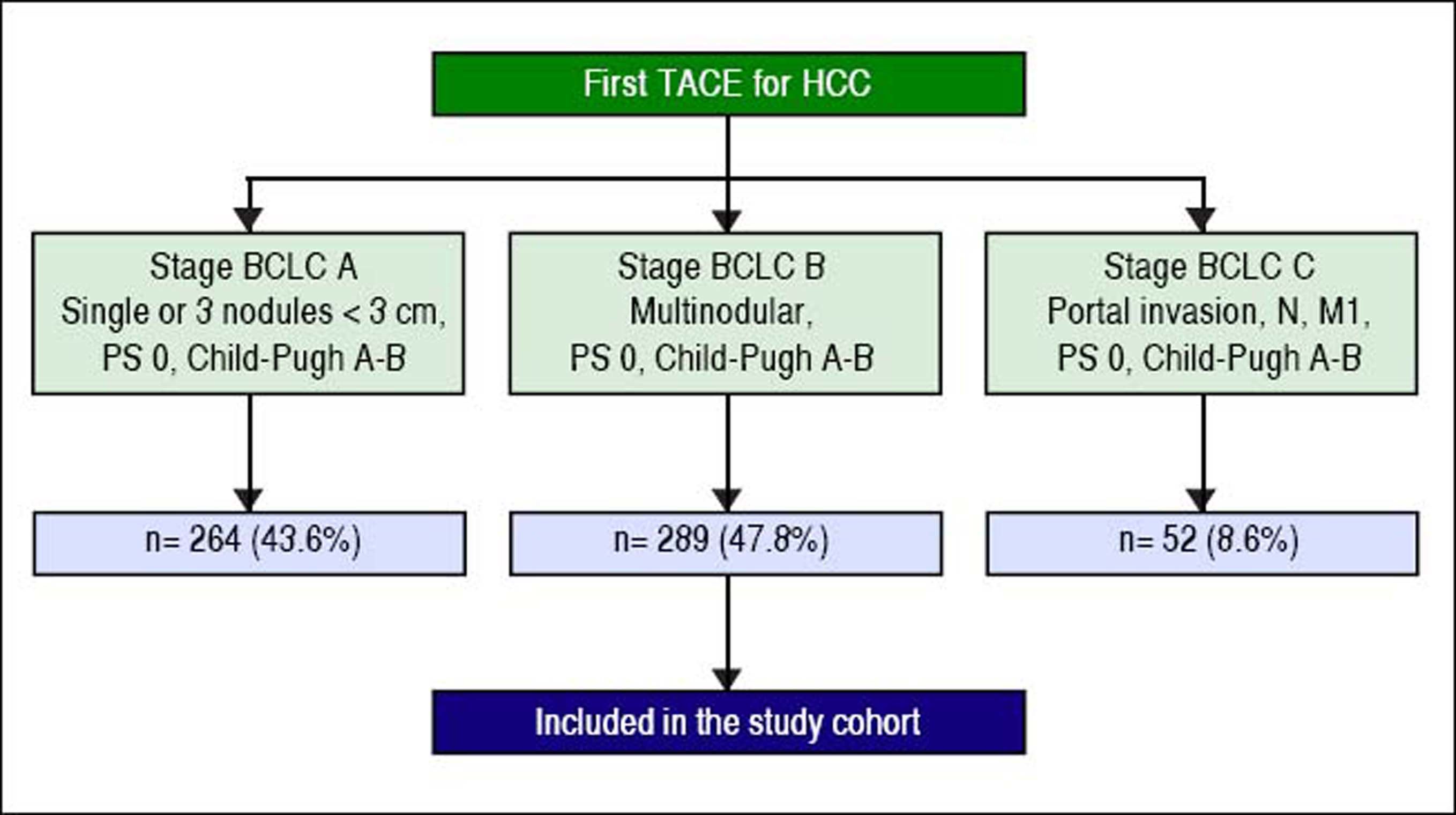
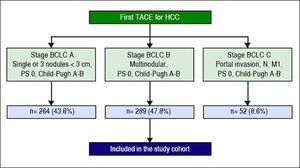
ResultsPatient characteristicsA total of 605 patients with HCC were treated with the first TACE procedure during the abovementioned period. 43.6% (n = 264) presented in BCLC stage A, 47.8% (n = 289) in stage BCLC-B (whole cohort) and 8.6% (n = 52) in BCLC-C (Figure 1). Two hundred and twenty-eight fit the restricted criteria for BCLC-B subclassification (restrict BCLC-B cohort). Table 2 shows the demographic, clinical, and tumour information for all patients. The continuous variables were not normally distributed. The majority of the patients were men (78%); the median age was 68 ± 12 years and only 14 (5%) showed cancer-related symptoms. Almost all of them (99%) had cirrhosis, the most common cause being hepatitis C virus (54%). Two hundred and eight (72%) patients were Child-Pugh class A; the median MELD score was 9 ± 3. Thirty-seven (13%) had non-tumour segmental portal vein thrombosis. One hundred and seventy-seven (61%) patients had a tumour burden beyond the up-to-seven criterion. Two hundred and twenty-five (78%) patients had never been treated with any other treatment for HCC; 70 (24%) patients had received DEB-TACE. Median Alpha-Fetoprotein (AFP) level was 22 ± 174.5 ng/mL.
Baseline predictors of survival in 289 patients with BCLC-B hepatocellular carcinoma at time of first TACE.
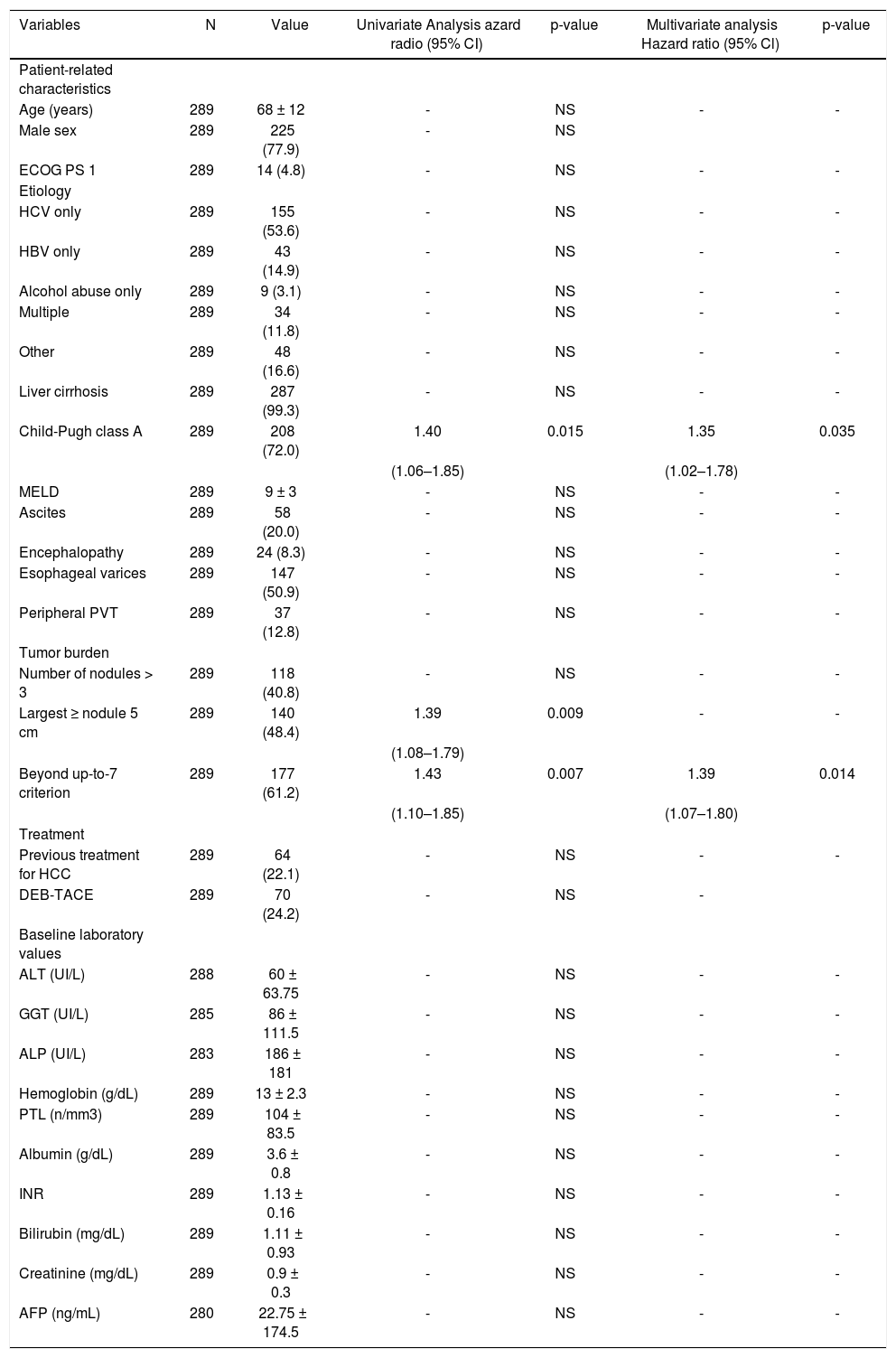
| Variables | N | Value | Univariate Analysis azard radio (95% CI) | p-value | Multivariate analysis Hazard ratio (95% Cl) | p-value |
|---|---|---|---|---|---|---|
| Patient-related characteristics | ||||||
| Age (years) | 289 | 68 ± 12 | - | NS | - | - |
| Male sex | 289 | 225 (77.9) | - | NS | ||
| ECOG PS 1 | 289 | 14 (4.8) | - | NS | - | - |
| Etiology | ||||||
| HCV only | 289 | 155 (53.6) | - | NS | - | - |
| HBV only | 289 | 43 (14.9) | - | NS | - | - |
| Alcohol abuse only | 289 | 9 (3.1) | - | NS | - | - |
| Multiple | 289 | 34 (11.8) | - | NS | - | - |
| Other | 289 | 48 (16.6) | - | NS | - | - |
| Liver cirrhosis | 289 | 287 (99.3) | - | NS | - | - |
| Child-Pugh class A | 289 | 208 (72.0) | 1.40 | 0.015 | 1.35 | 0.035 |
| (1.06–1.85) | (1.02–1.78) | |||||
| MELD | 289 | 9 ± 3 | - | NS | - | - |
| Ascites | 289 | 58 (20.0) | - | NS | - | - |
| Encephalopathy | 289 | 24 (8.3) | - | NS | - | - |
| Esophageal varices | 289 | 147 (50.9) | - | NS | - | - |
| Peripheral PVT | 289 | 37 (12.8) | - | NS | - | - |
| Tumor burden | ||||||
| Number of nodules > 3 | 289 | 118 (40.8) | - | NS | - | - |
| Largest ≥ nodule 5 cm | 289 | 140 (48.4) | 1.39 | 0.009 | - | - |
| (1.08–1.79) | ||||||
| Beyond up-to-7 criterion | 289 | 177 (61.2) | 1.43 | 0.007 | 1.39 | 0.014 |
| (1.10–1.85) | (1.07–1.80) | |||||
| Treatment | ||||||
| Previous treatment for HCC | 289 | 64 (22.1) | - | NS | - | - |
| DEB-TACE | 289 | 70 (24.2) | - | NS | - | |
| Baseline laboratory values | ||||||
| ALT (UI/L) | 288 | 60 ± 63.75 | - | NS | - | - |
| GGT (UI/L) | 285 | 86 ± 111.5 | - | NS | - | - |
| ALP (UI/L) | 283 | 186 ± 181 | - | NS | - | - |
| Hemoglobin (g/dL) | 289 | 13 ± 2.3 | - | NS | - | - |
| PTL (n/mm3) | 289 | 104 ± 83.5 | - | NS | - | - |
| Albumin (g/dL) | 289 | 3.6 ± 0.8 | - | NS | - | - |
| INR | 289 | 1.13 ± 0.16 | - | NS | - | - |
| Bilirubin (mg/dL) | 289 | 1.11 ± 0.93 | - | NS | - | - |
| Creatinine (mg/dL) | 289 | 0.9 ± 0.3 | - | NS | - | - |
| AFP (ng/mL) | 280 | 22.75 ± 174.5 | - | NS | - | - |
Data expressed as patients number (%) or median ± IQR. Univariate analysis by Kaplan-Meier method of survival function. Multivariate analysis by backward stepwise cox regression. For continuous variables, median values were used to determine the cut-off. HCC: Hepatocellular carcinoma. TACE: Transarterial chemoembolization. DEB: Drug-eluting beads. MELD: Model for End-Stage Liver Disease. ECOG: Eastern Cooperative Oncology Group. PS: Performace Status. PTV: Portal Vein Thrombosis. HCV: Hepatitis C Virus. HBV: Hepatitis B Virus. ALT: Alanine Aminotrasferase. GGT: Gamma-Glutamyltrasferase. ALP: Alkaline Phosphatase. PTL: Platelet count. INR: International Normalized Ratio. AFP: Alphafetoprotein.
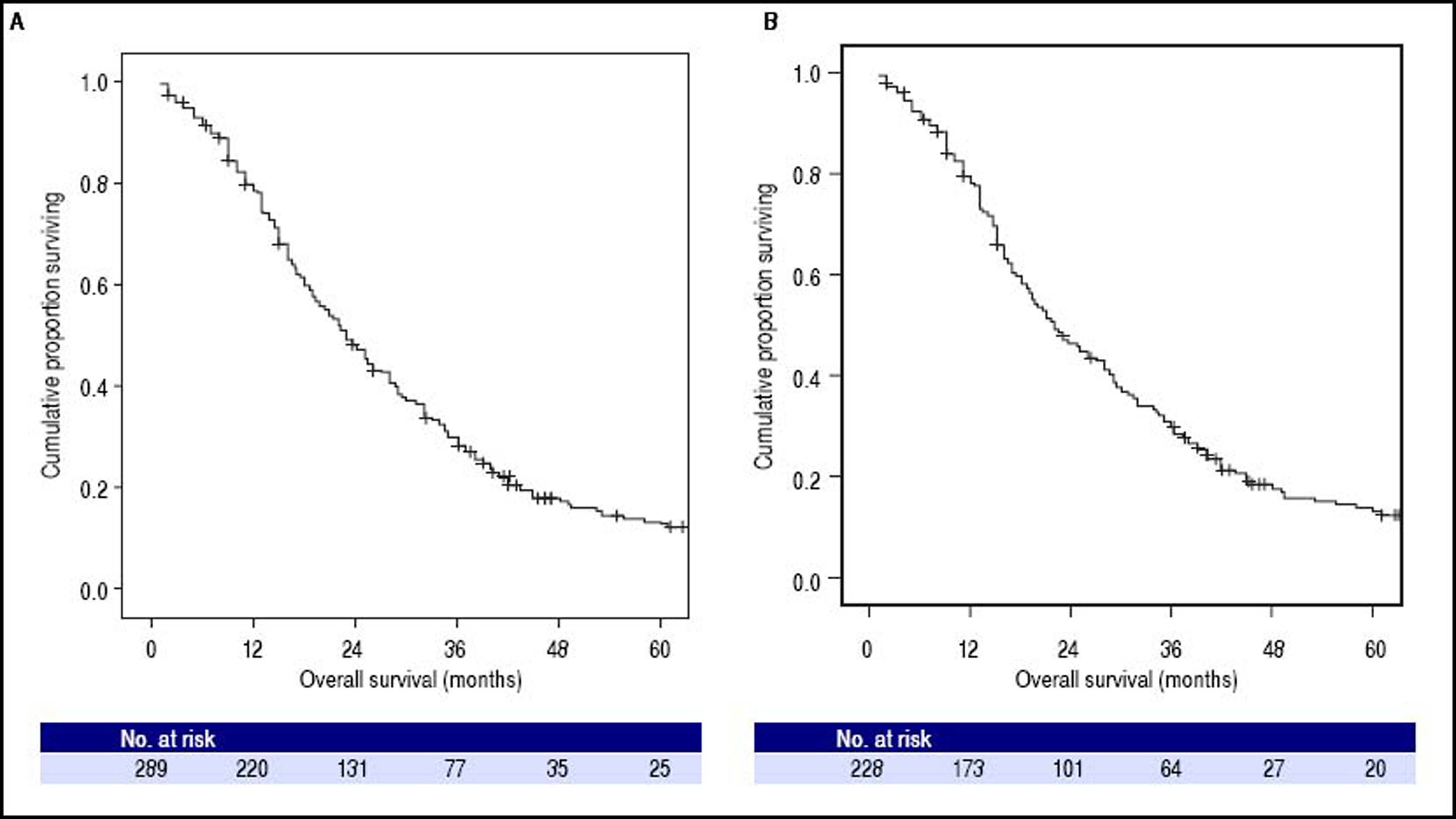
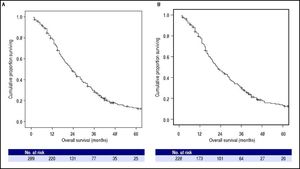
At the time of the present analysis, 247 (85%) patients had died. The overall median survival of the whole cohort was 23.0 months (95% CI: 20.2–25.8, Figure 2A) and the 1-, 2- and 3-year survival probability was 76%, 45% and 27% respectively. The Cox Proportional Hazard hypothesis was considered valid, since the curves look parallel.
Kaplan-Meier analysis of overall survival. A. Among whole cohort (289 patients), the median overall survival was 23.0 months (95%CI: 20.2–25.8). B. Among restrict BCLC-B cohort (228 patients), the median overall survival was 22.0 months (95%CI: 18.2-25.8); in this cohort patients with single nodule > 5 cm or performance status = 1, classified as BCLC A or C respectively according to origina BCLC proposal, were excluded.
The overall median survival of the restricted BCLC-B cohort was 22.0 months (95% CI: 18.2–25.8, Figure 2B) and the 1-, 2- and 3-year survival probability was 76%, 44% and 28% respectively.
Baseline predictors of survivalUnivariate analysis showed that Child-Pugh class, largest nodule diameter, and the up-to-seven criterion were significant baseline predictors of survival in HCC patients treated with TACE (Table 2). Cox regression analysis identified Child-Pugh class (p = 0.035) and the up-to-sev-en criterion (p = 0.014) as independent baseline predictors of survival for the entire cohort of HCC patients treated with TACE (Table 2). In the restricted cohort, the same variables were significantly associated with survival in univariate and multivariate analyses.
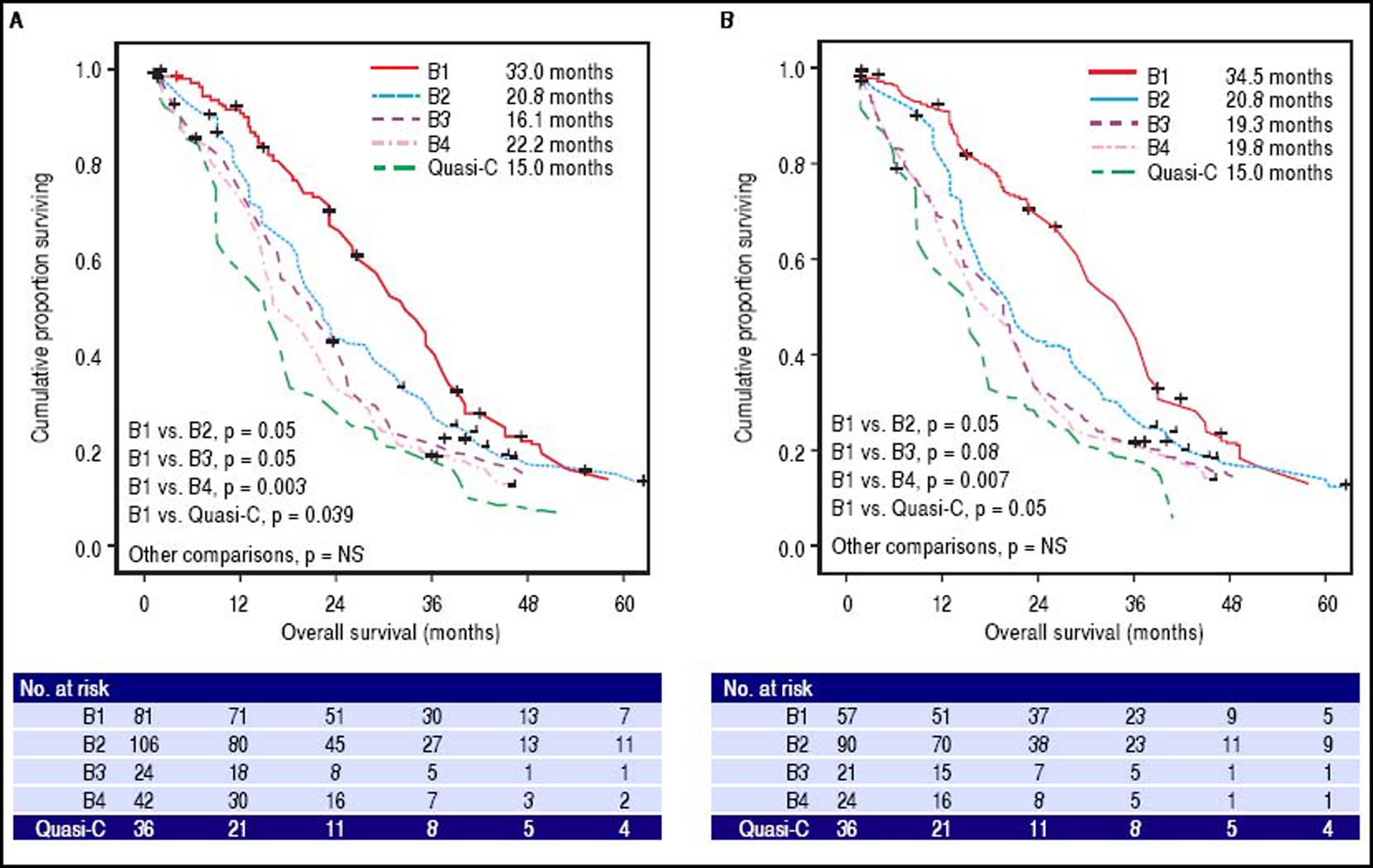
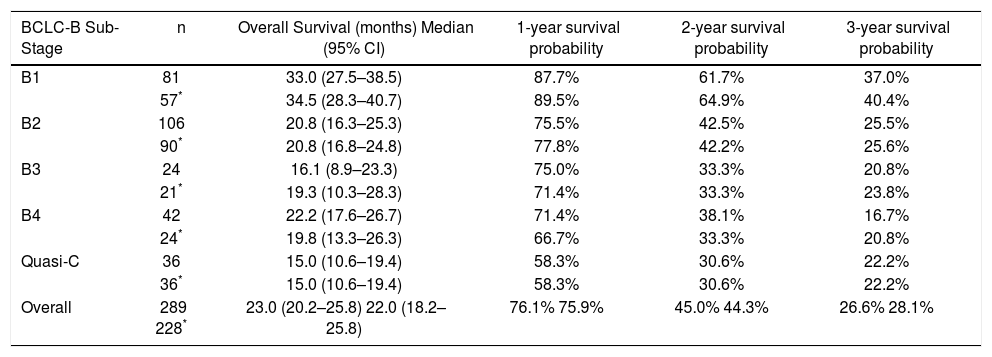
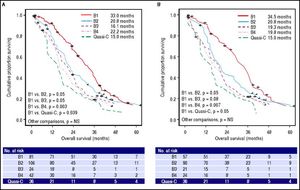
BCLC-B substage survivalSurvival curves according to the BCLC-B subclassification proposal are presented in table 3 and figure 3A for the whole cohort. Median survival was 33.0 months for B1 stage, 20.8 months for B2 stage, 16.1 months for B3 stage, 22.2 months for B4 stage and 15.0 months for quasi-C stage. Regarding the discriminatory ability of the substaging proposal, the log rank test showed a significant survival difference for B1 vs. B4 (p = 0.003) and B1 vs. quasi-C (p = 0.039) and a trend for B1 vs. B2 (p = 0.05) and B1 vs. B3 (p = 0.05).
Median survival times and survival rates of patients with BCLC intermediate hepatocellular carcinoma stratified in proposed substages.
| BCLC-B Sub-Stage | n | Overall Survival (months) Median (95% CI) | 1-year survival probability | 2-year survival probability | 3-year survival probability |
|---|---|---|---|---|---|
| B1 | 81 | 33.0 (27.5–38.5) | 87.7% | 61.7% | 37.0% |
| 57* | 34.5 (28.3–40.7) | 89.5% | 64.9% | 40.4% | |
| B2 | 106 | 20.8 (16.3–25.3) | 75.5% | 42.5% | 25.5% |
| 90* | 20.8 (16.8–24.8) | 77.8% | 42.2% | 25.6% | |
| B3 | 24 | 16.1 (8.9–23.3) | 75.0% | 33.3% | 20.8% |
| 21* | 19.3 (10.3–28.3) | 71.4% | 33.3% | 23.8% | |
| B4 | 42 | 22.2 (17.6–26.7) | 71.4% | 38.1% | 16.7% |
| 24* | 19.8 (13.3–26.3) | 66.7% | 33.3% | 20.8% | |
| Quasi-C | 36 | 15.0 (10.6–19.4) | 58.3% | 30.6% | 22.2% |
| 36* | 15.0 (10.6–19.4) | 58.3% | 30.6% | 22.2% | |
| Overall | 289 228* | 23.0 (20.2–25.8) 22.0 (18.2–25.8) | 76.1% 75.9% | 45.0% 44.3% | 26.6% 28.1% |
Survival probability according to BCLC-B subclassification. A. Among whole cohort (289 patients), results from log-rank test showed significant differences of B1vs.B4 (p = 0.003) and B1vs.Quasi-C (p = 0.039). B. Among restrict BCLC-B cohort (228 patients), results from log-rank test retained the significance only for B1vs.B4 (p = 0.007); in this cohort patients with single nodule > 5 cm or performance status =1, classified as BCLC A or C respectively according to origina BCLC proposal, were excluded.
Survival curves for the restricted BCLC-B cohort are presented in table 3 and figure 3B. Median survival was 34.5 months for B1 stage, 20.8 months for B2 stage, 19.3 months for B3 stage, 19.8 months for B4 stage and 15.0 months for quasi-C stage. Regarding the discriminatory ability of the substaging proposal, the log rank test showed a significant survival difference for B1 vs. B4 (p = 0.007) and a trend for B1 vs. B2 (p = 0.05), B1 vs. B3 (p = 0.08) and B1 vs. quasi-C (p = 0.05).
DiscussionThe purpose of this study was to analyze the prognostic ability of proposed BCLC-B subclassification14 in a multicenter Italian cohort of intermediate HCC patients treated with TACE. The main result of the present study was that the substage B1 showed significantly better survival than other substages, while when differences between stages from B2 to Quasi-C were analyzed, the prognostic power of the proposed B subclassification was lost. Three other studies have tried to validate the prognostic capacity of the BCLC-B subclassification proposal in HCC treated patients: two eastern cohorts (from Taiwan and Korea) that included only patients treated with TACE, like ours,15,16 and a German cohort that included all patients with intermediate HCC managed in the centre, regardless of the therapeutic allocation.17 All three studies confirmed the best survival for patients classified in substage B1 but failed to confirm the ability of the proposed subclassification14 to discriminate prognosis when other substages were considered. So while the Taiwan group suggested the addition of alpha-fe-toprotein,15 the Korean study proposed to merge of B3 and B4 to get a better prognostic predicition.16 The german study and the italian study on untreated patients also underline the need for a more refined assessment of liver function impairment through inclusion of MELD score.17,18
An interesting observation of our study is derived from the result of the surprising survival time of B4 subpopulation (22.2 and 19.8 months in the whole and restrict cohort respectively). Even in the above cited studies15–17 it can be observed that the B4 survival was quite better of the B3, but while in these studies the B4 subgroup size was small, in our study the B4 subgroup is large enough to allowing some considerations. According to the expert panel B4 patients should have been received only best supportive care.14 But in the real world we encounter relatively frequently patients with a Child-Pugh score B8-9 or PS = 1 that are sufficiently well compensated and may also bear a small tumour burden; not allocating them to a TACE programme (or possibly to a percutaneous treatment) would result in a severe under treatment, even outside the perspective of transplantation. In effect, the Bolondi subclassification for patients with intermediate HCC was created primarily with the aim of optimising treatment allocation and only secondarily has a prognostic classification.14 Recently, Kudo, et al. have proposed and validated Kinki criteria, a simplification of the BCLC-B subclassification in only three substages: B1 (Child-Pugh score 5–7 and within up-to-7), B2 (Child-Pugh score 5-7 and beyond up-to-7) and B3 (Child-Pugh score B8,9 and any tumor status).33,34
Child-Pugh class and the up-to-seven criterion are the cornerstones on which the proposed subclassification of intermediate HCC, also known as BCLC-B, was built.14 In our population, Child-Pugh class, a measure of liver function, and the up-to seven criterion, were independent baseline predictors of survival. Conversely, the presence of portal vein thrombosis and the performance status, that are also constructive elements of BCLC-B subclassification, were not independent baseline predictors of survival in our cohort. The quasi-C substage includes patients with undefined peripheral portal vein thrombosis, which were included in the advanced stage in the original classification.30 In our cohort, these patients have a survival comparable or even better than those treated with TACE in study of Luo, et al.35 and those treated with sorafenib in the SHARP study.36
Forner3 observed that the proposed subclassification includes in the intermediate stage either patients classified as early (patients with single nodule > 5 cm; candidates for surgery) either patients classified as advanced (patients with PS = 1; candidates, if possible, for oral therapy with sorafenib) in the original BCLC classification.30 To overcome this limitation, we conducted a separate analysis on a restricted cohort that excluded these cases of controversial allocation; the results of the restricted cohort reflected quite well the results obtained for the whole cohort.
Limitations of our study include its retrospective nature, the homogeneous treatment modality (meaning that all patients received TACE as treatment) and incomplete knowledge of the clinical history of patients from the time of TACE to death, especially with regard to time to progression, the onset of hepatic decompensation, and number of treatments performed after first TACE. Moreover, the external validity could be influenced by the fact we used the median of the continuous variables as a cutoff value for the univariate and multivariate analysis. The multicenter design and the high number of cases (85%) for which data on patient survival is available strengthens the findings of our analysis.
In conclusion, apart from substage B1, BCLC-B subclassification does not discriminate perfectly patients treated with TACE. Since also some patients in substage B4 can benefit from TACE, we believe that a multidisciplinary approach is advisable based on the single patient clinical, laboratory and radiological conditions.
Abbreviations- •
AASLD: Association for the Study of Liver Diseases.
- •
AFP: alpha-fetoprotein.
- •
BCLC: Barcelona Clinic Liver Cancer.
- •
CT: computed tomography.
- •
DEB: drug eluting beads.
- •
EASL: European Association for the Study of the Liver.
- •
ECOG: Eastern Cooperative Oncology Group.
- •
HbsAg: hepatitis B surface antigen.
- •
HCC: hepatocellular carcinoma.
- •
HCV: hepatitis C virus.
- •
IQR: interquartile range.
- •
MELD: Model for End-stage Liver Disease.
- •
MRI: magnetic resonance imaging.
- •
OLT: orthotopic liver transplantation.
- •
PS: performance status.
- •
TACE: transarterial chemoembolization.
Nothing to declare.
Conflict of Interest StatementNothing to declare.
Authorship StatementProfessor Antonio Grieco, M.D., is guarantor of this article and takes responsibility for the integrity of the work as a whole, from inception to published article. Biolato M. and Grieco A. designed the research study, Racco S., Gallusi G., Iavarone M., Della Corte C., Cabibbo G., Maida M., collected the data, Biolato M. and La Torre G. analysed the data, Biolato M. and Grieco A. wrote the paper, and Gasbarrini A., Attili A.F., Cammá C. and Sangiovanni A. critically revised the manuscript for important intellectual content.
All authors approved the final version of the manuscript.