Introduction. Hepatitis C (HCV) continues to be the leading indication for liver transplantation (LT). Sustained virological response (SVR) rates to pegylated interferon (PEG-IFN) and ribavirin (RBV) therapy for recurrent HCV in Genotype 1 (G1) LT recipients have been disappointing (30-40%). Experience with triple therapy using protease inhibitors (PI) boceprevir (BOC), telaprevir (TVR) in these patients has been limited.
Material and methods. This national multicenter retrospective study included 76 patients (64 male, mean age 57 ± 6 years), treated for G1 HCV recurrence with either BOC (n = 41) or TVR (n = 35), who were non-responders or relapsers (n = 54), treatment naïve (n = 22) or had fibrosing cholestatic HCV (n = 3). 53 patients were on cyclosporine, 22 on tacrolimus and one patient on prednisone alone.
Results. On treatment virologic response was observed in 84% (64/76), 83% in BOC and 85% in TVR group. A higher week 4 response after starting triple therapy (RVR) was noted in TVR group 25/35 (81%) as compared to BOC group 26/41 (63%); p value = 0.02. The end of treatment response was 78% and 75% in BOC and TVR group, respectively. SVR 12 weeks after treatment discontinuation was observed in 59.5% (22/37); 58.3% in the BOC group and 61.5% in TVR group. Treatment was discontinued early in 23 patients (serious adverse effects n = 19, treatment failure n = 4). Infections occurred in 5 patients with 2 deaths (all in BOC). Anemia was the most common side effect (n = 55, 72%) requiring erythropoietin and RBV dose reduction. In the BOC group, cyclosporine dose reduction was 2.2 ± 1.0 fold and 8.6 ± 2.4 fold with tacrolimus. In TVR group, dose reduction was 3.0 ± 1.4 with cyclosporine and 12 ± 5.7 fold with tacrolimus. Conclusions. PI-based triple therapy appears more effective in producing HCV-RNA clearance than dual therapy. Tolerability is a serious issue and drug-drug interactions are manageable with close monitoring.
Chronic hepatitis C virus (HCV) infection is one of the leading causes of end-stage liver disease and hepatocellular carcinoma worldwide, and remains the leading indication for liver transplant (LT).1–3
Spontaneous clearance of HCV post-LT is rare and HCV recurrence is universal in individuals with HCV viremia at the time of transplantation. Compared with other etiologies of liver disease, patient and graft survival rates are inferior due to more rapidly progressive fibrosis driven by HCV recurrence.4,5 Fibrosis is accelerated in post LT patients as compared with non-transplant, resulting in cirrhosis, graft loss and consideration for re-transplantation.5–7 Anti-viral therapy (AVT) can alter fibrosis progression and improves graft and patient survival.8,9 Response to AVT with pegylated interferon (PEG-IFN) and ribavirin (RBV) in genotype 1 (G1) has been disappointing with sustained virologic response (SVR) rates of 30% to 40%.10–12 The benefits of achieving an SVR are clear with improvement in liver fibrosis, lower probability of decompensation and lower cumulative mortality post transplantation.13,14
It is clear that in the post-transplant population there is a desperate need for more effective therapy for HCV recurrence especially given the tragic disparity between organ need and availability. Since 2011, the standard of care in non-transplant patients with G1 chronic hepatitis C infection became triple therapy combining PEG-IFN, RBV and the NS3/ 4A protease inhibitors (PI) telaprevir (TVR) or boceprevir (BOC). Phase III trials have shown that PegIFN/RBV plus BOC or TVR increased SVR rates in naïve and previously treated G1 non-transplant patients The use of triple therapy in the post-transplant setting in patients with G1-HCV recurrence has been limited.15–17
There have also been concerns regarding the safety profile and potential interactions with calcineurin inhibitors (CNIs) in post LT recipients. Early pharmacokinetic data on the use of TVR in healthy volunteers revealed significant increases in cyclosporine (5-fold) and tacrolimus levels (70-fold) due to the inhibition of the P450 3A cytochrome.18
In this national Canadian multicenter study we determined the on-treatment virologic response and SVR at week 12 in liver transplant recipients treated with triple therapy for HCV G1 recurrence in all Canadian liver transplant centers. We also determined the safety and tolerability of PI based triple therapy in post LT patients.
Material and MethodsStudy designThis retrospective multicenter observational study was approved by the research ethics board at the Toronto General Hospital.
Study subjectsAll Canadian LT recipients who received PI based triple therapy for biopsy proven HCV G1 recurrence between January 2012 and May 2013 were included. The indication for antiviral therapy was based on biopsy-proven chronic hepatitis defined using the METAVIR score.19 All the patients had significant fibrosis (stage ≥ 2) and/or moderate to severe lobular hepatitis (grade ≥ 3) according to the METAVIR system or suffered from fibrosing cholestatic hepatitis (FCH), defined according to the following criteria: presence of extensive, dense portal fibrosis with immature fibrous bands extending into the sinusoidal spaces, ductular proliferation, cholestasis and moderate mononuclear inflammation.19 The usual contraindications to PEG-IFN and RBV combination were applied (pretreatment neutrophils < 1.0 x 109/ L, platelets < 40 × 109/L, hemoglobin < 100 g/L, evidence of acute cellular rejection (ACR) within 3 months before starting therapy, evidence of chronic ductopenic rejection, history of or ongoing severe psychiatric disorders and HIV co infection).
Treatment protocol for recurrent HCV G1All patients received PEG-IFN and RBV. The dose and choice of PEG-IFNa2a (Pegasys®, Hoffman LaRoche, Mississauga ON) versus PEGIFNa2b (Pegetron®, Merck Canada, Kirkland, QC) was decided by the treating transplant Hepatologist. The RBV dose was adjusted to renal function parameters and could be escalated to maximally tolerated levels or reduced, depending on the degree of anemia and overall tolerance. The choice of PI was also left to the discretion of the treating physician. Patients were treated with standard regimens for TVR and BOC with the intended duration of therapy of 48 weeks with standard futility rules were applied.20 In the event of such a lack of response, the option of continuing PEG-IFN/RBV was considered on the basis of patients’ tolerance and biochemical responses on treatment along with the fibrosis stage.
Erythropoietin alpha 40,000 IU SC once weekly was started when the hemoglobin was less than or equal to 100 g/L and/or symptoms and earlier in patients with a history of coronary artery disease at the discretion of the transplant hepatologists. Granulocyte macrophage colony stimulating factor (filgrastim) 300 SC once weekly was administered when neutrophils fell to less than 1.0×109/L at the discretion of the transplant hepatologists.
ImmunosuppressionImmunosuppression was managed in all patients according to internal guidelines of each transplant centre. The use of calcineurin inhibitor (CNI), either cyclosporine or tacrolimus, was at the discretion of the treating Hepatologist. Mycophenolate mofetil was continued only in patients requiring CNI dose reduction; otherwise it was discontinued whenever possible. Similarly, some patients were converted to cyclosporine based immunotherapy at the discretion of the transplant Hepatologist prior to starting AVT.
Clinical, biochemical, and virological monitoring during/after therapyRoutine biochemical tests and a complete blood count were generally performed weekly during the lead in phase if applicable, and twice weekly during the first month of triple therapy and then after every two weekly in the remainder of AVT. Close monitoring was maintained with weekly labs in the first month post treatment.
Viral load (VL) was monitored in plasma using the Abbott Real Time HCV assay (Abbott Molecular, USA; lower limit of detection, 12 IU/mL) or COBAS® Taqman® HCV assay (Roche Diagnostics, Laval QC), depending on centre, at baseline and then at weeks 4, 8, 12, 24, 48 and 12 weeks after the end of treatment. A rapid virological response (RVR) was defined as an undetectable VL at week 4 of triple therapy. At week 12, a complete EVR (EVR) was defined as undetectable HCV RNA. An end of treatment response (ETR) was obtained when the VL remained negative at the time of treatment discontinuation. A sustained virological response 12 (SVR12) was defined as undetectable VL 12 weeks after the end of treatment. The lead-in phase represents an initial period of 4 weeks of dual therapy with PEG-IFN/RBV, in standard doses, followed by triple therapy. The concept of lead-in phase was to improve efficacy of BOC based triple therapy. Indeed, by lowering HCV RNA level, a short course of PEG-IFN/RBV may theoretically reduce the risk of viral breakthrough or resistance.
Statistical analysisStatistical analysis was performed with SPSS (SPSS for Windows 20.0, Chicago, IL) and STATA 12 (StataCorp LP, College Station, TX). Statistical differences in categorical variables were determined using chi squared or Fisher’s exact test as appropriate. Differences in continuous variables were analyzed using Student t test, analysis of variance, or Mann-Whitney test as appropriate for the variable and distribution. Univariate and Multivariate Exact logistic Regression analyses were used to estimate each independent variable separately with all the other variables conditioned out and fitted in the model. Model score and R squared are reported. A p value of 0.05 or less was considered statistically significant.
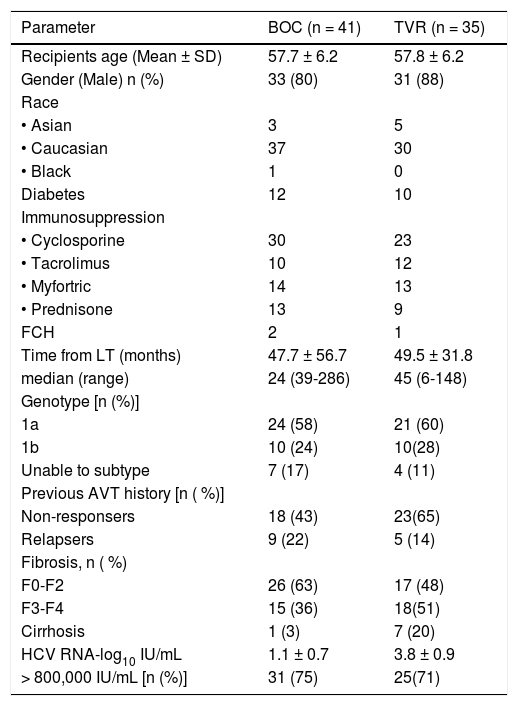
ResultsCharacteristics of the study populationA total of 76 patients were started on PI based triple therapy in seven Canadian transplant centers from January 2012 to May 2013 (BOC n = 41, TVR n = 35). Patient’s participation per center is as follow: Toronto 36, London 1, Edmonton 7, Vancouver 10, McGill 7, University of Montreal 11 and Calgary 4. At the time of analysis 69% (52/76) of patients were off therapy (29 completed, 23 discontinued early either due to intolerance or treatment failure) while 31% (24/76) were still on treatment. A PEG-IFN and ribavirin lead in period was utilized in 11/35 (31%) patients in TVR group and all but one of the patients in BOC group. Demographics and patients characteristics are shown in Table 1 for both treatment groups. There was no statistical difference in terms of age, sex and race, presence of diabetes, BMI, time from LT, HCV genotype 1 subtype, and history of previous treatment with PEG-IFN/RBV or fibrosis stage in both the treatment groups. Fifty three patients (70%) received cyclosporine, twenty two (28%) received tacrolimus and one patient was only on prednisone. There were three patients with FCH in this cohort one of them was on dual therapy for more than 1 year post FCH when BOC was added. One of the three FCH patients has been previously reported as a case report.21
Baseline demographics and patient characteristics.
| Parameter | BOC (n = 41) | TVR (n = 35) |
|---|---|---|
| Recipients age (Mean ± SD) | 57.7 ± 6.2 | 57.8 ± 6.2 |
| Gender (Male) n (%) | 33 (80) | 31 (88) |
| Race | ||
| • Asian | 3 | 5 |
| • Caucasian | 37 | 30 |
| • Black | 1 | 0 |
| Diabetes | 12 | 10 |
| Immunosuppression | ||
| • Cyclosporine | 30 | 23 |
| • Tacrolimus | 10 | 12 |
| • Myfortric | 14 | 13 |
| • Prednisone | 13 | 9 |
| FCH | 2 | 1 |
| Time from LT (months) | 47.7 ± 56.7 | 49.5 ± 31.8 |
| median (range) | 24 (39-286) | 45 (6-148) |
| Genotype [n (%)] | ||
| 1a | 24 (58) | 21 (60) |
| 1b | 10 (24) | 10(28) |
| Unable to subtype | 7 (17) | 4 (11) |
| Previous AVT history [n ( %)] | ||
| Non-responsers | 18 (43) | 23(65) |
| Relapsers | 9 (22) | 5 (14) |
| Fibrosis, n ( %) | ||
| F0-F2 | 26 (63) | 17 (48) |
| F3-F4 | 15 (36) | 18(51) |
| Cirrhosis | 1 (3) | 7 (20) |
| HCV RNA-log10 IU/mL | 1.1 ± 0.7 | 3.8 ± 0.9 |
| > 800,000 IU/mL [n (%)] | 31 (75) | 25(71) |
TVR: telaprevir. BOC: boceprevir. LT: liver transplant. AVT: anti-viral therapy. FCH: fibrosing cholestatic hepatitis.
64 of 76 patients (84%) developed undetectable HCV RNA while on treatment, 34/41 (83%) in BOC and 30/35 (85%) on TVR group. A higher week 4 response (RVR) was noted in the TVR group 25/35 (81%) as compared to the BOC group 26/41(63%) (p<0.02). Week 12 response (EVR) was obtained in 83% in BOC and 86% in TVR group. The ETR was 78% among 32 patients in BOC group and 75% among 20 patients in the TVR group. At the time of analysis SVR12 was achieved in 59.5% in this cohort (22 out of 37 evaluable patients), 58.3% in the BOC group (14 out of 24 evaluable patients) and 61.5% in TRV group (8 of the 13 evaluable patients).
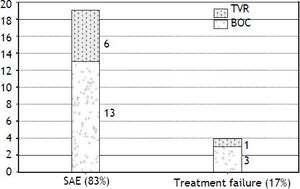
Twelve patients were considered non-responders within this cohort, 7 in BOC and 5 in TVR. All non-responders discontinued treatment early, 8 out of 12 (66.7%) patients stopped therapy early due to adverse effects and only four patients discontinued due to treatment failure. There were only three patients who relapsed after stopping the treatment and all were in the TVR group. One of the three patients with FCH achieved SVR in the BOC group and one met futility rules and was switched to sofosbuvir.
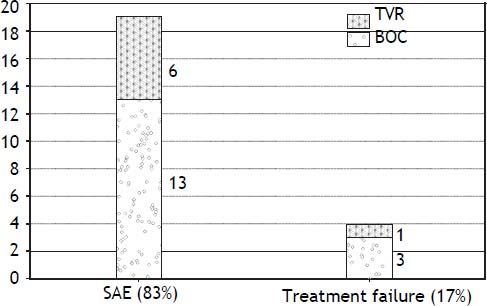
Intolerance and early discontinuationA total of twenty three patients (44%) discontinued therapy early in the course, 19/23 (82.6%) discontinued due to serious adverse effects and 4/23 (17.3%) were considered as treatment failure (Figure 1).
Major adverse events leading to treatment discontinuation included; infections in 5 patients; all in BOC group (2 with biliary sepsis and 3 had respiratory infection). Two patients developed acute pancreatitis (one in each treatment group) at week 8 and week 28 respectively and all treatment was stopped. Only one patient discontinued triple therapy due to acute rejection at week 12. Two patients decompensated on TT, one with ascites and the other one with both ascites and hepatic encephalopathy. Two patients in BOC group developed significant rash leading to treatment discontinuation. Only one patient in TVR group stopped treatment due to renal dysfunction. Two patients developed neuropsychiatric complications and 1 patient had a cardiac event leading to treatment cessation. Two patients died in the context of sepsis in the BOC group, one with chest infection while on treatment whereas the other patient died 2 months after stopping the treatment due to liver failure secondary to extensive portal venous and hepatic artery thrombosis.
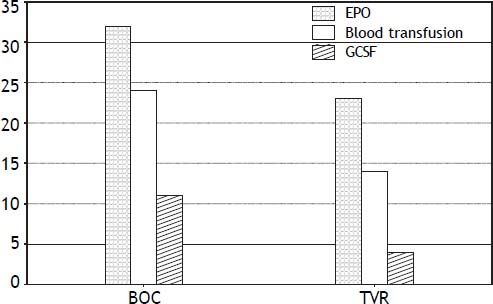
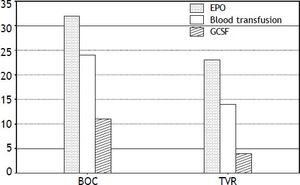
CytopeniasAnemia was the most common side effect, and was observed in approximately two thirds of the patients, necessitating erythropoietin (EPO) 78% in the BOC group and 65% in the TVR group, and prompting RBV dose reduction in 63% of patients. Severe anemia was seen in 43% (33/76) of patients in this cohort. Despite use of EPO333, 58% of BOC and 40% of TVR patients required blood transfusions (Figure 2). Thirteen (25%) patients required PEG-IFN dose reduction due to anemia and neutropenia. Neupogen (G-CSF) was administered for neutropenia in 11 BOC (26%) treated and 4 TVR (11%) treated patients. Only one patient required platelet transfusion for severe thrombocytopenia in the cohort.
ImmunosuppressionAll patients achieved a steady state of immunosuppression before initiation of PI and CNI dose was reduced on the day of PI introduction. In BOC group the cyclosporine dose reductions were 2.2 ± 1.0 fold and were 8.6 ± 2.4 fold with tacrolimus. In the TVR group the dose reductions were 3.0 ± 1.4 and 12 ± 5.7 fold with cyclosporine and tacrolimus, respectively. At the time of discontinuation of PI the CNI dose was increased in all the patients.
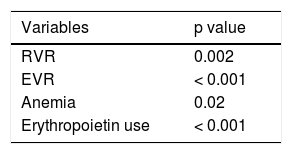
Predictors for responseEarly virological response including both RVR and EVR, anemia and erythropoietin use was found to be the predictors of SVR on the univariate analysis (Table 2). The multivariate exact logistic regression model confirmed that the variables independently associated with the individual response to SVR (Table 3) were the rapid virologic response (RVR) and early discontinuation of therapy, presumably reflecting early virologic success. Previous treatment history, naïve vs. treatment experienced and immunosuppression were not found to be significant.
Univariate analysis of baseline prognostic factors for the prediction of sustained virologie response (SVR12).
| Variables | p value |
|---|---|
| RVR | 0.002 |
| EVR | < 0.001 |
| Anemia | 0.02 |
| Erythropoietin use | < 0.001 |
RVR: rapid virological response. EVR: early virological response. SVR: sustained virological response.
Multivariate exact logistic regression model for the prediction of sustained virologic response (SVR12).
| Parameters | Odds Ratio estimated for SVR | 95.0% CI | p value |
|---|---|---|---|
| RVR | 26.7 | 1.5-2005.6 | 0.015 |
| Anemia | 12.3 | 0.7-926.0 | 0.103 |
| Early discontinuation | 20.7 | 1.7-1206.8 | 0.008 |
Model score 20.16, p ≤ 0.001. R2 0.5663. RVR: rapid virological response. SVR: sustained virological response.
This is the largest national multicenter cohort of LT recipients treated with PI based triple therapy for HCV G1 recurrence. In this cohort we have demonstrated the efficacy and safety of triple therapy in post LT patients, with on treatment virologic response rate of 84% and SVR 12 rate of 59.5%.
Our results suggest that triple therapy achieves higher SVR rates than those previously obtained with a dual therapy consisting of PEG-IFN/RBV in a post-LT setting. A meta-analysis, that included all genotypes, found a pooled SVR rates of only 30% (range 8-50%).10,22 Our data is superior to the European multicentre cohort presented by Coilly and colleagues at the recent meeting of the American Association for the Study of the Liver Diseases meeting in 2013 with SVR 12 rates of 48.6% (n = 79).16 We found early virological response (RVR, EVR), anemia and early discontinuation were the predictors of SVR on univariate analysis. RVR and early discontinuation of AVT continued to be the predictors of SVR on multivariate exact logistic regression analysis.
The current study demonstrated a high RVR 80% in TVR as compare to BOC group 63.4% (p value = 0.02). The response rate at week 12 was similar in both the groups (83% in BOC and 86% in TVR). The end of treatment response was also noted to be comparable in both BOC and TVR (78% and 75% respectively). Many studies have demonstrated that EVR is the principal predictive factor associated with SVR, even in liver transplant recipients.23–25 Similarly in our study both RVR and EVR were found to be the predictors for the SVR 12 (p value < 0.0001 for both). On multivariate exact logistic regression analysis the odds for SVR in the patient who had achieved RVR was about 26 times than those who did not achieve RVR. (95% CI: 2.1-880.9, p = 0 .014). We observed a low relapse rate in our cohort, with only 3 patients relapsing, and all were in the BOC group.
Triple therapy encountered higher early discontinuation rate (44%) in post-transplant population predominantly due to poor tolerance. The French post-LT cohort reported 23% of discontinuation rate.26 We noted a higher number of patients who discontinued therapy were on BOC (16/23) than on TVR (7/23). This could also be related to PI treatment duration, TVR being shorter than BOC. We also observed that early discontinuation had a profound effect on SVR, and on multivariate analysis, early discontinuation of therapy was found to be inversely related with SVR (OR: 20.7, 95%CI: 1.7-1206, p = 0.008). However, nearly one quarter (23%, 5/22) of patients achieved SVR despite early discontinuation (3 were in BOC and 2 in TVR group). One patient stopped BOC at week 8 but continued dual therapy for 48 weeks, the remaining patients stopped all treatment at week 8, 10, 20 and 24 weeks.
Serious adverse events leading to early discontinuation in our study included infections, rash, pancreatitis, neuropsychiatric and hepatic decompensation. While the rate of serious infections was 6.5%, a higher infection rate of 27% was reported in the French cohort by Coilly.22 There were two deaths in our study, both related to sepsis and in the BOC group. Only one patient discontinued therapy due to acute rejection and one due to renal dysfunction. Prophylactic antibiotics were not used in our patients, but our threshold for prescribing antibiotics was much lower.
The most common adverse effect observed in our study was anemia, requiring RBV dose reduction in 63% and erythropoietin use in 71% of patients. Mechanisms involved in the development of anemia in post-transplant population are same as described in the immune competent population. Interferon-related bone marrow suppression, myelosuppression due to concomitant therapies, renal insufficiency, HCV-interference with erythropoietin production, and above all, RBV dose-dependent hemolysis all contribute to anemia. Since the prevalence of these factors, particularly renal insufficiency and myelosuppression due to concomitant therapies, is higher in the liver transplant setting, the addition of a protease inhibitor to Peg-IFN/ RBV results in increased incidence and severity of anemia.27
Blood transfusions were required in half of our patients despite erythropoietin use and RBV dose reduction were frequently required as not all centers were able to provide erythropoietin because of variable re-imbursement policies amongst the Canadian provinces. Severe anemia (Hb < 80 gm/L) was observed in 43% of patients, but none discontinued therapy. Similar results were observed by both Verna, et al.28 (49% transfusion, 87% growth factors), and Coilly, et al. (92% anemia and treated with EPO, 35% blood transfusion).22 In a U.S. study an interim analysis of patients undergoing TVR or BOC based TT post-LT, found that 77% of patients in the TVR group received EPO.15 Interestingly, in our study the development of anemia and use of erythropoietin have a favorable impact on SVR only on univariate analysis (p value = 0.02). Previously, with dual therapy, the development of anemia, despite its association with poorer quality of life and potential development of severe complications, was considered a desirable effect since it was an indirect marker of higher efficacy. The development of anemia was an indirect confirmation that the patient had adhered to the treatment, and that the RBV molecule had entered not only the hepatocyte but also the erythrocyte leading to adverse as well as desired effects at the same time. Higher SVR rates were noted with BOC in a post hoc analysis of Phase III trials (SRINT-2 & RESPOND-2) in patients who developed anemia compared to those who did not develop this complication.29
Drug-drug interactions were manageable in the present cohort, although there has been a major concern in post liver transplant population, as PIs are potent inhibitors of the CYP3A4 enzyme.30 Significant dose reduction was required more with TVR than with BOC. The mean reduction with BOC was about 2-fold and 3- fold with cyclosporine and tacrolimus, respectively; while in TVR group the interactions were more potent with the mean reductions were 8-fold and 12-fold with cyclosporine and tacrolimus, respectively. We ensured close monitoring with frequent labs till the steady drug levels were achieved both at the initiation and at the discontinuation of PI.
This study presents some limitations. First of all, it was not a prospective randomized study. Secondly early discontinuation rules and immunosuppressive regimens were not fully standardized. Third, we could not provide data about the interleukin 28B genotype of both the recipient and donor liver which has been shown to influence SVR rates, being significantly higher for CC compared with CT/TT genotypes.31 These limitations, however, also reflect the “real world” aspect of treating HCV post-transplant and the variability in clinical practice that currently exists amongst transplant centers in Canada. Therefore, our study is most likely an accurate reflection of the current clinical outcomes post-transplant in Canada.
ConclusionPI-based triple therapy appears more effective in transplant recipients in producing HCV RNA clearance than dual therapy. Tolerability is a serious issue with high rates of early withdrawal. Drug-drug interactions are manageable with close monitoring, significant renal dysfunction is rare, and allograft rejection is not a major issue. We also noted that almost a third of patients achieved SVR despite early withdrawal, suggesting that shortening of treatment may be an option in some cases.
Abbreviations- •
ACR: acute cellular rejection.
- •
AVT: anti viral therapy.
- •
BOC: boceprevir.
- •
CNI: calcineurin inhibitors.
- •
EPO: erythropoietin.
- •
ETR: end of treatment response.
- •
EVR: early virological response.
- •
FCH: fibrosing cholestatic hepatitis.
- •
G1: genotype 1.
- •
HCV: hepatitis C virus.
- •
LT: liver transplantation.
- •
NR: non-responder.
- •
PEG-IFN: pegylated interferon.
- •
PI: protease inhibitors.
- •
RBV: ribavirin.
- •
RVR: rapid virological response.
- •
SVR: sustained virological response.
- •
TVR: telaprevir.
- •
VL: viral load.
We would like to thank all the patients and investigators involved in the study.
Disclosure- •
Nabiha Faisal: Nothing to disclose.
- •
Eric Yoshida: has been an investigator of clinical trials sponsored by Merck Inc, Vertex Inc, Gilead Sciences, Janssen Inc, Boeringher Ingle-heim Inc, Pfizer Inc, Norvartis Inc, Abbie Vie Inc, Hoffman LaRoche Inc. He has received honouria for CME lectures sponsored by Vertex Canada, Gilead Canada, Hoffman LaRoche Canada He has been a paid speaker for Advisory Board meetings of Boeringher Ingleheim Canada, Vertex Canada and Hoffman LaRoche Canada. He has received an honouariaum for attending an Advisory Board Meeting of Hoffman LaRoche Canada.
- •
Kelly Burak: has been an investigator of clinical trials sponsored by Bayer, Bristol-Myers-Squib, Pfizer, Genentech. He has received honorarium from Astellas for speaking and for attending advisory boards from Vertex, Gilead, Janssen, Astellas and Novartis.
- •
Bandar Al-Judaibi: Nothing to disclose.