Introduction and aim. Critically ill patients in states of circulatory failure are at risk of acute liver injury, from mild elevations in aminotransferases to substantial rises consistent with hypoxic hepatitis or “shock liver”. The present study aims to quantify the national prevalence of acute liver injury in patients with hemodynamic instability, identify risk factors for its development, and determine predictors of mortality.
Material and methods. The 2009-2010 Nationwide Inpatient Sample was interrogated using ICD-9-CM codes for hospital admissions involving states of hemodynamic lability. Multivariable logistic regression was used to evaluate the risks of acute liver injury and death in patients with baseline liver disease, congestive heart failure, malnutrition, and HIV.
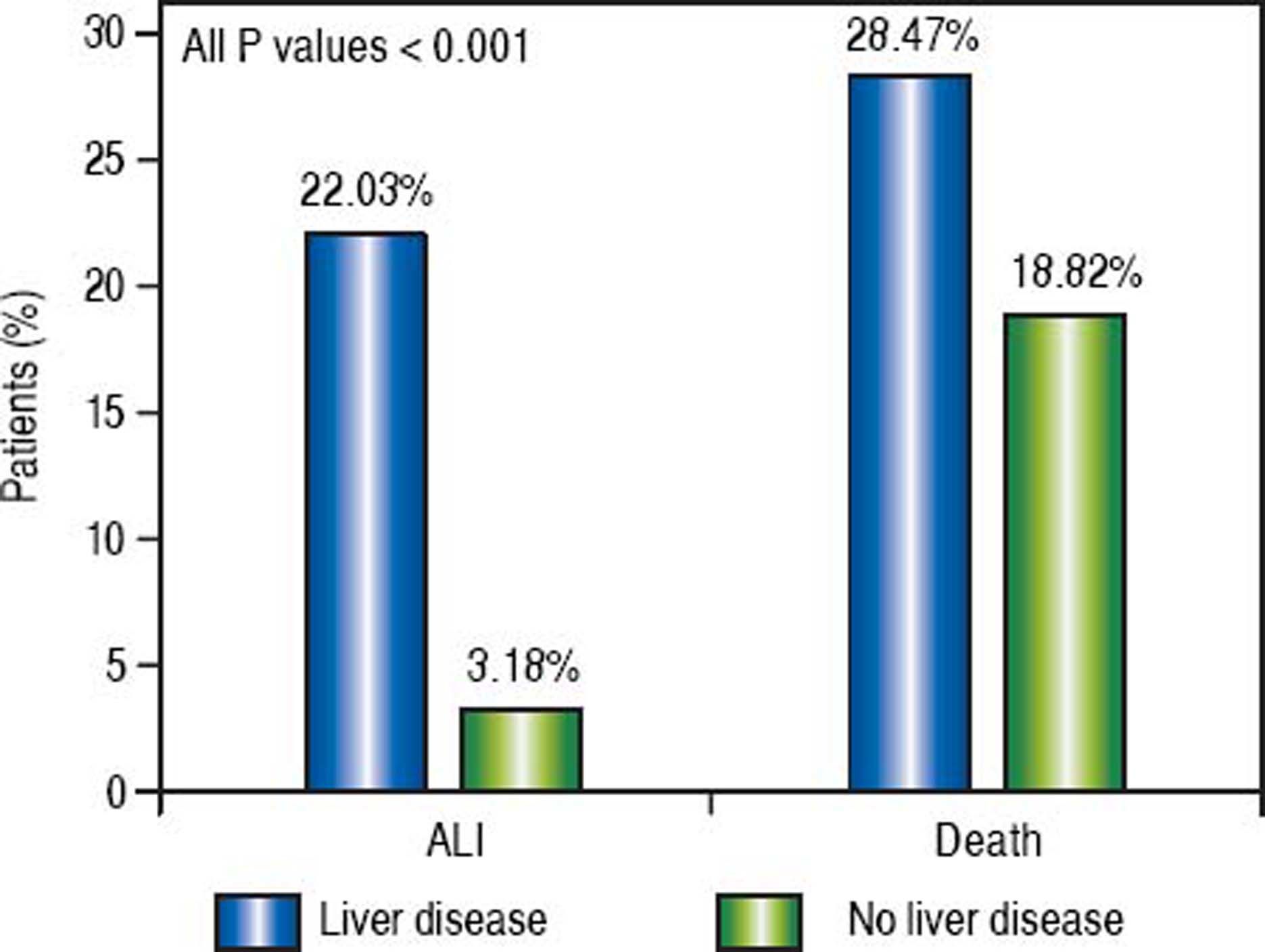
Results. Of the 2,865,446 patients identified in shock, 4.60% were found to have acute liver injury. A significantly greater proportion of patients with underlying liver disease experienced acute liver injury (22.03%) and death (28.47%) as compared to those without liver disease (3.18% and 18.82%, respectively). The odds of developing acute liver injury were increased in all baseline liver diseases studied, including all-cause cirrhosis, hepatitis B, hepatitis C, alcoholic liver disease, and non-alcoholic fatty liver disease, as well as in congestive heart failure and malnutrition. All-cause cirrhosis and alcoholic liver disease, however, conferred the greatest risk. Similar trends were seen with mortality. HIV was not a predictor for acute liver injury.
Conclusion. Liver injury is a major concern among patients with protracted circulatory instability, especially those suffering from underlying liver disease, heart failure, or malnutrition.
Critically ill patients with hemodynamic instability are often at risk for acute liver injury (ALI) from a number of different causes with varying levels of clinical significance. ALI in this population can range from idiopathic or iatrogenic elevations in aminotransferases to distinct clinical entities such as hepatic congestion, sepsis-associated cholestasis, or at worst hypoxic hepatitis (HH). ALI has important ramifications for critically ill patients as liver dysfunction is associated with poorer outcomes independently of other organ dysfunction.1
Congestive hepatopathy is often seen in patients with impaired forward flow leading to increased venous pressure in the inferior vena cava and hepatic veins. Patho-physiologically, this leads to congestion and dilatation of hepatic sinusoids, followed by perisinusoidal edema and atrophy of hepatocytes. If sufficiently sustained, this can lead to collagen deposition and areas of liver fibrosis.2 Congestive hepatopathy is a spectrum of disorders, however, as passive congestion can be present without affecting liver function in some patients, while in others venous congestion can predispose the liver to hypoxic events.3
Sepsis-associated cholestasis, also sometimes referred to under the umbrella of Intensive Care Unit (ICU) jaundice, is the observation of elevated levels of bilirubin in critically ill patients. The etiology of hyperbilirubinemia is often difficult to pinpoint. Sepsis alone can cause jaundice, especially in gram-negative infections, as hepatocytes lose the ability to process bilirubin and are predisposed to cell death.4 This liver dysfunction, however, can also worsen with antibiotics, the use of positive ventilation, or any major surgery in addition to sepsis/shock states alone.1
Finally, critically ill patients can suffer from HH, also known as ischemic hepatitis or “shock liver”. It is defined as a dramatic but transient increase in serum aminotrans-ferases to 20 times the upper limit of normal in the setting of acute cardiac, circulatory, or respiratory failure without other identifiable causes of liver damage like viral or drug-induced hepatitis.5 The prevalence of HH in critical care units ranges from 1%6–8 to as high as 12%9 as reported in several small studies.5 Hypoxic damage of the liver is associated with an in-hospital mortality of 56%6 and one-year mortality near 75%.7,8 In predisposed patients, the theorized length of hypotension needed for ischemic injury to the liver can be as short as 15 to 20 min.10
The present study analyzes a national sample of hospital discharges to both quantify the frequency of ALI on a large scale and estimate the risk of mortality based upon the presence of medical comorbidities in hemodynamically unstable patients.
Material and MethodsData sourceAll data were gathered from the Nationwide Inpatient Sample (NIS) between 2009 and 2010. The NIS is the largest national database of hospital discharges in the United States, estimating a 20% stratified sample of non-federal, acute care hospitals participating in the Healthcare Cost and Utilization Project but excluding rehabilitation and long-term care facilities.11 Included in each discharge record are patient demographics, primary and secondary diagnoses and procedures, length of stay, expected payment source, and hospital characteristics (e.g., region, size, and teaching status). We used the Clinical Modification of the International Classification of Diseases, Ninth Revision (ICD-9-CM) diagnosis codes to identify patients with ALI in the NIS using an algorithm previously validated by Myers, et al.12
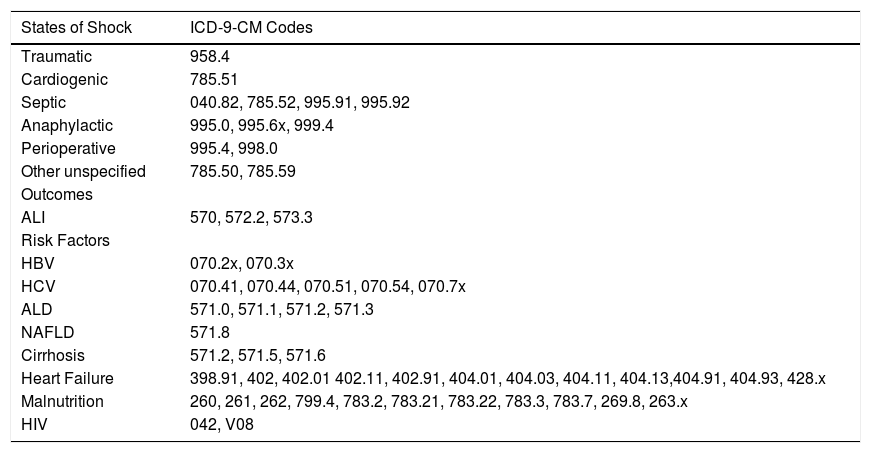
Predictor and outcome variablesICD-9-CM diagnostic codes (Table 1) were used to identify all inpatient admissions listed in the NIS involving traumatic shock, cardiogenic shock, septic shock, anaphylactic shock, postoperative shock, and other unspecified states of shock or hemodynamic lability. In accordance with the previously validated methodology by Myers, et al.,12 primary outcomes were development of ALI (ICD-9-CM codes 570, 572.2, 573.3) and death. These records were also analyzed for potential risk factors including all-cause cirrhosis, hepatitis B virus (HBV), hepatitis C virus (HCV), alcoholic liver disease (ALD), non-alcoholic fatty liver disease (NAFLD), heart failure, malnutrition, and human immunodeficiency virus (HIV).
Clinical Modification of the International Classification of Diseases, Ninth Revision (ICD-9-CM) codes used in this study.
| States of Shock | ICD-9-CM Codes |
|---|---|
| Traumatic | 958.4 |
| Cardiogenic | 785.51 |
| Septic | 040.82, 785.52, 995.91, 995.92 |
| Anaphylactic | 995.0, 995.6x, 999.4 |
| Perioperative | 995.4, 998.0 |
| Other unspecified | 785.50, 785.59 |
| Outcomes | |
| ALI | 570, 572.2, 573.3 |
| Risk Factors | |
| HBV | 070.2x, 070.3x |
| HCV | 070.41, 070.44, 070.51, 070.54, 070.7x |
| ALD | 571.0, 571.1, 571.2, 571.3 |
| NAFLD | 571.8 |
| Cirrhosis | 571.2, 571.5, 571.6 |
| Heart Failure | 398.91, 402, 402.01 402.11, 402.91, 404.01, 404.03, 404.11, 404.13,404.91, 404.93, 428.x |
| Malnutrition | 260, 261, 262, 799.4, 783.2, 783.21, 783.22, 783.3, 783.7, 269.8, 263.x |
| HIV | 042, V08 |
ALI: Acute liver injury. HBV: Hepatitis B virus. HCV: Hepatitis C virus. ALD: Alcoholic liver disease. NAFLD: Non-Alcoholic Fatty Liver Disease. HIV: Human immunodeficiency virus.
Data analysis was performed in accordance with the stratified and weighted sampling design of the NIS. Prevalence of the primary outcomes with and without liver disease and congestive heart failure (CHF) were compared using the χ2 test. Multivariable logistic regression was used to estimate the relative odds of the outcomes given the aforementioned potential risk factors, adjusting for age, gender, health insurance, hospital size, hospital teaching status, and geographic region. All controlled covariates were chosen a priori for their potential to confound. Analyses were performed using SAS software (release 9.3; SAS Institute, Cary, NC) and Stata/IC version 13.1 (Stata Corp, College Station, Texas). P values < 0.05 were considered statistically significant.
Ethical considerationsThe NIS contains entirely anonymous, publically available data with no threat of compromise to patient confidentiality. The study protocol was reviewed and approved by the Johns Hopkins Institutional Review Board (number NA_00086245).
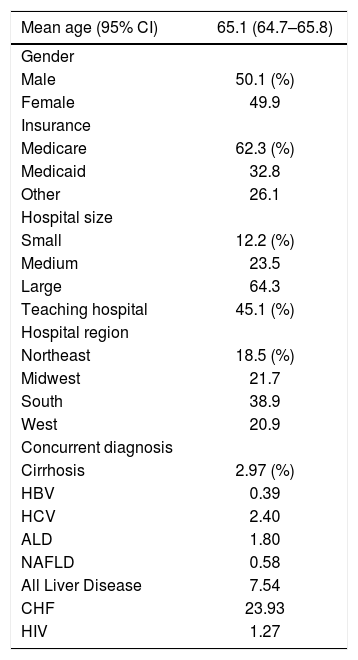
ResultsThere were 2,865,446 admissions in 2009 and 2010 that satisfied the inclusion criteria of shock or hemodynamic instability. The mean age of patients included was 65.1 years, with 50.1% of the population male and most patients insured with Medicare (Table 2). Among the included cohort, 4.60% were found to have ALI and 19.55% died. The overall frequency of concomitant liver disease was 7.54%, including all-cause cirrhosis 2.97%, HBV 0.39%, HCV 2.40%, ALD 1.80%, and NAFLD 0.58%. Concurrent diagnosis of CHF was noted in 23.93% of these admissions (Table 2).
Demographics of patients admitted for shock.
| Mean age (95% CI) | 65.1 (64.7–65.8) |
|---|---|
| Gender | |
| Male | 50.1 (%) |
| Female | 49.9 |
| Insurance | |
| Medicare | 62.3 (%) |
| Medicaid | 32.8 |
| Other | 26.1 |
| Hospital size | |
| Small | 12.2 (%) |
| Medium | 23.5 |
| Large | 64.3 |
| Teaching hospital | 45.1 (%) |
| Hospital region | |
| Northeast | 18.5 (%) |
| Midwest | 21.7 |
| South | 38.9 |
| West | 20.9 |
| Concurrent diagnosis | |
| Cirrhosis | 2.97 (%) |
| HBV | 0.39 |
| HCV | 2.40 |
| ALD | 1.80 |
| NAFLD | 0.58 |
| All Liver Disease | 7.54 |
| CHF | 23.93 |
| HIV | 1.27 |
CI: Confidence interval. HBV: Hepatitis B virus. HCV: Hepatitis C virus. ALD: Alcoholic liver disease. NAFLD: Non-Alcoholic Fatty Liver Disease. HIV: Human immunodeficiency virus.
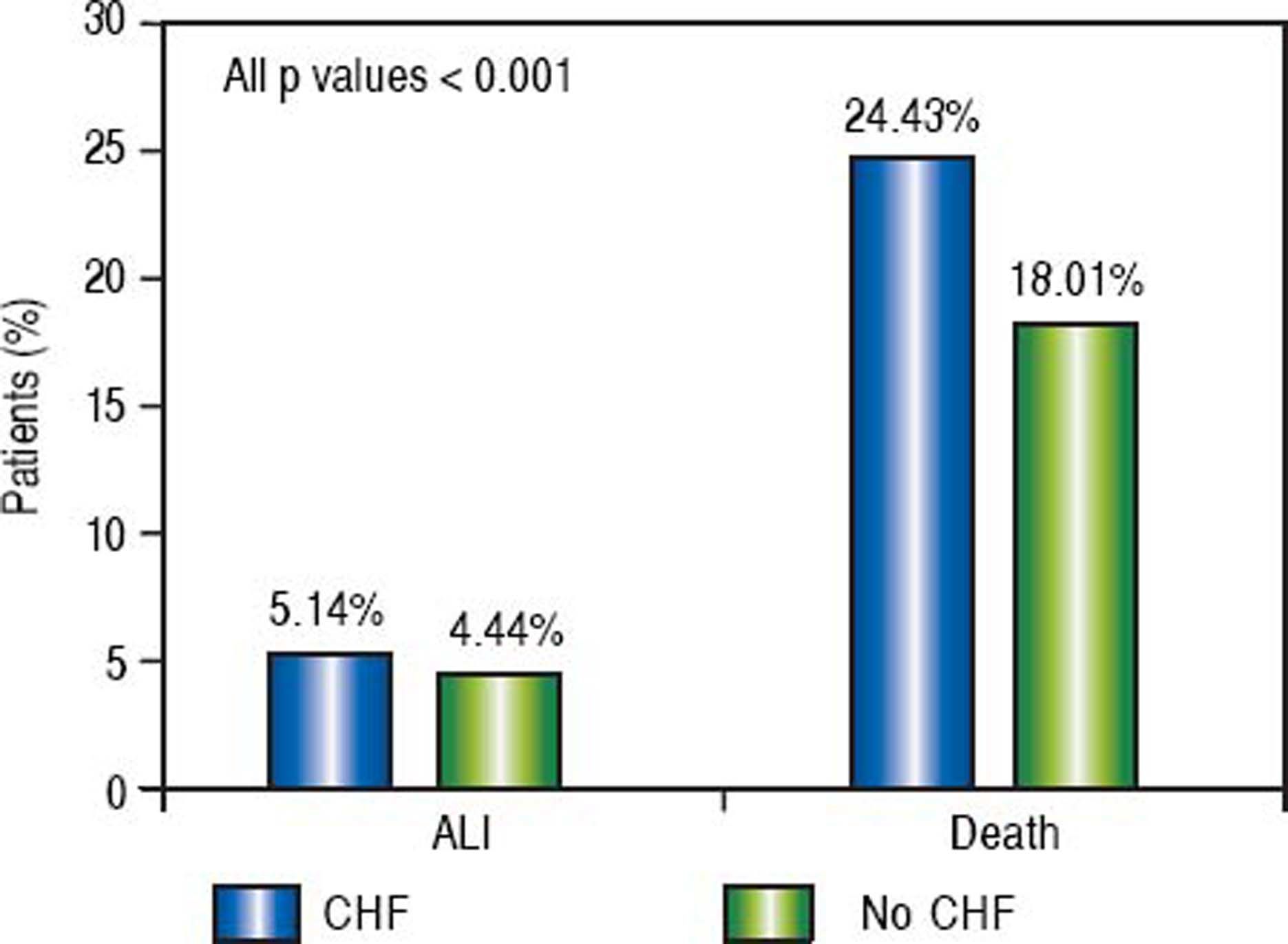
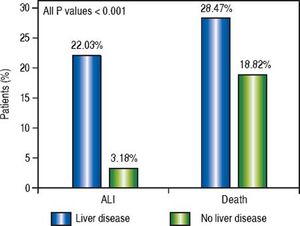
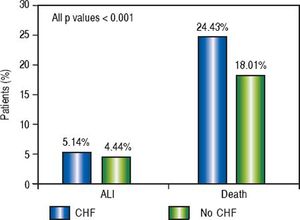
Of the patients with any form of liver disease, 22.03% suffered from ALI and 28.47% death compared to 3.18% and 18.82% respectively of patients without liver disease (P < 0.001) (Figure 1). A similar pattern was observed in CHF patients as 5.14% suffered from ALI and 24.43% death compared to 4.44% and 18.01% respectively in those without CHF (P < 0.001) (Figure 2).
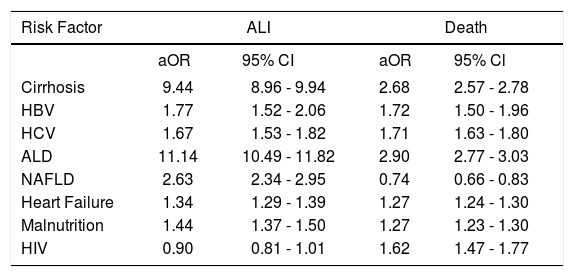
In multivariable analysis, the odds of developing ALI were increased with predisposing liver diseases including all-cause cirrhosis [adjusted odds ratio (aOR) 9.44, 95% confidence interval (CI) 8.96–9.94], underlying HBV (aOR 1.77, 95% CI 1.52–2.06), HCV (aOR 1.67, 95% CI 1.53– 1.82), ALD (aOR 11.14, 95% CI 10.49–11.82), and NAFLD (aOR 2.63, 95% CI 2.34–2.95). Odds of developing ALI were also increased with heart failure (aOR 1.34, 95% CI 1.29-1.39) and malnutrition (aOR 1.44, 95% CI 1.37-1.50), but not with HIV (aOR 0.90, 95% CI 0.81-1.01).
Similarly, increases in odds of mortality were seen with most underlying liver disease, heart failure, malnutrition, and HIV (Table 3). Odds of mortality were increased in all underlying liver conditions including all-cause cirrhosis (aOR 2.68, 95% CI 2.57-2.78), HBV (aOR 1.72, 95% CI 1.50-1.96), HCV (aOR 1.71, 95% CI 1.63-1.80), ALD (aOR 2.90, 95% CI 2.77-3.03), but not in NAFLD (aOR 0.74, 95% CI 0.66-0.83). Odds of mortality were also increased in heart failure (aOR 1.27, 95% CI 1.24-1.30), malnutrition, and HIV (aOR 1.62, 95% CI 1.47-1.77).
Odds of developing ALI or death based on risk factors.*
| Risk Factor | ALI | Death | ||
|---|---|---|---|---|
| aOR | 95% CI | aOR | 95% Cl | |
| Cirrhosis | 9.44 | 8.96 - 9.94 | 2.68 | 2.57 - 2.78 |
| HBV | 1.77 | 1.52 - 2.06 | 1.72 | 1.50 - 1.96 |
| HCV | 1.67 | 1.53 - 1.82 | 1.71 | 1.63 - 1.80 |
| ALD | 11.14 | 10.49 - 11.82 | 2.90 | 2.77 - 3.03 |
| NAFLD | 2.63 | 2.34 - 2.95 | 0.74 | 0.66 - 0.83 |
| Heart Failure | 1.34 | 1.29 - 1.39 | 1.27 | 1.24 - 1.30 |
| Malnutrition | 1.44 | 1.37 - 1.50 | 1.27 | 1.23 - 1.30 |
| HIV | 0.90 | 0.81 - 1.01 | 1.62 | 1.47 - 1.77 |
ALI: Acute liver injury. aOR: Adjusted Odds Ratio. CI: Confidence interval. HBV: Hepatitis B virus. HCV: Hepatitis C virus. ALD: Alcoholic liver disease. NAFLD: Non-Alcoholic Fatty Liver Disease. HIV: Human immunodeficiency virus. * Multivariable model adjusted for age, gender, health insurance, hospital size, teaching status, and geographic region.
Our use of the NIS allowed the study of millions of hospital admissions to characterize the risk of underlying liver disease in the development of ALI and death in patients with shock. ALI was identified in 4.60% of all admissions included in this study. In patients without baseline liver pathology, however, the prevalence of ALI was only 3% in contrast to 22% with underlying liver disease. The likelihood of ALI and death was elevated in cirrhosis, viral hepatitis, and ALD, as well as in CHF and malnutrition.
This dramatic increase in ALI supports the idea of a secondary liver injury in those who are patho- physiologically predisposed - an entity now recognized as acute-onchronic liver failure (ACLF). The first consensus definition of ACLF proposed by the Asian Pacific Association for the Study of the Liver in 2009 defined ACLF as “an acute hepatic insult manifesting as jaundice and coagulopathy, complicated within 4 weeks by ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease”.13 Subsequent definitions have included modifiers such as high 28-day mortality14 and the presence of organ failure.15 The susceptibility of the liver to ACLF depends on both the etiology of the acute insult16 and, as seen in these results, the etiology of the underlying liver disease.
Of the risk factors included in this analysis, all-cause cirrhosis had among the largest increases in odds of ALI and death. These results support previous studies suggesting cirrhosis portends a poorer outcome in HH as well as other forms of ALI.9,17 Cirrhotic livers are predisposed to poor oxygen consumption as a result of portosystemic shunting and impaired functional blood flow.18 These patients are therefore more sensitive to hemodynamic shifts causing ALI in states of shock.
Also notable was ALD, which had the highest adjusted odds ratios in both outcomes. Alcohol induces a hyper-metabolic state with elevated oxygen demand in the centrilobular zones of the liver, thereby increasing susceptibility to hypoxia during times of low hepatic blood flow.19,20 Chronic alcohol use also leads to the upregulation of cytochrome P450 2E1 (CYP2E1), a vital enzyme in the hepatic metabolism of drugs and certain toxic metabolites, which in turn may exacerbate damage to the liver in times of severe stress.21
Viral hepatitides including HBV and HCV also portended increased risks of liver injury and death, though less drastically than ALD. Given the natural history of viral hepatitis infection, patients sampled range on the disease spectrum from clinically silent or mild liver disease to chronic inflammation, fibrosis, and eventual cirrhosis.22,23 As stated above, cirrhosis itself may predispose the liver to circulatory damage, but we know of no existing studies that measured whether chronic HBV or HCV infection independently reduced the hepatic capacity for extracting oxygen from systemic circulation. Further laboratory studies are needed to better explore this relationship.
Patients with NAFLD were found to have increased odds of ALI, but curiously a lower odds of mortality. In NAFLD, there is reduced portal vein flow velocity,24 which could explain why in times of shock such livers might be more prone to damage, as is the case in cirrhosis. One potential explanation for the lower odds of mortality is that patients with NAFLD are more likely to have metabolic syndrome and be prescribed statins. In one study, statin therapy was associated with a reduction in 28-day mortality for all patients admitted to the ICU, including those with HH, possibly owing to a protective effect of statins in ischemia/reperfusion injury.25
Non-liver diseases like heart failure were also found to have moderate increases in odds of ALI and death. The risk of liver damage conferred by CHF has been well-studied,3,7,8 and our results support the findings that heart failure portends a higher risk of ALI. This is most likely due to chronic venous congestion affecting the liver directly in addition to CHF patients having preexisting impaired blood flow exacerbated by times of hemodynamic lability.
Malnutrition also confers a greater risk of ALI and death, and malnutrition leading to liver injury has been studied previously with elevation of liver enzymes in anorexia nervosa. One study posited that in addition to circulatory abnormalities experienced in states of malnutrition, anorexia patients had depleted glycogen stores and autophagosomes on liver biopsy indicative of malnutrition-induced cell death.26 Furthermore, vitamin D deficiency has been associated with hepatic steatosis.27 The risk of ALI and death conferred by malnutrition is likely multifactorial, ultimately leading to increased susceptibility to hepatic injury induced by bodily stressors.
HIV, unlike heart failure and malnutrition, did not lead to increased risk for ALI, but did lead to an increased risk of death. HIV monoinfection has been associated with hepatic fibrosis,28 and chronic liver disease can occur in HIV patients with co-morbid viral hepatitis and/or alcohol abuse.29 However, these scenarios may not occur with sufficient frequency to significantly influence the likelihood of developing ALI. The increased risk of death is consistent with previous literature showing that HIV infection portends poorer outcomes including mortality in patients suffering from septic shock or circulatory compro-mise.30,31
The present analysis has some limitations, mostly those inherent to the use of administrative data. First, the accuracy of ICD-9-CM codes is susceptible to variations in regional and national coding practices. The ALI coding algorithm was previously validated by Myers, et al. to have both excellent sensitivity and specificity.12 However, that study was conducted using a large Canadian health registry, so its results may not be wholly reproducible in a U.S.-based cohort. The NIS also does not contain personally identifiable information or link to individual medical records; therefore, it is not possible to further confirm an ALI diagnosis by examining the precise liver enzyme abnormalities. Second, our study inclusion criteria grouped various hemodynamic lability processes (i.e., cardiogenic shock, septic shock, anaphylactic shock, etc.) into one broad category, but there are physiologic differences between these clinical classifications that may additionally modify the risk for developing ALI. These subtleties may be further explored in future studies. Despite these shortcomings, our use of the NIS in analysis permitted a robust sample size that sufficiently powered statistical tests for even relatively uncommon conditions, and minimized the referral bias associated with single-center tertiary hospital studies.
In conclusion, the present analysis illustrated that in the setting of protracted circulatory instability, hospitalized patients with baseline liver disease or heart failure had increased risks of ALI and death. Not all pre-existing disorders conferred the same level of risk; future studies may help elucidate the differences in pathophysiological mechanisms including most interestingly ALD and NAFLD. Given the relative frequency and poor prognosis of ALI in critically ill patients, early identification of individuals with high risk features highlighted in this study may be crucial to providing the optimal care that improves the consequent outcome.
Abbreviations- •
ACLF: Acute-on-Chronic Liver Failure.
- •
ALD: Alcoholic Liver Disease.
- •
ALI: Acute Liver Injury.
- •
aOR: Adjusted Odds Ratio.
- •
CHF: Congestive Heart Failure.
- •
HBV: Hepatitis B.
- •
HCV: Hepatitis C.
- •
HH: Hypoxic Hepatitis.
- •
ICU: Intensive Care Unit.
- •
NAFLD: Non-alcoholic Fatty Liver Disease.
- •
NIS: Nationwide Inpatient Sample.
None.
Funding/SupportNot applicable.
Previous PresentationDigestive Disease Week 2013, Orlando, FL.
Author ContributionsStudy concept and design: BL, BK, TW, PC.
Acquisition of data: BL.
Analysis and interpretation of data: NW, BL, BK, TW, PC.
Drafting of the manuscript: NW, PC.
Critical revision of the manuscript for important intellectual content: NW, BL, BK, TW, AG, PC.
Statistical analysis: BL, PC.
Obtained funding: not applicable.
Administrative, technical, or material support: AG.
Study supervision: PC.
Funding SourcesNot applicable.