Background.Centaurea americana, Krameria ramosissima, Juglans mollis and Turnera diffusa are used by traditional healers in the northeastern region of Mexico to protect against liver damage. However, the hepatoprotective properties of these plants have not been investigated scientifically. This study reports on the protective effects of these plants using an in vitro assay.
Material and methods. Extracts of plants were tested for antioxidant activity using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging method. The effects of extracts from these plants on a human hepatoma cell line (Huh7) were evaluated according to cell viability and aspartate aminotransferase and malondialdehyde levels before and after exposure of the cells to carbon tetrachloride (CCl4).
Results. All extracts reduced DPPH levels by more than 50%. C. americana flower and stem/leaf extracts, the aerial part of T. diffusa, and the nut, leaf and bark of J. mollis extracts were used to assess hepatoprotective activity. The extract of the aerial part of K. ramosissima was toxic. Pretreatment of Huh7 cells with extracts from the flower of C. americana (FCA), the stem/leaf fraction of C. americana (S/LCA), the leaf of J. mollis (LJM) and the bark of J. mollis (BJM) prior to the CCl4 challenge, protected against CCl4-induced liver damage, as evidenced by a significant decrease in the activity of the medium enzyme. The FCA, S/LCA, LJM and BJM extracts showed significant antilipid peroxidant effects in vitro. In conclusion, the hepatoprotective effects of the FCA, S/LCA, LJM and BJM extracts observed in this study may result from their antioxidative properties.
Chronic liver disease is an increasing burden to society. The World Health Organization estimates that 46% of global disease and 59% of mortality is due to chronic disease.1 In Mexico, it will be a significant cause of morbidity and mortality in the near future.2 As well as available treatments for liver diseases, the population uses herbal products, nutraceuticals and/or plant preparations, which may consist of powdered plant material, extracts, tinctures, fatty acids or essential oils.3,4 It is estimated that 70-80% of people worldwide rely chiefly on traditional, largely herbal, medicine to meet their primary health care needs.5
Plants have contributed immensely to Western medicine by providing ingredients for drugs. They have also played a central role in drug discovery. Some drugs are still extracted directly from plants. Others are produced by transformation of chemicals found in them, while still others are synthesized. Many have their historical origin in research on active compounds found in plants.6-8
Mexico is a country with a large biodiversity and a historical tradition of using medicinal plants.9,10 However, although more than 1,000 plants are used to treat diseases, less than 20% of these have been scientifically evaluated.11
Centaurea americana, Krameria ramosissima, Juglans mollis and Turnera diffusa are used by traditional healers in northeastern Mexico to protect against liver damage.12 However, these protective effects have not been evaluated. We assessed the hepatoprotective effects of these plants using a cell culture model consisting of a human hepatoma cell line that was treated with CCl4 to induce hepatocyte damage.
Materials and MethodsPlant material and chemicalsC. americana, K. ramosissima, T. diffusa and J. mollis specimens were collected in the Mexican states of Coahuila and Nuevo León during the spring and summer of 2006. The plants were authenticated and voucher specimens of each plant (UAN-17624, UAN-4850, UAN-8272 and UAN-17854, respectively) were deposited in the institutional herbarium located in the Facultad de Ciencias Biológicas of the Universidad Autónoma de Nuevo León.
Absolute anhydrous ethyl alcohol was obtained from Mallinckrodt Baker (México City, México). Si-libinin, 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) and carbon tetrachloride (CCl4, 99.9%) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Dimethyl sulfoxide was purchased from ACS Research Organics (Cleveland, OH, USA). Thiobarbituric acid reactive substances were purchased from Kit OXItek (Buffalo, NY, USA). Dulbecco's modified Eagle's medium, fetal bovine serum trypsin 0.25% (1x), penicillin G (100 μg/mL), streptomycin (100 pg/mL) and phosphate-buffered saline were purchased from Gibco Invitrogen (Carlsbad, CA, USA).
Hydroalcoholic extractsC. americana samples were separated into flower and stem/leaf portions and both were used; the aerial parts of K. ramosissima and T. diffusa were used, and J. mollis samples were separated into nut, leaf and bark portions and were used. Each portion was dried at room temperature for two weeks and then ground finely. The extracts were obtained by shaking 50 g of dried material in a 300 mL volume of ethanol and water (90:10) for 30 minutes. Three extracts of each sample were prepared. The extracts were filtered and concentrated under reduced pressure at 37 °C, dried in an oxygen-free environment at 37 °C, and stored at -80 °C until used.
Determination of antioxidant activitysThe antioxidant activity of the hydroalcoholic extracts was evaluated using the DPPH radical scavenging method.13 A 1,000 μg/mL extract and a 0.2 mg/mL solution of DPPH in pure ethanol were prepared. Five hundred microliters of each solution were then mixed and allowed to stand at room temperature in the dark for 30 minutes. Absorbance at 517 nm was then measured using a Beckman DU 7500 spectrophotometer (Beckman Coulter, Fullerton, CA, USA). Quercetin was used as a positive control. Three analytical replicates were carried out on each sample.
The reduction percentage was estimated using the following equation: Scavenging effect (%) = (A-B) × 100/A, where A = absorbance in the presence of DPPH alone (blank) and B = absorbance in the presence of DPPH and the extract.
Cell cultureCells were grown in Dulbecco’s modified Eagle’s medium supplemented with heat-inactivated fetal bovine serum (10%), penicillin G (100 IU/mL), streptomycin (100 μg/mL) and 1% nonessential amino acids at 37 °C in a humidified atmosphere containing 95% O2 and 5% CO2. At 80-90% confluence, the cells were trypsinized and plated (1 × 106 cells per well). The cells were used after attachment.
Effects of plant extracts on Huh7 cellsThe cytotoxicity of plant extracts on Huh7 cells were evaluated using cell viability and levels of aspartate aminotransferase (AST) and malondialdehyde (MDA) in the medium after exposure to extracts at concentrations of 10, 100, and 1,000 μg/ mL in phosphate-buffered saline for one hour. Silibinin was used as a control. Each assay was performed in triplicate and experiments were repeated three times.
Cell viability assayCell viability was assessed using the MTT reduction assay with slight modifications.14 This colorimetric assay involves the conversion of MTT to a purple formazan derivative by mitochondrial succinate dehydrogenase, which is present only in viable cells. Huh7 cells (1 μ 106) were treated with each plant extract (10, 100 or 1000 ×g/mL in phosphate-buffered saline) for one hour. After removal of the extracts from the wells, the cells were washed with phosphate-buffered saline. The cells were then incubated with 150 μL of MTT (0.5 mg/mL) for two hours, after which the medium was removed and 560 μL of dimethyl sulfoxide was added to dissolve the formazan crystals that had formed. Absorbance was measured in an ELISA microplate reader at 570 nm (Multiskan Ex; Thermo Labsystems, Vantaa, Finland). Viability was defined as the ratio (expressed as a percentage) of absorbance of treated cells to untreated control cells.
AST measurementAST activity in the supernatant after exposure of cells to various extract concentrations of plant extracts for one hour was determined. The supernatant was stored at -80 °C until analysis. AST activity was determined using slides in a biochemical autoanalyzer (Vitros DTII Systems Chemistry, module DTSCII; Johnson & Johnson Ortho-Clinical Diagnostics, New Brunswick, NJ, USA).
Lipid peroxidation assayLipid peroxidation was measured by quantifying thiobarbituric acid reactive substances in supernatants after exposure of cells to various extract concentrations for one hour according to the method of Kit OXItek. The supernatants were stored at -80 °C until analysis. Fluorescence intensity was determined using a 530 nm excitation filter and a 550 nm emission filter in an LS45 spectrophotometer (Perkin Elmer, Waltham, MA, USA). The results were expressed as MDA equivalents.
In vitro assay for hepatoprotective effectsCells were treated with various concentrations of plant extracts (10, 100 and 1,000 μg/mL in phosphate-buffered saline) for one hour, followed by the addition of 40 mM CCl4 in 0.05% dimethyl sulfoxide and subsequent incubation for two hours. The supernatant medium was then collected and stored at 80 °C until quantification of AST and MDA levels. We incubated Huh7 cells with silibinin (100 μg/mL) under the same conditions as a hepatoprotective positive control.
Statistical analysisAll variables were measured in triplicate and experiments were repeated three times. Results are expressed as the mean ± standard deviation. Data were analyzed using Student’s t-test, a multivariate linear model and Pearson’s correlation method. SPSS software (v.15.0; SPSS Inc., Chicago, IL, USA) was used for all analyses. A P < 0.05 was considered significant.
ResultsAntioxidant activityAt a concentration of 1,000 μg/mL, the flower extract of C. americana, the aerial extract of T. diffusa and the leaf and bark extract of J. mollis exhibited DPPH free radical scavenging activity (79 ± 1.6%, 82.4 ± 4.6%, 80 ± 2%, 76.8 ± 1.2%, respectively), followed by the nut extract of J. mollis (68 ± 3%), the C. americana stem/leaf extract (62.5 ± 4.8%) and the K. ramosissima extract (53 ± 1.78%). Quercetin exhibited a DPPH free radical scavenging activity of 90.08 ± 0.9% at a concentration of 1,000 μg/mL.
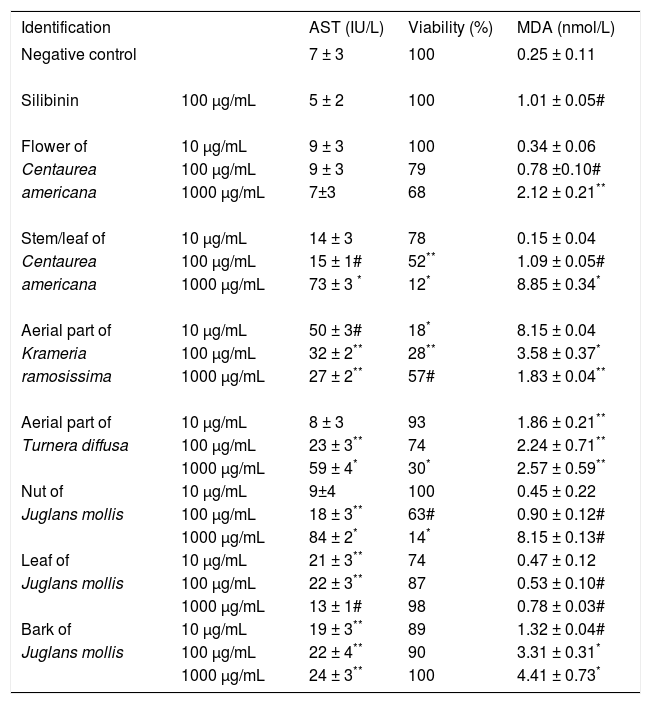
Effects of the hydroalcoholic extracts on Huh7 cellsThe cytotoxicity of plant extracts on Huh7 cells is listed in Table 1. The plant extracts were toxic when there was a decrease in cell viability of more than 60% compared with untreated cells, an AST level > 50 IU/L or an MDA level greater than that of the control. According to these parameters, 1,000 μg/mL of the C. americana stem/leaf extract, the aerial extract of T. diffusa, the nut extract of J. mollis and all concentrations of the aerial extract of K. ramosissima were toxic. All the other extracts were used to evaluate hepatoprotective activity.
Effect of hydroalcoholic extract of Centaurea americana, Krameria ramosissima, Turnera diffusa and Juglans mollis on Huh7 cell line.
| Identification | AST (IU/L) | Viability (%) | MDA (nmol/L) | |
|---|---|---|---|---|
| Negative control | 7 ± 3 | 100 | 0.25 ± 0.11 | |
| Silibinin | 100 μg/mL | 5 ± 2 | 100 | 1.01 ± 0.05# |
| Flower of | 10 μg/mL | 9 ± 3 | 100 | 0.34 ± 0.06 |
| Centaurea | 100 μg/mL | 9 ± 3 | 79 | 0.78 ±0.10# |
| americana | 1000 μg/mL | 7±3 | 68 | 2.12 ± 0.21** |
| Stem/leaf of | 10 μg/mL | 14 ± 3 | 78 | 0.15 ± 0.04 |
| Centaurea | 100 μg/mL | 15 ± 1# | 52** | 1.09 ± 0.05# |
| americana | 1000 μg/mL | 73 ± 3 * | 12* | 8.85 ± 0.34* |
| Aerial part of | 10 μg/mL | 50 ± 3# | 18* | 8.15 ± 0.04 |
| Krameria | 100 μg/mL | 32 ± 2** | 28** | 3.58 ± 0.37* |
| ramosissima | 1000 μg/mL | 27 ± 2** | 57# | 1.83 ± 0.04** |
| Aerial part of | 10 μg/mL | 8 ± 3 | 93 | 1.86 ± 0.21** |
| Turnera diffusa | 100 μg/mL | 23 ± 3** | 74 | 2.24 ± 0.71** |
| 1000 μg/mL | 59 ± 4* | 30* | 2.57 ± 0.59** | |
| Nut of | 10 μg/mL | 9±4 | 100 | 0.45 ± 0.22 |
| Juglans mollis | 100 μg/mL | 18 ± 3** | 63# | 0.90 ± 0.12# |
| 1000 μg/mL | 84 ± 2* | 14* | 8.15 ± 0.13# | |
| Leaf of | 10 μg/mL | 21 ± 3** | 74 | 0.47 ± 0.12 |
| Juglans mollis | 100 μg/mL | 22 ± 3** | 87 | 0.53 ± 0.10# |
| 1000 μg/mL | 13 ± 1# | 98 | 0.78 ± 0.03# | |
| Bark of | 10 μg/mL | 19 ± 3** | 89 | 1.32 ± 0.04# |
| Juglans mollis | 100 μg/mL | 22 ± 4** | 90 | 3.31 ± 0.31* |
| 1000 μg/mL | 24 ± 3** | 100 | 4.41 ± 0.73* |
Results are expressed as the mean ± standard deviation for nine experiments. AST, aspartate aminotransferase; MDA, malondialdehyde. Each experiment included an untreated control.
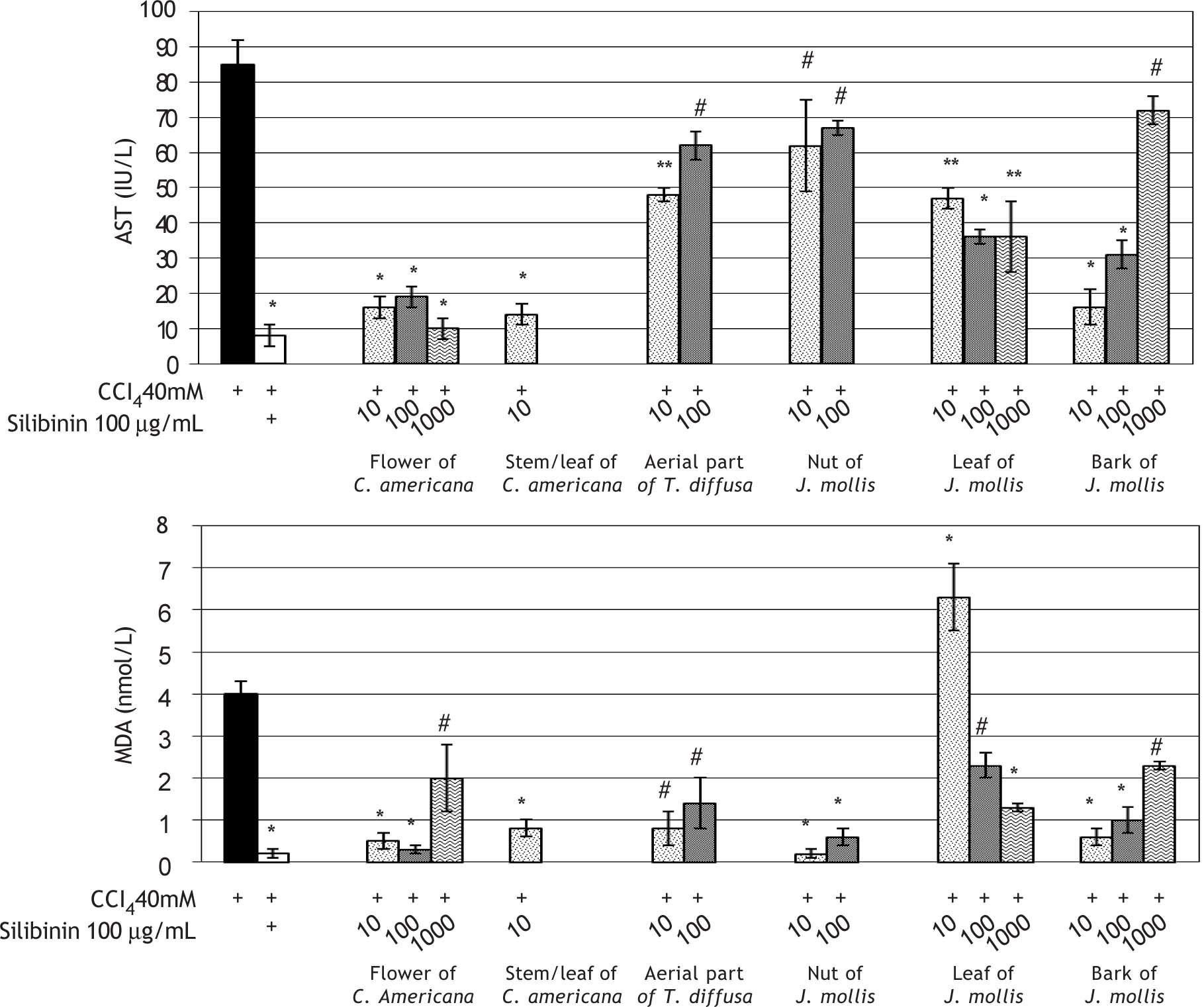
The effects of the six plant extracts on cells treated with CCl4 are shown in Figure 1. AST levels were significantly elevated after treatment with CCl4. Treatment with 100 μg/mL silibinin reduced CCl4-induced elevation of AST level at all concentrations of C. americana flower extract, C. americana stem/leaf extract (10 μg/mL), J. mollis leaf extract (100 and 1000 μg/mL) and J. mollis bark extract (10 and 100 μg/mL) (P < 0.001) (Figure 1A).
Hepatoprotective effects of various concentrations of extracts of the flower or stem/leaf portions of C. americana, Aerial part of T.diffusa, Nut or Leaf or Bark portions of J.mollis on Huh7 cells exposed to CCl4. AST: Aspartate aminotransferase. MDA: Malondialdehyde equivalents. (A) AST (IU/L) and (B) MDA (nmol/mL). Values are expressed as the mean ± standard deviation for nine experiments. *P<0.001; **P=0.001; #, P=0.05.
Cells exposed to CCl4 showed a significant increase in MDA level. All extracts prevented lipid peroxidation when added at levels of 10 pg/mL, 100 pg/mL and 1000 pg/mL, except for the 10 pg/mL J. mollis leaf extract, which resulted in an increase in MDA level greater than that induced by CCl4 (Figure 1B).
DiscussionIn recent years, in vitro systems have been used extensively in several scientific disciplines for various applications: screening of plant extracts or compounds, evaluation of protective effects and determination of characteristic liver lesions and associated biochemical mechanisms induced by toxic compounds.15,16 Model systems involving primary cell cultures, immortalized cell lines (Huh7 and HepG2), partial or whole livers and perfused livers are well established and have been used to investigate potential hepatoprotective drugs and compounds.17,18 To the best of our knowledge, this is the first study to report the in vitro hepatoprotective effects of C. americana, K. ramosissima, T. diffusa and J. mollis extracts using a cell culture model consisting of a human hepatoma cell line that was treated with CCl4 to induce hepatocyte damage.
Many plant-derived natural products worldwide have the potential to be protective and therefore can be used to treat acute and chronic liver diseases.19-23 The hepatoprotective effects of plants are mainly attributed to the presence of flavonoids, coumarins, phenolic acids and antioxidants.24-27 The scavenging activity of the DPPH free radical is used to assess the antioxidant properties of amines, phenols, vitamins, plant extracts and medicinal drugs.28 In the DPPH radical-scavenging assay, all hydroalcoholic crude extracts exhibited an antioxidant activity greater than 50%. The C. americana flower extract, the aerial extract of T. diffusa, the J. mollis leaf extract and the J. mollis bark extract exhibited antioxidant activity greater than 80% in the DPPH assay, which was more than that of the other extracts.
Based on the results of the MTT assay, AST level and the level of thiobarbituric acid reactive substances, C. americana flower and stem/leaf extracts, the aerial extract of T. diffusa and the nut, leaf and bark extracts of J. mollis extracts were assessed for hepatoprotective activity. In contrast with the aerial extract of K. ramosissima, these extracts were not toxic.
CCl4 is a toxic compound commonly used to evaluate the hepatoprotective effects of plant extracts in vivo and in vitro.29 CCl4 is metabolized by hepatocytes to the highly reactive trichloromethyl peroxy radical (CCl3O2•), which interacts with a hydrogen atom in the fatty acid membrane of the endoplasmic reticulum, inducing malondialdehyde production and the release of enzymes from the cell.30,31 Our results showed that pretreatment of cells with all concentrations of C. americana flower extract, 10 μg/mL of C. americana stem/leaf extract, 100 μg/mL and 1,000 μg/mL of J. mollis leaf extract and 10 μg/ mL and 100 μg/mL of J. mollis bark extract caused a significant reduction in CCl4-induced serum enzyme and MDA levels, indicating that they exerted hepatoprotective effects.
The hepatoprotective effect of plants may be attributed to their antioxidant content because antioxidants scavenge free radicals in cases of liver disease.32,33 For example, the hepatoprotective effect of Silybum marianum, a member of the Asteraceae family, is mainly due to its antioxidant content.26,34,35
C. americana Nutt belongs to the Asteraceae (Compositae) family and is commonly known as the “Jolly Joker” or “Basket” flower. It is an annual plant that grows in the southern United States and the bordering states of México, such as Nuevo León.36 It is claimed to have anti-inflammatory properties and is used to treat gastrointestinal distress and as a muscle relaxant.37 In addition, its antioxidant activity has been described and a lethality assay of lignan metabolites and phytoecdysteroids extracted from the seeds has been conducted.38
Several plants belonging to the genus Juglans are known to possess liver protective effects,39 antioxidative activity, human cancer cell antiproliferative effects40,41 and an antidiabetic effect.42 The walnut tree Juglans mollis (family Juglandaceae, genus Juglans) commonly known as “wild walnut”, “walnut horse” or “nogalillo”, is distributed in northeast Mexico.43 It is traditionally used for lung pain, hair loss and for washing wounds.44 It is also known for its antimycobacterial effects.45 Previous studies conducted by our group have demonstrated that C. americana extract and J. mollis extract have antioxidant properties.12
The hepatoprotective effect of the C. americana and J. mollis extracts against CCl4-induced liver damage may be associated with inhibition of lipid peroxidation or free radical scavenging activity. Although these in vitro results are encouraging, the question remains as to whether they may be transferred to an in vivo situation in experimental animals. There is also uncertainty whether the in vitro protection may be observed in human liver diseases, which are quite different regarding pathogenetic causes compared with the animal model using carbon tetrachloride. Therefore, a wide range of additional studies is required to solve these uncertainties.
Further research using the bioassay-guide fractionation procedure to identify the compound or compounds responsible for the hepatoprotective effects of the C. americana flower and stem/leaf extracts as well as the J. mollis leaf and bark extracts should be carried out.
AcknowledgmentsWe acknowledge UANL grant fellowships PRO-MEP/103.5/04/757 and PAICYT SA992-04 for PCP. We thank biologist Humberto Sánchez for selection and identification of the plants used in this trial.
Abbreviations- •
DPPH: 2,2-diphenyl-1-picrylhydrazyl free radical.
- •
MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide.
- •
AST: Aspartate aminotransferase.
- •
MDA: Malondialdehyde
- •
CCl4: Carbon tetrachloride