The aim of this study was to investigate prophylactic and curative effect of polyphenolic extract of Ichnocarpus frutescense against carbon tetrachloride and tamoxifen induced hepatotoxicity in rats. Carbon tetrachloride and tamoxifen caused liver damage in rats manifested by significant rise in serum enzymes levels. Models of carbon tetrachloride and tamoxifen intoxication elicited significant declines in the reduced glutathione concomitant with significant elevations in malondialdehyde levels. The oral administration of polyphenolic extract to carbon tetrachloride and tamoxifen intoxicated ats, produced significant increments in the reduced glutathione concomitant with significant decrements in malondialdehyde and liver transaminases levels. Prophylactic and curative treatments with the polyphenolic extract generally resulted in a relatively good protection against both carbon tetrachloride and tamoxifen intoxicated rats. The histopathological changes of liver sections showed an improved histological appearance. The extract inhibits CYP monoxygenases aminopyrine-N-demethylase and aniline hydroxylase, suggesting a plausible hepatoprotective mechanism. However prophylactic treatment with the polyphenolic extract exhibited a higher activity compared to curative treatment. The normalization of phenobarbitone induced sleeping time suggests the restoration of liver CYP enzymes. The study shows that hepatoprotective activity of polyphenol extract is by regulating the levels of hepatic microsomal drug metabolising enzymes. These results supported the use of this plant for the treatment of hepatitis in oriental traditional medicine.
The liver is an important organ actively involved in metabolic and detoxification functions and is a frequent and primary target for several toxicants.1 Ingestion of toxicants leads to liver cell necrosis in human as well as in experimental animals.2,3 Drugs continue to be removed from the market with disturbing regularity because of the late discovery of hepatotoxicity. Now, the incidence of acute exposure to xenobiotics has a significant trend to increase and becomes a hazard for the people. Tamoxifen citrate, is an anti-estrogenic, non-steroidal compound widely used for adjuvant therapy in breast cancer.4 This strong hepatotoxic and hepatocarcinogenic effect of tamoxifen in rats raises the issues bearing on the prophylactic chronic administration to healthy women.5 Tamoxifen is metabolized by human CYP 3A4 and 2D6 and is also an effective inducer of CYP 2B2, 2B1, and 3A in rat liver at doses comparable with the therapeutic doses used in humans.6,7
Carbon tetrachloride is highly volatile and is relatively stable in the environment. Carbon tetrachloride produces acute systemic toxicity following ingestion or inhalation. Symptoms of hepatic toxicity (5 ppm or greater) may appear after a delay of one to four days following acute exposure. Since CCl4 toxicity results from its bioactivation mainly by CYP2E1.8-10 Toxicant-or drug-induced liver injury is a potential complication of nearly every medication. We needed to rule out the possibility that the observed hepatic toxicity was due to toxicant or therapeutic drugs for treatment. It is well known that a number of non-toxic herbs are having activities like membrane stabilizing, hepatoprotective, anti-oxidative and CYP2E1 inhibitory effects. Several herbal formulation prevented hepatotoxicity significantly and improved the disease outcome as well as patient compliance without any toxicity or side effects.11
Several endogenous protective mechanisms have been evolved to limit reactive oxygen species and the damage caused by them.12 It was obvious that, certain toxicants (may be drug overdose or harmful substances) have been characterized by their ability to induce liver injury following the cleavage by CYP to form free radicals.13-14 The body has developed mechanisms to remove free radicals. However, since this protection may not be complete, or when the formation of reactive oxygen species is excessive, additional protective mechanisms of dietary antioxidants may be of significance. Therefore, many natural and artificial agents possessing anti-oxidative properties have been proposed to prevent and treat hepatopathies induced by oxidative stress.15 There is an increasing evidence for the hepatoprotective role of flavonoids, polyhydroxy organic compounds and terpenoids particularly from vegetables, fruits and some herbs.16-19
Leaves of Ichnocarpus frutescens (L.) R.Br. (Apocynaceae) boiled in gingelley oil are applied for headaches, fevers and wounds.20 Survey of literature revealed that different pentacyclic triterpenoids and flavonoids have been isolated 21-22. The utilization of decoction of leaves of I. frutescens in the treatment of jaundice, and diabetes is noteworthy.23-24 Some of the active constituents of this plant, such as triterpenoids and flavonoids were shown to present hepatoprotective, antioxidant and related biological activities.25 Our earlier report revealed the antiinflammatory, free radical scavenging, anti-cancers and anti-diabetic activities of the polyphenolic extract of I.frutescens.26-28 In spite of the reported use, no systematic experimental studies have been carried out to assess the hepatoprotective activity of I. frutescens. Therefore, the polyphenolic extract of I. frutescens leaves was investigated for its hepatoprotective effect to assess ethnomedical uses.
Materials and MethodsChemicalsTamoxifen citrate was purchased from Sigma (St. Louis, USA). Phenobarbitone sodium was obtained from Nicholas Piramal India Limited (Mumbai, India). Assay kits aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, γ-glutamyl transferase, total bilirubin and the total proteins were purchased from Span Diagnostic Ltd (Mumbai, India). Reduced glutathione, thiobarbituric acid and 5, 5’-dithiobis (2-nitrobenzoic acid) were purchased from SISCO Research Laboratories Pvt. Ltd (Mumbai, India). Carbon tetrachloride and dimethyl sulfoxide were obtained from Merck (Mumbai, India). All other chemicals were of high purity and obtained from commercial sources.
Plant materialFresh leaves of Ichnocarpus frutescens (L.) R.Br. (Apocynaceae) were collected from the delta region of the Cauvery river, Thiruchirappalli, India, during February 2005 and were authenticated at the Botanical Survey of India, Central National Herbarium Howrah, India (Ref No: CNH/I -I/87/2005-TECH/ 1326). An authentic voucher specimen was deposited at the Herbarium of the Division of Pharmacognosy, Department of Pharmaceutical Technology, Jadavpur University, Kolkata, India.
Preparation of the polyphenolic extract (PPE)Leaves were air dried under room temperature without exposure to sunlight and then coarsely powdered. Powdered leaves (200 g) were macerated with 70 % aqueous/ethanol (500 mL) by stirring at room temperature for 7 days. The extract was decanted, filtered under vacuum, concentrated in a rotary vacuum evaporator (Buchi, India), and then lyophilized (Lyophilization Systems India Pvt Limited, Hyderabad). The powder extract (yielded 23 % w/v) was used for the present study.
Animals and ethical approvalWistar rats of either sex, weighing about 200-250 g body wt were used in the present study (M/s Ghosh Enterprises, Kolkata, India). Animals were collected from breeding colony and acclimatized to the laboratory condition for two weeks. They were housed in macrolon cages under standard laboratory conditions (light period 7.00 am to 7.00 pm, 21 ± 2 °C, and relative humidity 55-70%). The animals were fed with commercial diet from Hindustan Lever Ltd (Bangalore, India) and had free access to water (ad libitum) during the experiments. Experiments were performed complied with the rulings of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) New Delhi, India (Registration No: 0367/01/C/CPCSEA) and the study was permitted by the institutional animal ethical committee (IAEC) of the Jadavpur University, Kolkata, India.
Carbon tetrachloride-induced hepatotoxicityAfter acclimatization, the rats were divided into four groups of six rats each. Liquid paraffin was injected (i.p) to Group I (vehicle group) at a dose of 1 ml/kg body wt (i.p), for 16 days. Concentrated carbon tetrachloride in liquid paraffin (1:2) was injected (i.p) to Group-II (control group) at a dose of 1ml/kg body wt, every 72 h for 16 days (i.e 1,4,7,10,13 and 16 days). Group III (prophylactic group) was treated with the polyphenolic extract (200 mg/kg body wt) for 7 days before carbon tetrachloride injection (1 ml/kg body wt (i.p) in liquid paraffin every 72 h for 16 days) and continued for 16 days. Group IV (curative group) was simultaneously treated with the polyphenolic extract (200 mg/kg body wt) orally along with carbon tetrachloride injection (1 ml/kg body wt, (i.p) in liquid paraffin at every 72 h for 16 days) and continued for 16 days. The details of the groups and treatments are given in Table 1. All animals were sacrificed 24 h after the last dose of carbon tetrachloride injection29.
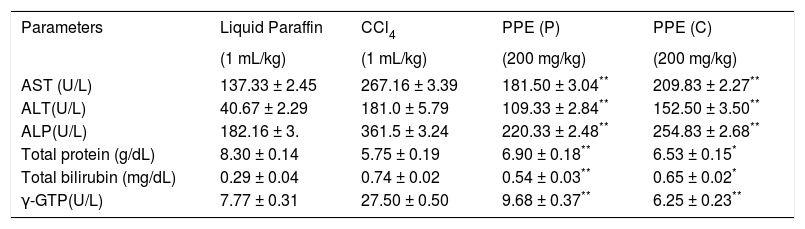
Effects of the polyphenolic extract of I. frutescens on serum biochemical parameters in carbon tetrachloride-induced hepatotoxic rat liver.
| Parameters | Liquid Paraffin | CCl4 | PPE (P) | PPE (C) |
|---|---|---|---|---|
| (1 mL/kg) | (1 mL/kg) | (200 mg/kg) | (200 mg/kg) | |
| AST (U/L) | 137.33 ± 2.45 | 267.16 ± 3.39 | 181.50 ± 3.04** | 209.83 ± 2.27** |
| ALT(U/L) | 40.67 ± 2.29 | 181.0 ± 5.79 | 109.33 ± 2.84** | 152.50 ± 3.50** |
| ALP(U/L) | 182.16 ± 3. | 361.5 ± 3.24 | 220.33 ± 2.48** | 254.83 ± 2.68** |
| Total protein (g/dL) | 8.30 ± 0.14 | 5.75 ± 0.19 | 6.90 ± 0.18** | 6.53 ± 0.15* |
| Total bilirubin (mg/dL) | 0.29 ± 0.04 | 0.74 ± 0.02 | 0.54 ± 0.03** | 0.65 ± 0.02* |
| γ-GTP(U/L) | 7.77 ± 0.31 | 27.50 ± 0.50 | 9.68 ± 0.37** | 6.25 ± 0.23** |
PPE: Polyphenolic extract. P: Prophylactic. C: Curative. Values are expressed as mean ± SEM, (n = 6).
The experiment was performed as described by El-Beshbishy with slight modifications30. Rats were randomly divided into four groups of 6 animals each. Group I served as normal untreated, which received a normal saline (5 ml/ kg body wt). Group II (tamoxifenintoxicated rats) rats were treated with tamoxifen at a dose of 45 mg/kg/day (i.p.) for 7 successive days. Group III rats were treated orally with the polyphenolic extract (200 mg/ kg body wt daily) for 10 days before and 7 days after tamoxifen intoxication (dose identical to Group II) for 18 days as protection against (prophylactic group) liver injury induced by tamoxifen. Group IV rats were simultaneously treated with the polyphenolic extract along with tamoxifen intoxication (dose identical to Group II) for 18 days as protective against (curative treatment) liver injury induced by tamoxifen.
Determination of serum biochemical parametersAt the end of each experiment, the animals were fasted, anesthetized (ketamine 60 mg/kg, i.p.) and killed by cervical decapitation. Blood was obtained by direct cardiac puncture for serum biochemical estimations. Blood samples were kept at room temperature for 1 h and centrifuged at 3000 rpm for 30 min to obtain the serum, which was kept at -20° C until the assay. The levels of AST, ALT, ALP, γ-GT, bilirubin and the total proteins were measured spectrophotometrically (Spekol 1200, Japan) using the Span Diagnostic Kits (Mumbai, India). The abdomen was excised and their livers were removed immediately by dissection, washed in ice-cold isotonic saline and blotted between two filter papers. Their livers were wrapped in aluminium foil and stored at - 80 °C. A 10 % (w/v) liver homogenate was prepared in ice-cold 0.1 M potassium phosphate buffer, pH 7.5 using Teflon homogenizer (Bio-Lab, India). The fresh aliquot from their livers homogenate was used for the estimation of glutathione and lipid peroxidation.31,32
HistopathologyFor histopathological study, liver from each animal was removed after dissection and preserved in 10% formalin. Then representative blocks of liver tissues from each lobe were taken and possessed for paraffin embedding using the standard microtechnique. The paraffin sections, 6 micron thickness were cut and stained with hematoxylin and eosin (H&E) and observed under the light microscope (Zeiss Photomicroscope, India).
Effect of phenobarbitone-induced sleep timeThe antihepatotoxic activity was assessed by determining one functional parameter, i.e., barbiturate sleep time against phenobarbital-induced sleep of rats poisoned with carbon tetrachloride. The time of lost reflex induced by short acting barbiturate is significantly prolonged if any hepatic damage and this can be used as a measure for the function of the liver drug metabolizing enzymes.33-34 Eighteen rats were selected and divided into three groups of six each. Group A was kept as normal and sleep time was determined after injecting phenobarbital (40 mg/kg i.p.). Group B was administered with carbon tetrachloride (1 mL/kg i.p). Group C was given orally a dose of the polyphenolic extract (200 mg/kg). After 24 h a dose of carbon tetrachloride was given to B and C groups, duration of sleep and sleep latency was determined against sodium phenobarbital (40 mg/ kg body wt.) after 2 h of carbon tetrachloride injection to rats.
Inhibition of phase I enzymesThe enzymes were induced in rats by oral administration of phenobarbital sodium (80 mg/ kg body wt) for 5 days35. The animals were sacrificed after an overnight fasting by ether anesthesia and the liver was excised. A 10 % homogenate was used for the assay. Various concentrations of the extract (50-500 ìg/ml) were added to the reaction mixture and incubated for 2 h at 37 °C. p-aminophenol formed during the enzyme action reacts with phenol in alkaline medium to form a blue colored product, which was measured at 630 nm. The percentage inhibitions of aniline hydroxylase and aminopyrene-N-demethylase were calculated by comparing the absorbance of control to that of the drug treated samples.
Statistical analysisThe experimental data were expressed as means ± SEM. The significances of difference among the various treated groups and the control group were analyzed by one-way ANOVA followed by Dunnett’s multiple comparison test using the Graphad Instat Software (San Diego, USA). p < 0.05 was considered as statistically significant.
ResultsEffects of carbon tetrachloride-induced hepatotoxicityEffect on carbon tetrachloride induced hepatotoxicity on rats revealed severe hepatocellular damages evident by the marked elevation in the serum of the levels of AST, ALT, ALP, γ-GT and bilirubin content when compared with the carbon tetrachloride treated rats (Table 1). The different biochemical parameters were found to recover to nearly normal in prophylactic treated and curative treated rats with the polyphenolic extract when compared to carbon tetrachloride. A significant increase was observed in the melondialdehyde concentration of the liver tissue after carbon tetrachloride administration. On the contrary, a marked fall was observed in the reduced glutathione level. Both treatments (prophylactic and curative) with the polyphenolic extract when given orally, prevented progression of carbon tetra-chloride-induced chronic liver injury and lipid peroxidation and level of reduced glutathione was also recouped (Table 3). Analysis of variance (p < 0.01) showed maximum recoupment in the serum biomarkers, lipid peroxidation and reduced glutathione level with the extract at a dose of 200 mg/kg in the prophylactic study.
Effect of polyphenolic extract of I. frutescens on reduced glutathionevand lipid peroxidation level in carbon tetrachloride-induced hepatotoxic rat livers.
| Treatment | Dose | Reduced glutathione | Lipid peroxidation |
|---|---|---|---|
| (μg/mg protein) | (mol MDA/mg protein) | ||
| Liquid paraffin | 1 mL/kg | 15.49 ± 0.51 | 1.57 ± 0.03 |
| CCl4 | 1 mL/kg | 10.24 ± 0.35 | 1.97±0.49 |
| PPE (C) | 200 mg/kg | 13.34 ± 0.38** | 1.61 ± 0.02** |
| PPE (C) | 200 mg/kg | 12.48 ± 0.31** | 1.49 ± 0.25** |
PPE: Polyphenolic extract. P: Prophylactic. C:Curative. Values are expressed as mean ± SEM, (n = 6).
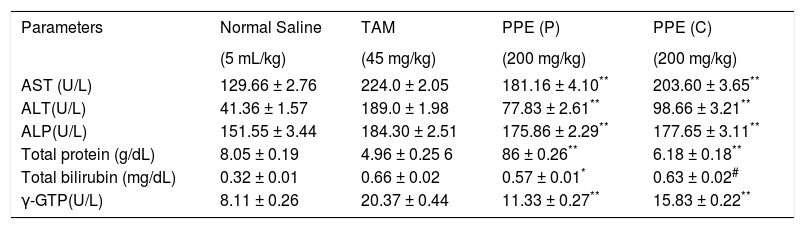
The tamoxifen-treated group manifested a severe liver damage with elevation of AST, ALT, ALP, γ-GT levels. On the contrary, the total protein level was decreased as compared to the tamoxifen treated rats. The higher concentration of bilirubin and the lower of total protein confirm the depth and intensity of liver necrosis. The results on tamoxifen-intoxicated rats are shown in Table 2. These higher levels were markedly suppressed by prophylactic and curative treatments of the polyphenolic extract. But significant reverse changes in all of the parameters were observed in the prophylactic treatment (200 mg/kg) when compared to the curative group. The latter group showed a tendency towards the normal. Intraperitoneal administration of tamoxifen (45 mg/ kg for 7 days) caused a significant increase of lipid peroxidation. In addition, the level of reduced glutathione was drastically reduced in tamoxifen-intoxicated rats. Oral treatment with the polyphenolic extract significantly (p < 0.01), prevented elevated lipid peroxidation (Table 4). Pre-administration of the polyphenolic extract significantly increased the level of reduced glutathione in the liver when compared to the post treated tamoxifen intoxicated groups.
Effects of the polyphenolic extract of I. frutescens on serum biochemical parameters in tamoxifen-induced hepatotoxic rat liver.
| Parameters | Normal Saline | TAM | PPE (P) | PPE (C) |
|---|---|---|---|---|
| (5 mL/kg) | (45 mg/kg) | (200 mg/kg) | (200 mg/kg) | |
| AST (U/L) | 129.66 ± 2.76 | 224.0 ± 2.05 | 181.16 ± 4.10** | 203.60 ± 3.65** |
| ALT(U/L) | 41.36 ± 1.57 | 189.0 ± 1.98 | 77.83 ± 2.61** | 98.66 ± 3.21** |
| ALP(U/L) | 151.55 ± 3.44 | 184.30 ± 2.51 | 175.86 ± 2.29** | 177.65 ± 3.11** |
| Total protein (g/dL) | 8.05 ± 0.19 | 4.96 ± 0.25 6 | 86 ± 0.26** | 6.18 ± 0.18** |
| Total bilirubin (mg/dL) | 0.32 ± 0.01 | 0.66 ± 0.02 | 0.57 ± 0.01* | 0.63 ± 0.02# |
| γ-GTP(U/L) | 8.11 ± 0.26 | 20.37 ± 0.44 | 11.33 ± 0.27** | 15.83 ± 0.22** |
PPE: Polyphenolic extract. TAM: Tamoxifen. P: Prophylactic. C: Curative. Values are expressed as mean ± SEM, (n = 6).
Effects of the polyphenolic extract of I. frutescens on reduced glutathione and lipid peroxidation level in tamoxifen-induced hepatotoxic rat livers.
| Treatment | Dose | Reduced glutathione (μg/mg protein) | Lipid peroxidation (mol MDA/mg protein) |
|---|---|---|---|
| (μg/mg protein) | (mol MDA/mg protein) | ||
| Normal saline | 5 mL/kg | 16.93 ± 0.34 | 1.61 ± 0.01 |
| Tamoxifen | 45 mL/kg | 11.61 ± 0.22 | 3.37 ± 0.13 |
| PPE (C) | 200 mg/kg | 13.54 ± 0.24** | 2.44 ± 0.15** |
| PPE (C) | 200 mg/kg | 13.18 ± 0.22** | 2.84 ± 0.07** |
PPE: Polyphenolic extract. P: Prophylactic. C: Curative. Values are expressed as mean ± SEM, (n = 6).
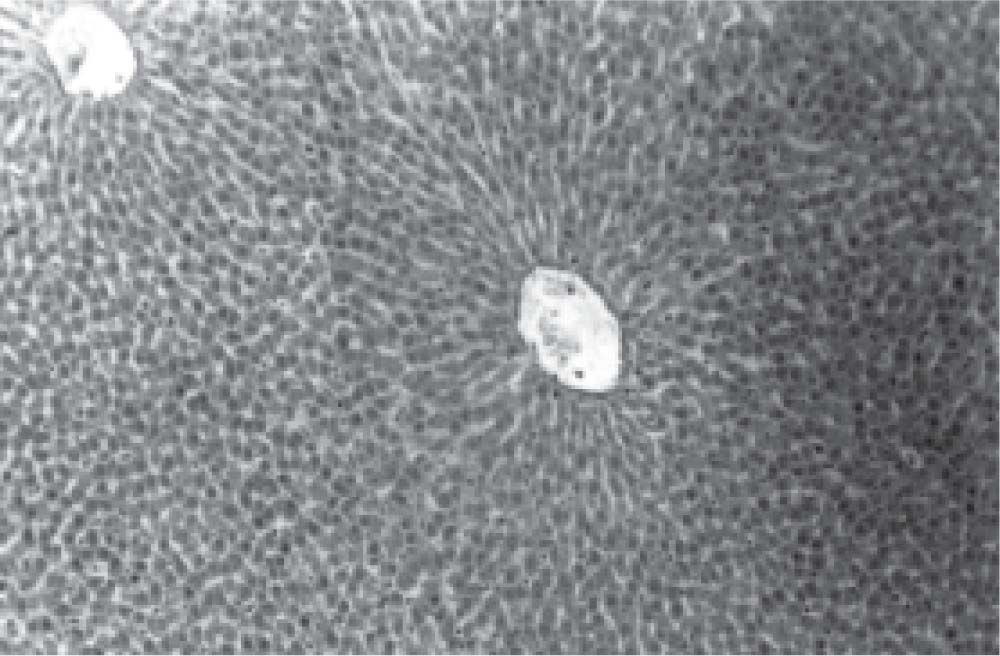
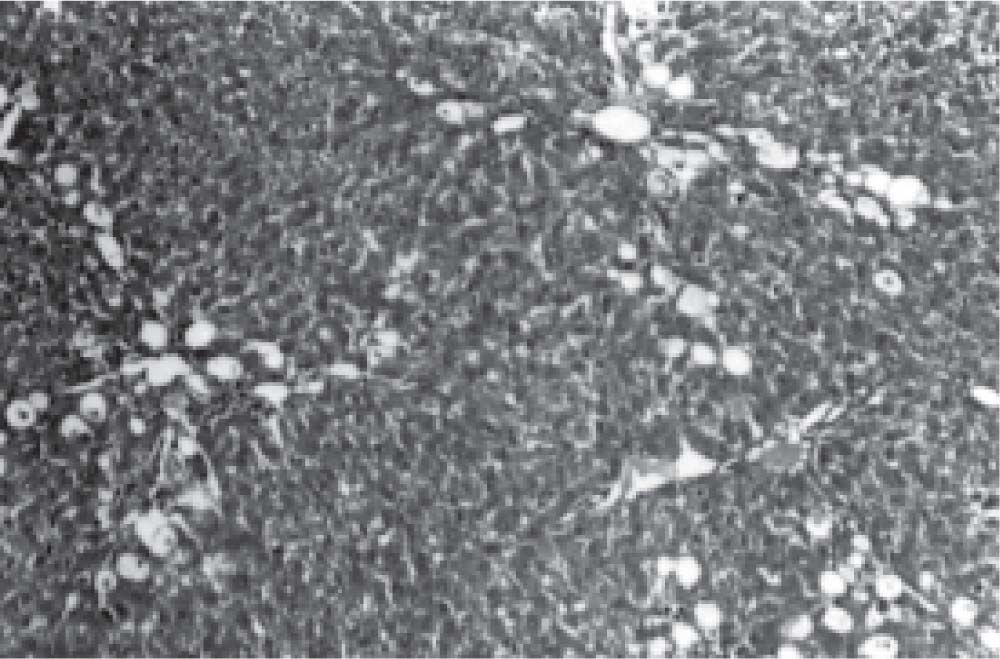
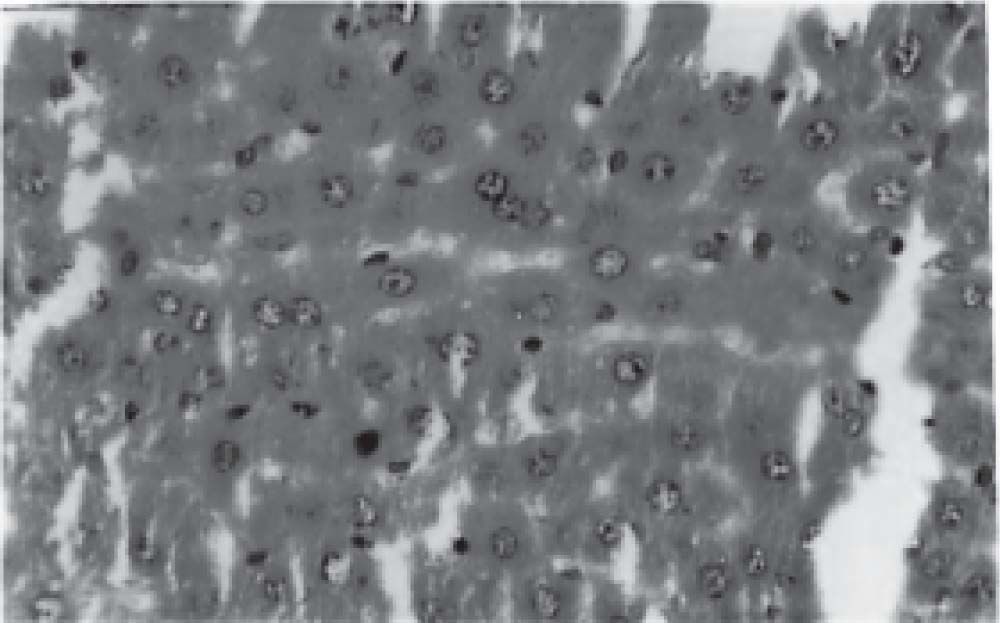
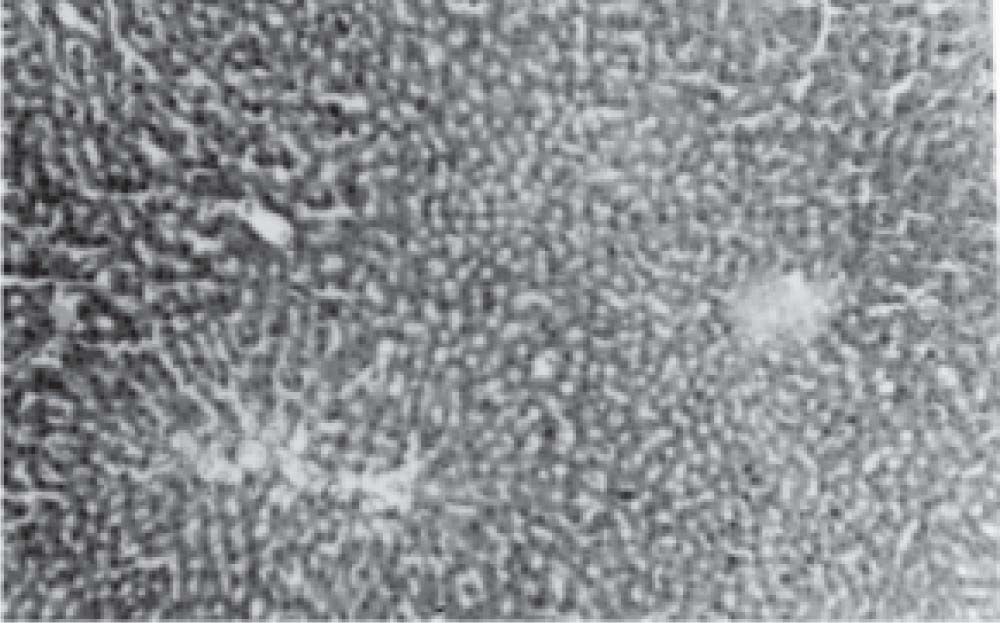
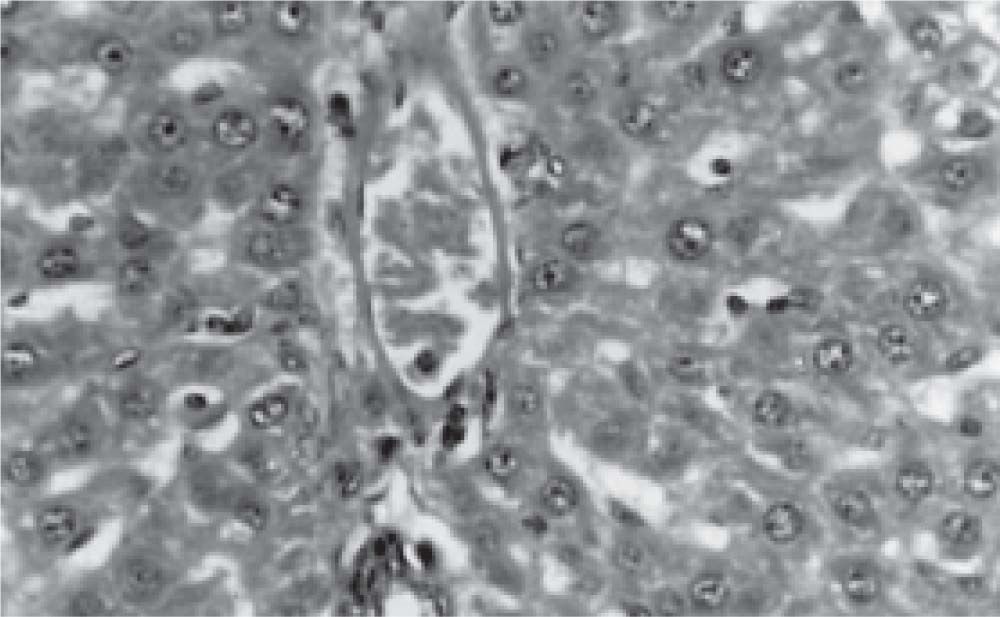
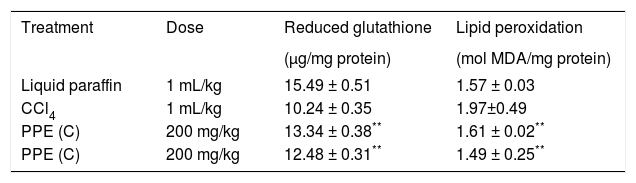
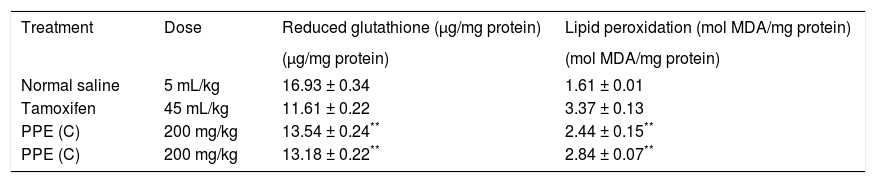
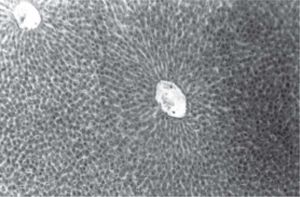
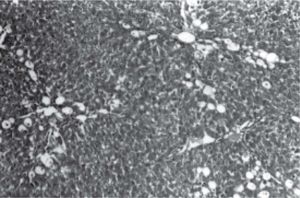
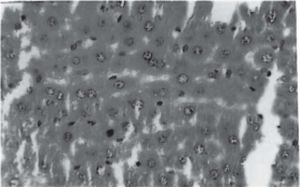
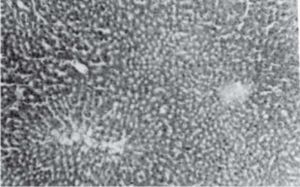
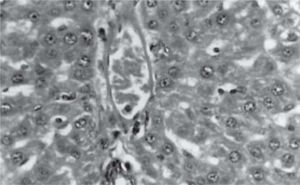
Figure 1 exhibits the normal anatomy of the rat liver treated with liquid paraffin. Figure 2 & 3 shows an abnormal anatomy with loss of hepatic lobules, degeneration of fatty cells and cloudy swelling of the liver treated with carbon tetrachloride and tamoxifen respectively; pictures were similar to that was reported earlier.36 Kupffer and Sinusoidal cells demonstrated an arrest in distribution. On the other hand, no changes or necrosis in fatty cells were observed and the arrangements of the hepatocytes were almost normal in the rats treated with the polyphenolic extract in carbon tetra chloride (Figure 4) and tamoxifen (Figure 5) intoxicated rats.
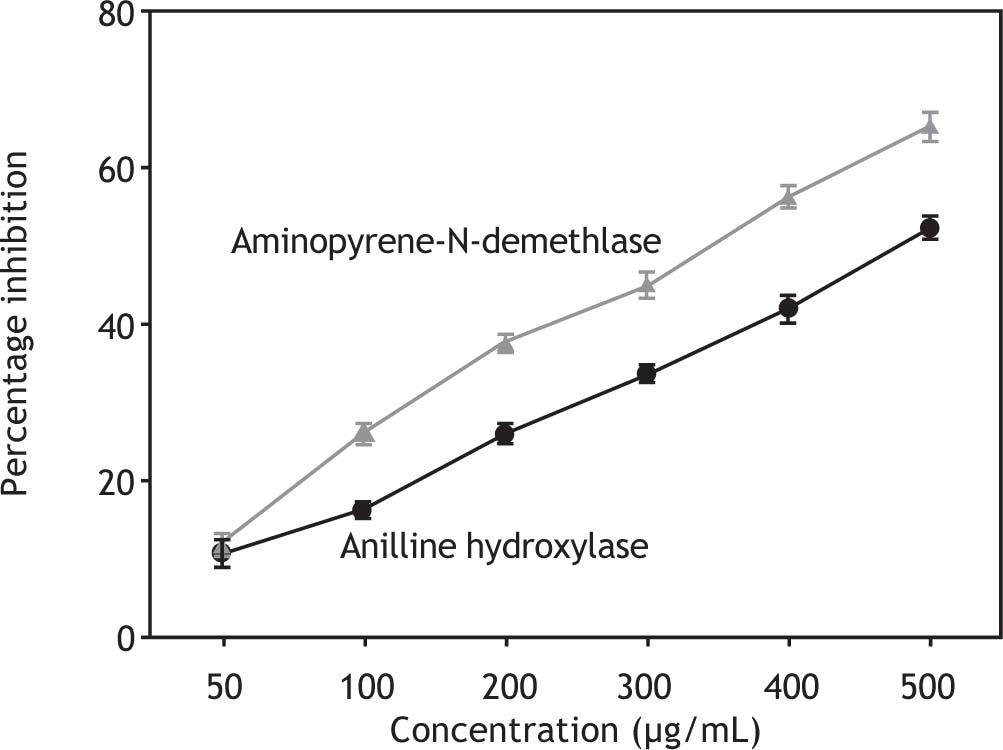
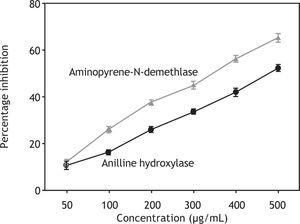
Administration of the polyphenolic extract (200 mg/kg) 1 h before carbon tetrachloride injection significantly shortened the phenobarbital-induced sleep time (Table 5). Aniline hydroxylase and aminopyrene-N-demethylase, which are phase I enzymes and are present in the CYP, becomes implicated in the activation of carcinogens to their ultimate form. The polyphenolic extract inhibits the activities of both enzymes in a concentration-dependent manner (Figure 6) and the concentration needed for 50 % inhibition was found to be 479.05 μg/mL and 344.97 μg/ mL for aniline hydroxylase and aminopyrene-N-demethylase, respectively.
Effects of the polyphenolic extract of I. frutescens on Phenobarbital-induced sleep time in carbon tetrachloride-induced intoxicated rats.
| Treatment | Sleep Latency (min) | Duration of sleep (min) |
|---|---|---|
| Control | 3.83 ± 0.57 | 84.33 ± 2.60 |
| CCl4 + Phenobarbitol | 3.42 ± 0.18 | 187.66 ± 2.49 |
| CCl4 + Phenobarbitol+ PPE | 2.43 ± 0.17 | 106.0 ± 2.47 |
Dose: CCl4 -1 ml/kg; Phenobarbital -40 mg/kg; PPE -200 mg/kg.PPE -polyphenolic extract; Values are expressed as mean ± SEM, (n = 6).
*Significantly different from control group at p < 0.01.
Inhibition of aniline hydroxylase and aminopyrene-N-demethylase enzyme activities by polyphenolic extract. Values were shown as mean ± SEM of triplicate experiments. Statistical significance of the treatment was done by using Student’s-test. Results were expressed as percentage inhibition of CYP enzymes. Concentration needed for 50% inhibition (IC50) of aniline hydroxylase was 4.6 479.05 μg/ml while concentration needed for aminopyrene-N-demethylase was 344.97 μg/ml.
Carbon tetrachloride is an extensively studied liver toxicant, and its metabolites trichloro methyl radical (CCl3 • -) and trichloro methyl peroxy radical (CCl3O2 • -) are known to be involved in the pathogenesis of liver.37-39 These free radicals may bind directly to microsomal lipids and other cellular macro-molecules contributing to the breakdown of membrane structure and disrupting cell energy processes and reactions. Prophylactic administration of the polyphenolic extract at a dose of 200 mg/kg for 7 days was effective to ameliorating morphological and biochemical indices of carbontetrachloride induced hepatotoxicity. Simultaneous treatment with the polyphenolic extract for 18 days was also effective in mitigating a few biochemical signs of carbon tetrachloride-induced liver damage. The prophylactic treatment mediated suppression of the increased AST, ALT, ALP, γ-GT activities suggesting a possible protection against liver injury, and restoration of the level of total serum protein confirming thus its hepatoprotective action. A significant increase in hepatic lipid peroxidation was observed after carbon tetrachloride exposure. Alterations in the structure of the endoplasmic reticulum and other membranes, and loss of the metabolic enzyme activation may lead to liver damage.40
The pretreatment of carbon tetrachloride-intoxicated rats with polyphenolic extract elicited the strongest effect in inducing a highly significant decline in TBARS and a highly significant increase in the level of reduced glutathione in comparison to tamoxifen-intoxicated rats. Results suggest that the polyphenolic extract reduces the oxidative stress in rats with carbon tetrachloride and tamoxifen treatment. Taken together, the current study shows that prophylactic administration of the polyphenolic extract enhances the protection against hepatic damage caused by free radicals and lipid peroxidation, and strengthens the detoxification by carbon tetrachloride and tamoxifen in rats. The ability of a hepatoprotective drug to reduce the injurious effects or to preserve the hepatic physiological mechanisms showed the index of its protective effects. Although serum enzyme levels are not a direct measure of hepatic injury, they show the functional status of the liver. Lowering of the enzyme level is a definite indication of hepatoprotective action of the polyphenolic extract.
On the other hand, liver injury caused by the administration of tamoxifen, shows an increased activities of AST, ALT, ALP, γ-GT. Tamoxifen citrate is an anti-estrogenic drug widely used for the treatment of breast cancer.41 It was obvious that tamoxifen in toxic doses lead to oxidative liver damage as it has been elucidated to be a hepatocarcinogen in rats.42 It may be more toxic to liver because it has much higher affinity to hepatic tissues than to any other tissues. The status of oxidative stress after tamoxifen administration accompanies the decreased hepatic glutathione content and the increased lipid peroxidation. One week pre-treatment with the polyphenolic extract significantly improved serum biochemical parameters. These results show that the polyphenolic extract significantly inhibited lipid peroxidation in liver induced by tamoxifen. The decrease in antioxidant defense systems in animals renders them more susceptible to the hepatotoxcity. In the tamoxifen model prophylactic treatment with the polyphenolic extract also reduced the degree of hepatocellular injury as evidenced by the improved serum biochemical parameters and malondialdehyde levels in tamoxifen-treated rat livers.
Oral administration of the polyphenolic extract shortened the phenobarbital-induced sleep time in carbon tetrachloride-treated rats, since barbiturates are metabolized exclusively in the liver34. Prolonged sleep time after exposure of rats to carbon tetrachloride in rats shows an impaired metabolism of these hypnotics possibly due to carbon tetrachloride-induced liver damage. The treatment of animals with carbon tetrachloride caused damage to the microsomal drug-metabolizing enzymes in the hepatocytes leading to substantial decrease of the hepatic drug metabolizing capacity, which reflects a prolongation of the phenobarbital-induced sleep time.
CYP is involved in several biological interactions involving hydroxylation, and oxidative deamination, demethylation, dehydrogenation.43,44 While some of the biological reactions of CYP are highly essential for the removal of xenobiotics from the body, these reactions are also involved in the activation of xenobiotics to active metabolites, which can be highly injurious to the body. Hence CYP can be considered as double-edged weapon and its control is highly needed in the physiological balance of the body. Tamoxifen has been found to be metabolized by liver primarily into three metabolites, tamoxifen-N-oxide, N-desmethyl and 4- hydroxytamoxifen, formed by CYP. The N-demethylation was demonstrated to be catalyzed by CYP3A4 in rat and human liver. The polyphenolic extract has also been found to inhibit enzymes responsible for the activation of tamoxifen, such as, aniline hydroxylase and aminopyrene-N-demethylase. The inhibition of enzymes activity in vitro could be due to the direct inactivation of the enzymes or in vivo inhibition of enzyme expression induced by phenobarbitone.
The polyphenolic extract may interfere with the CYP and ultimately kinder the formation of free radicals and metabolites (4-hydroxy tamoxifen). The ability of the polyphenolic extract of I. frutescens to reduce the prolongation of phenobarbital-induced sleep in carbon tetrachloride-exposed rats is further supportive for the anti-hepatotoxic potential of the polyphenolic extract. Despite being an inhibitor of phase I enzymes, the polyphenolic extract does not cause harmful bioactivation. In vivo studies have showed that the constituents of I. frutescens decrease the hepatotoxicity of carbon tetrachloride and tamoxifen by favourably influencing hepatic metabolism. The probable reason for this favourable effect is inhibition of phase I enzymes, which results in the rapid clearance of the potentially toxic metabolite.
Medicinal practioners have prescribed Ayurveda and drug from herbal origin as a system of medicine in India over centuries. Treatment options for common liver diseases such as cirrhosis, fatty liver and chronic hepatitis are problematic. Physician and patients are in need of effective therapeutic agents with a low incident of side effects. In India, more than 87 plants are used in different combinations in the preparation of 33 patented herbal formulations for liver disorders. Plants traditionally used in the alleviation of liver dysfunctions might therefore provide a useful source of new hepatoprotective compounds for development as pharmaceutical entities or as simple adjuncts to existing clinical therapies.
Our results showed that polyphenolic extract of I. frutescens (both prophylactic and curative) could protect the experimental animals from the deleterious effects of carbon tetrachloride and tamoxifen on liver. Moreover, the use of polyphenolic extract as prophylactic is more effective than its curative against carbon tetrachloride and tamoxifen induced liver injury. The polyphenolic extract alleviates the symptoms of liver damage as evidenced by biochemical and histopathological studies. Further clinical studies will justify the use of polyphenolic extract of I. frutescens as an adjuvant to any kind of patients with tamoxifen and carbontetra chloride intoxication.
AcknowledgementsThe authors are grateful to All India Council of Technical Education (AICTE), Government of India, New Delhi, India, for financial support.
Abbreviations- •
PPE: Polyphenolic extract.
- •
CPCSEA: Committee for the Purpose of Control and Supervision of Experiments on Animals.
- •
SEM: Standard error mean.
- •
ANOVA: Analysis of variance.
- •
CCl4: Carbon tetrachloride.
- •
AST: Aspartate aminotransferase.
- •
ALT: Alanine aminotransferase.
- •
TBARS: Thiobarbituric acid reactive substances.
- •
ALP: Alkaline phosphatise.
- •
γ-GT: Gamma glutamyl transferase.
- •
AICTE: All India Council of Technical Education.
- •
AICTE, bw: body weight, ip: intraperitoneal, w/v: weight/volume, g: gram, kg: kilogram, ml: milliliter.
- •
TAM: Tamoxifen.
- •
MDA: Malondialdehyde.
- •
h: hour.