Background. Pulmonary complications are common in acute liver failure (ALF). The role of the lungs in the uptake of harmful soluble endogenous macromolecules was evaluated in a porcine model of ALF induced by hepatic devascularization (n = 8) vs. controls (n = 8). In additional experiments, pulmonary uptake was investigated in healthy pigs. Fluorochrome-labeled modified albumin (MA) was applied to investigate the cellular uptake.
Results. As compared to controls, the ALF group displayed a 4-fold net increased lung uptake of hyaluronan, and 5-fold net increased uptake of both tissue plasminogen activator and lysosomal enzymes. Anatomical distribution experiments in healthy animals revealed that radiolabeled MA uptake (taken up by the same receptor as hyaluronan) was 53% by the liver, and 24% by the lungs. The lung uptake of LPS was 14% whereas 60% remained in the blood. Both fluorescence and electron microscopy revealed initial uptake of MA by pulmonary endothelial cells (PECs) with later translocation to pulmonary intravascular macrophages (PIMs). Moreover, the presence of PIMs was evident 10 min after injection. Systemic inflammatory markers such as leukopenia and increased serum TNF-α levels were evident after 20 min in the MA and LPS groups.
Conclusion. Significant lung uptake of harmful soluble macromolecules compensated for the defect liver scavenger function in the ALF-group. Infusion of MA induced increased TNF-α serum levels and leukopenia, similar to the effect of the known inflammatory mediator LPS. These observations suggest a potential mechanism that may contribute to lung damage secondary to liver disease.
Pulmonary complications in patients with liver related diseases are common.1 In acute liver failure (ALF), lung complications may comprise acute lung injury and adult respiratory distress syndrome. Clinical and radiological evidence of pulmonary edema may occur in patients with liver failure.2 The pathophysiological mechanisms of lung injury in ALF are not completely elucidated.3 Systemic inflammatory response syndrome (SIRS) and sepsis are associated with ALF4 with many factors contributing to the risk of developing sepsis in ALF, such as impaired function of circulating leukocytes and acute lung injury.1
Elevated blood levels of hyaluronic acid (HA) are reported in ALF.5 In ALF induced by paracetamol, one study demonstrated elevated circulatory levels of HA in both survivors and non-survivors, and significantly greater levels among the non-survivors.6 Under physiologic conditions, serum HA exists as a high-molecular weight substance but changes to a low molecular weight substance in disease states.7 Low molecular weight HA is known to elicit acute lung injury via Toll-like receptors-2 and 4 with subsequent MyD88 mediated inflammatory response.8
The highly endocytic liver sinusoidal endothelial cells (LSECs) which make up the liver sinusoidal walls take up colloids and soluble macromolecules from the blood, whereas Kupffer cells clear larger particles (> 200 nm) by phagocytosis.9 Chronic liver diseases induce vascular clearance of particles by the lungs,10 which has been attributed to pulmonary intravascular macrophages (PIMs).11 The existence of PIMs in healthy humans is controversial and believed to be inducible in certain pathological conditions such as cirrhosis.12 During ALF and SIRS a variety of inflammatory substances, including HA and lipopolysaccharide (LPS), are released.5 These substances may play a role in the lung damage induced by liver failure.
The aim of this study was to assess the lung uptake of harmful soluble macromolecules in a porcine model of ALF. We also aimed to investigate the kinetics of cellular absorption and clearance of modified albumin (MA), a soluble probe that is taken up by the same receptor as HA,13 and LPS by the lungs.
Material and MethodsMaterialsAll animals received human care in accordance with the institution’s guidelines. Chemicals were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. Modification of serum albumin was done with formaldehyde as described.14 Modified Albumin (MA) (0.8 mg/mL) was incubated with the fluorochrome FITC in a sodium carbonate buffer (0.5 mol/L, pH 9.5) in a protein/dye weight ratio of 5:1 at 4 °C overnight. Unbound fluorochrome was removed by dialysis against PBS without Ca2+ or Mg2+. E. coli lipopolysaccharide (serotype 0111; B4). Monoclonal goat anti-mouse IgG, TRITC-conjugate, was obtained from Zymed, San Francisco, CA. Monoclonal rabbit anti-FITC was from DAKO A/S, Denmark. Monoclonal rabbit anti-TRITC was from Molecular Probes Inc., OR. Anti-angiotensin antibody (RP183). Monodisperse polymer particles (MDPPs) with a diameter of 2.0 μm were obtained from SINTEF, University of Trondheim, Norway.
Animal preparationsAll animals (Sus scrofa domesticus) received care according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the NIH (publication 86-23, revised 1985).
All animals were fasted overnight with free access to water. Anesthesia was induced by giving 10 mg/kg ketamine and 0.5 mg/kg atropine intramuscularly. Further anesthesia induction was performed via an ear vein intravenous bolus of 10 mg/kg pentobarbital sodium and 10 microg/kg fentanyl. All animals had a tracheotomy performed with subsequent intubation and connection to a volume-controlled ventilator (Servo 900, Elema-Schönander, Stockholm, Sweden). FiO2 was kept at 0.5. Tidal volumes were adjusted by means of repeated arterial blood gas analyses to maintain PaCO2 between 4.5 to 5.0 kPa during surgery. Ventilation was not altered after zero hours. A 16-G central venous catheter (Secalon T, Ohmeda, Swindon, UK) was introduced into the left external jugular vein. An arterial line was placed in the left carotid artery. A Swan-Ganz catheter (Baxter Healthcare, Irvine, CA) was inserted into the pulmonary artery via the right external jugular vein. Continuous total intravenous anesthesia was maintained with 4 mg/kg/h pentobarbital sodium, 0.02 mg/kg/h fentanyl, and 0.3 mg/kg/ h midazolam. The depth of the anesthesia was assessed by corneal reflex and heart rate. The thermo dilution method was applied for measuring cardiac output, where 5 mL of cold (< 5 °C) normal saline was injected (Com-1, American Edwards Laboratories, Santa Ana, CA). Cardiac output was measured in triplicate and the results expressed as the mean value. A single investigator made all measurements over a period of < 4 s at end-expiration. Heating pads and blankets were used to keep normal body temperature (38.5 ± 1 °C). All animals were euthanized with an overdose of intravenous pentobarbital sodium and potassium chloride.
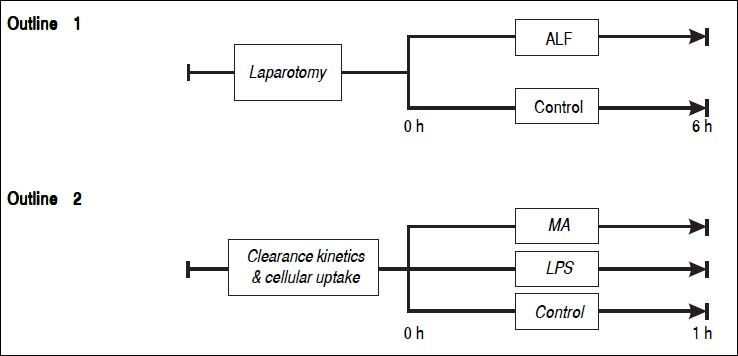
Study outline 1 - Lung mediated clearance of blood borne macromoleculesSixteen pigs weighing between 23 to 30 kg were randomly allocated into an ALF group (n = 8) or a sham-operated control group (n = 8) (Figure 1). ALF was induced by ligation of the hepatic arteries and the proximal portal vein. Following transection of the distal portal vein, the distal end of the portal vein was anastomosed end-to-side to the vena cava as described earlier.15 The controls underwent sham surgery: laparotomy and mobilization of the portal vein. Blood samples were drawn from the tip of the Swan-Ganz catheter within the pulmonary artery, and the arterial line placed in the left carotid artery. The samples were analyzed for soluble endogenous macromolecules such as HA, tissue plasminogen activator (t-PA) and lysosomal enzymes (acid phosphatase, hexosaminidase, acid lipase and aryl sulfatase). Net lung uptake was calculated by subtracting the venous concentration from the arterial concentration and multiplying with the cardiac output: AV-difference = (A-V) x flow
Outline 1 shows a large animal model of ALF (n = 8) and controls (n = 8). ALF was induced by hepatic arterial devascularization and creation of end-to-side portacaval anastomosis. Controls underwent only laparotomy and mobilization of the portal vein. Outline 2 shows intravenous injections of the soluble macromolecules MA (n = 5), the partially insoluble LPS (n = 4), and controls (n = 4). ALF: acute liver failure. MA: modified albumin. LPS: lipopolysaccharide.
Thirteen pigs were randomly allocated into 3 groups that were injected with either MA (n = 5) or LPS (n = 4), or a control group that did not receive any macromolecule (n = 4) (Figure 1).
- •
Distribution of macromolecules in healthy animals. Organ distribution of intravenously administered radiolabeled trace amounts of MA and LPS were determined as follows. Approximately 100 x 106 cpm 125I-MA, or 125I-LPS were injected into the left jugular vein. Blood samples were collected in 2 mL aliquots from an intravenous catheter positioned in the right jugular vein with its tip in the right atrium. The organs were dissected out and weighed after one hour. Radioactivity was measured in a γ-counter (Cobra II, Packard Instrument Co., CT).
- •
Investigation of lung uptake. Based on dose-response curves and matching mean pulmonary artery pressure profiles, the concentrations of 3 mg/kg MA and 5 microg/kg LPS were chosen. An MA dose > 3 mg/kg triggered a lethal cor pulmonale. Both MA and LPS were diluted in normal saline, and given as an infusion over 30 sec via the left external jugular vein. A median sternotomy was performed prior to infusion to gain access to the lung parenchyma. Thirty mg (approximately 10 billion) of an intravenous bolus of TRITC-labeled monodisperse polymer particles (MDPPs) were administered to saturate the PIM uptake.
To examine the cellular localization of intravenously infused FITC-labeled MA, multiple small lung biopsies were taken prior to, and after the infusion for fluorescence microscopy and immuno-transmission electron microscopy. Fluorescence microscopy biopsies were fixed in 4% paraformaldehyde and embedded in paraffinblocks. Transmission electron microscopy biopsies were prepared as described.16 Briefly, small cubes were cut out of the lung tissue, fixed in McDowell’s fixative and incubated in 2.3 mol/l sucrose overnight before immersion in liquid nitrogen on aluminum pins. Prior to immunolabeling, sections were retrieved in sucrose and mounted on carbon-coated grids. Sections were blocked for 15 min in 1% cold water fish skin gelatin, followed by incubation with anti-TRITC antibodies (diluted in cold water fish skin gelatin), washing in PBS and incubation with protein A-gold (University of Utrecht, The Netherlands)17 diluted in cold water fish skin gelatin for 15 min. The sections were examined in a JEOL JEM 1010 transmission electron microscope (Tokyo, Japan) at 80 kV.
AssaysPlasma HA concentrations were measured with a radio-immunometric assay (Pharmacia Diagnostics AB, Sweden). Lysosomal enzyme assays (acid phosphatase, hexosaminidase, acid lipase and aryl sulfatase) were performed as described.18 Enzyme activity, expressed in U, was expressed as the number of substrate molecules transformed (1U = microM product transformed per microl serum/h).
StatisticsTwo-way (time and group) analysis of variance for repeated measurements was applied to test whether any statistical significance existed between the groups followed by the LSD post hoc comparison test. An overall significance in analyses of variance for repeated measurements (F-test P < 0.05) may be attributable to either the effect of time (PT) or the interaction of group and time (PGT). PGT < 0.05 denoted a significant difference between the groups dependent of time, while PG < 0.05 denoted a significant difference between the groups independent of time. If both PGT and PG were significant, then only PGT was denoted since PGT is more robust than PG. PT<0.05 denoted a significant change in time for the groups. We used the SPSS statistical package (Chicago, Il).
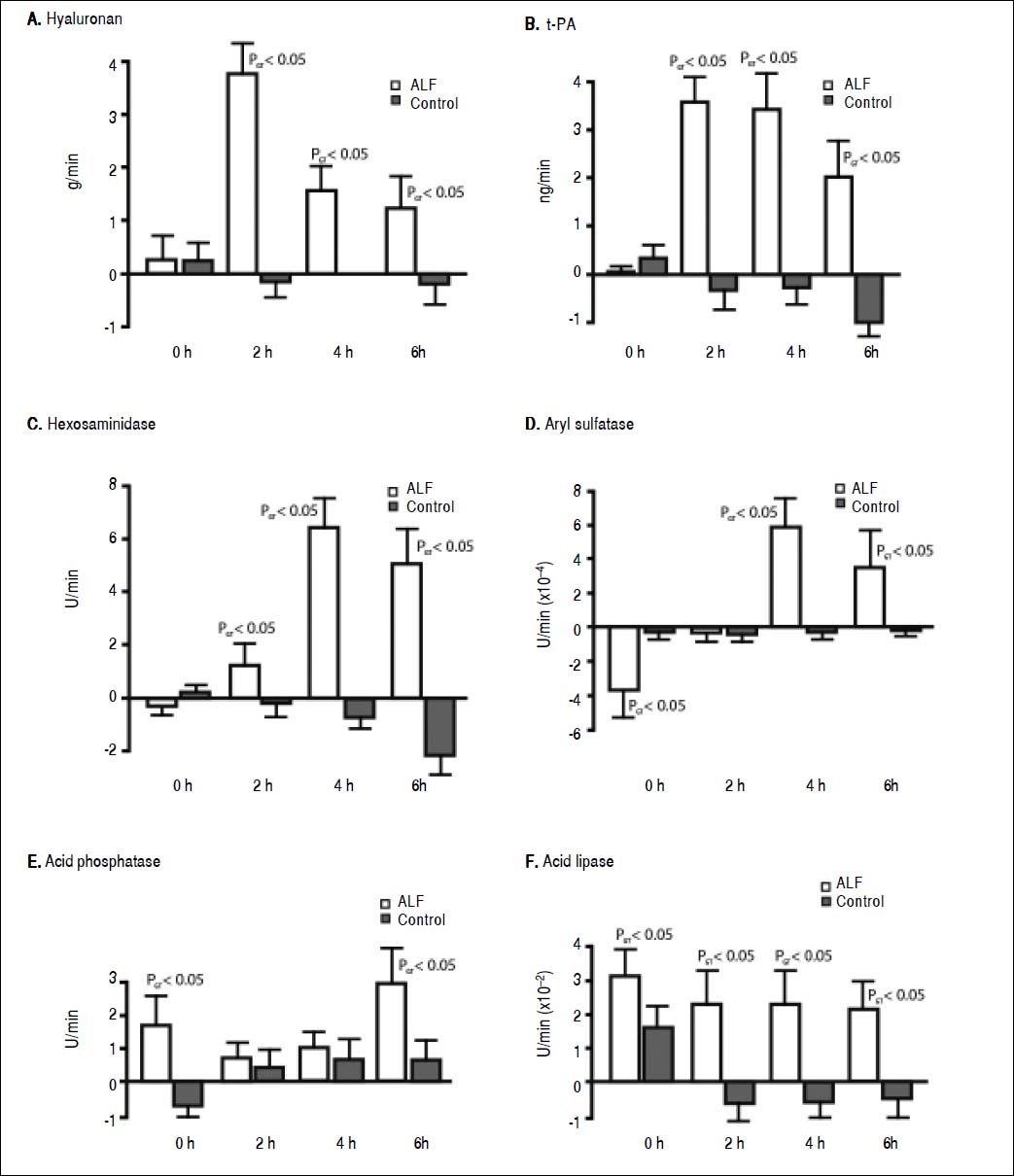
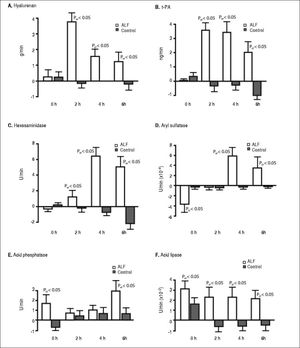
ResultsStudy outline 1Uptake of endogenous HA across the lungs in the ALF group was significantly greater than the control group (Figure 2A). The HA lung uptake rate 2 h after ALF induction was over 30-fold greater compared with the control group (PGT < 0.05) with a subsequent 10-fold decrease 6 h after ALF onset compared with the control group (PGT < 0.05). No significant increase in rate of uptake after the different time intervals was observed in the control group. This suggests an increase and a subsequent saturation of lung uptake in ALF. This notion is strengthened by the over 10-fold increase in HA concentrations in the pulmonary artery in the ALF group, an increase from 0.53 ± 0.43 mg/L at 0 h to 5.60 ± 0.42 mg/L at 6 h (PGT < 0.05). No significant increase was observed in the control group; 0.20 ± 0.57 mg/L at 0 h and 0.83 ± 0.32 mg/L at 6 h.
Figure 2. Lung clearance of soluble endogenous macromolecule in ALF. Lung uptake was given by the aortic concentrations subtracted by the pulmonary artery concentrations, and multiplied with the cardiac output. A positive value denoted uptake of circulating soluble macromolecule by the lungs, while a negative value denoted secretion. In ALF, note the initia large clearance by the lungs (2 to 4 h), and thereafter reduction of uptake indicating a saturation of the lung uptake. A. Hyaluronan. B. t-PA. C. Hexosaminidase. D. Aryl sulfatase. E. Acid phosphatase, and F. Acid lipase. ALF: acute liver failure. HA: hyaluronan. tPA: tissue plasminogen activator.
As with HA, t-PA uptake across the lung also increased 30-fold after 2 h (PGT < 0.05) (Figure 2B) that subsequently decreased throughout the experimental period. After ALF induction, there was a 5-fold increase in t-PA concentrations in the pulmonary artery at 6 h (PGT < 0.05). No significant increase was observed in the control group at 6 h.
There was also a 30-fold increase in t-PA lung flux after 2 h (PGT < 0.05) (Figure 2B). This uptake decreased throughout the experimental period. After ALF induction, there was a 5-fold increase in t-PA concentrations in the pulmonary artery, from 1.52 ± 0.20 ng/mL at 0 h to 7.93 ± 0.59 ng/mL at 6 h whereas no significant increase was observed in the control group; from 0.53 ± 0.12 ng/mL at 0 h to 0.72 ± 0.06 ng/mL at 6 h (PGT < 0.05).
Lysosomal enzymes were also taken up by the lungs in the ALF group. The greatest rate of uptake was observed for hexosaminidase, 7-fold more than the control group (PGT < 0.05) (Figure 2C). The uptake of aryl sulfatase increased gradually (PGT < 0.05) (Figure 2D), while acid phosphatase had a 3-fold greater uptake than the control group (PGT < 0.05) (Figure 2E). Acid lipase showed a continuous lung uptake with no indication of saturation in the ALF group compared with the control group (PGT < 0.05) (Figure 2F).
Study outline 2- •
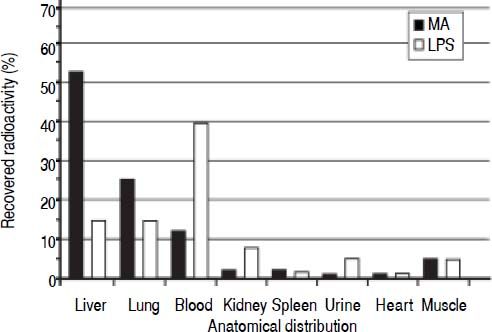
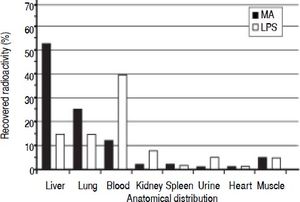
Clearance and distribution of macromolecules in healthy animals. The anatomical distributions of trace amounts of radiolabeled MA (a soluble probe that is taken up by the same receptor as HA13), and LPS were investigated in 13 healthy animals: MA group (n = 5), LPS group (n = 4), and control group (n = 4) (Figure 1). Sixty minutes after injection, the organs were analyzed for anatomical distribution of radioactivity (Figure 3). The liver was the main site of uptake for MA (52%), while LPS uptake was similar in both the lungs and the liver (15%). Moreover, more than 90% of the injected doses were recovered in the organs listed.
Figure 3.Clearance and anatomical distribution of intravenously administered macromolecules in healthy animals. Trace amounts of radiolabeled MA, and partially insoluble LPS were injected into the left jugular vein. Organs were analyzed for anatomical distribution 60 min after injection. Note that almost 40% of LPS remained in the blood after 60 min. More than 90% of the injected doses were recovered in the organs listed. Results are expressed as percent total radioactivity recovered. Organs that were analyzed with no uptake and therefore not included in the figure, were aorta, thyroi-dea, pancreas, thymus, stomach, duodenum, lymph nodes, pancreas, and colon. MA: modified albumin. LPS: lipopolysaccharide.
(0.02MB). - •
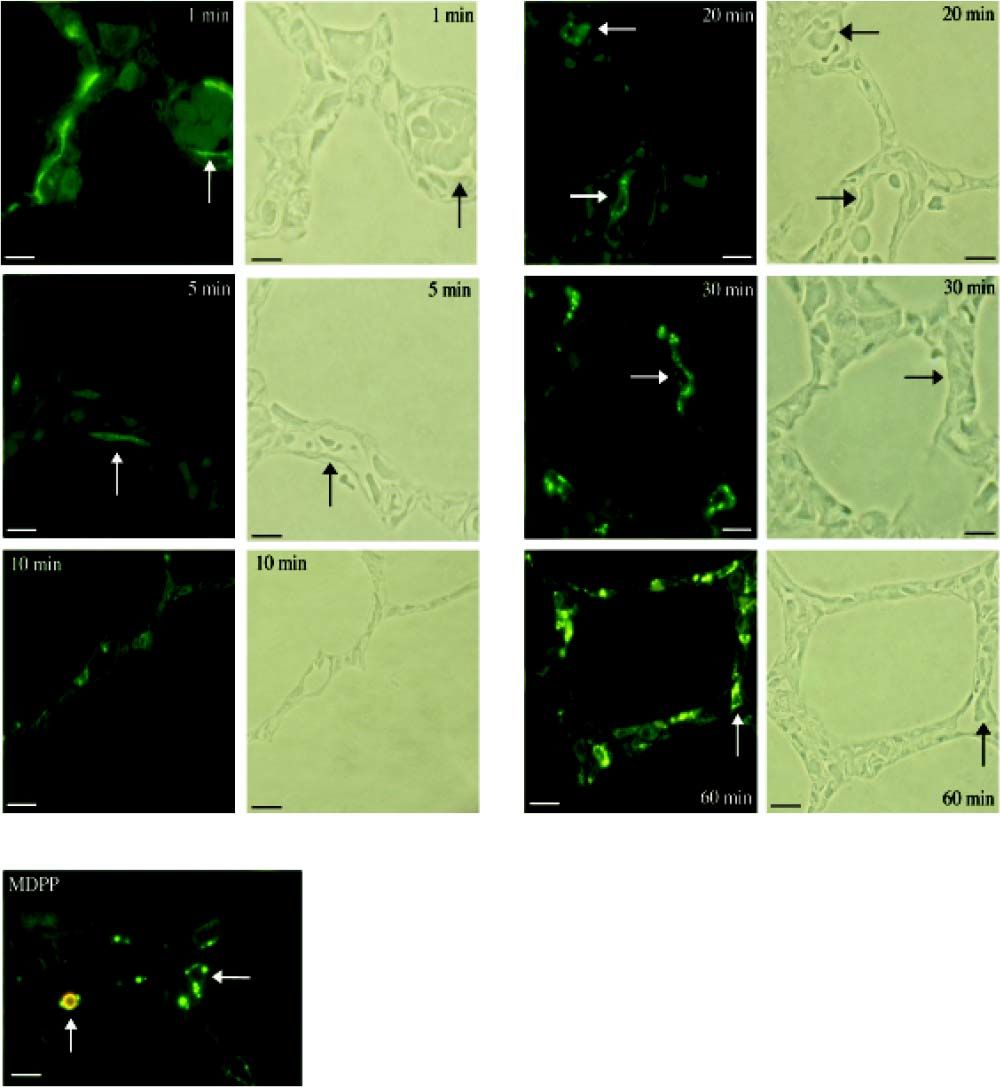
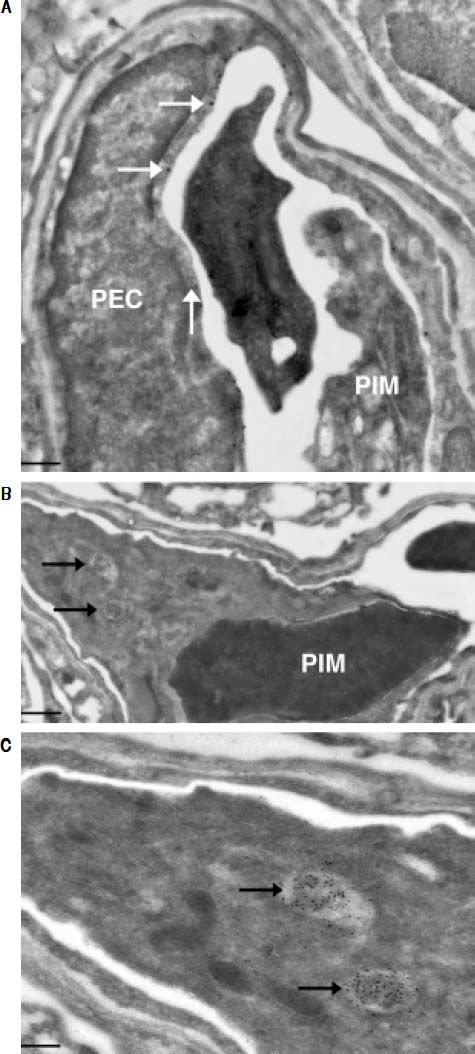
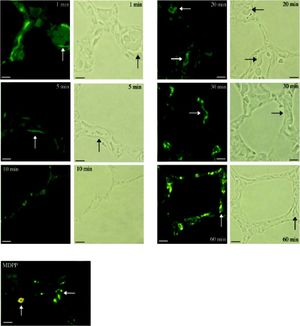
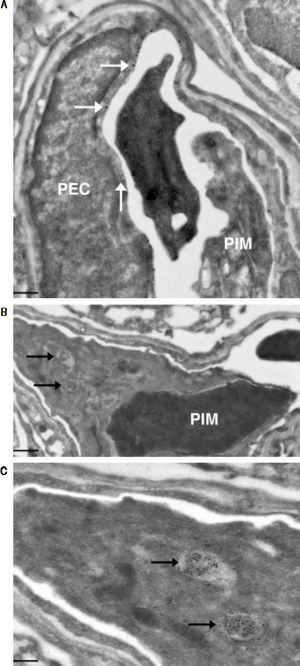
Investigation of cellular uptake in the lungs. PECs endocytosed detectable amounts of MA already 10 min after intravenous injection (Figure 4). After 10 min, note the presence of PIMs, with subsequent translocation of the green fluorescence from PECs to PIMs. Particulate matter (MDPP, 2 microns in diameter) was phagocytosed by PIMs (Figure 4). FITC-MA and TRITC-MDPP colocalized in PIMs after 60 min. The same pattern of translocation from PECs to PIMs was confirmed with electron microscopy (Figure 5), which also showed that PECs containing MA was co-labeled with the endothelial marker angiotensin converting enzyme (Figure 5A). Moreover, after 10 min MA translocated and aggregated in phagolysosomes of the PIMs (Figures 5B and 5C).
Figure 4.Cellular site of uptake in lung following intravenous administration of macromolecules and microparticles. Lung biopsies were obtained following infusion of soluble MA probe. Baseline lung biopsy prior to injection showed no green fluorescence in lung tissues. One minute after infusion green fluorescence was seen lining the lung capillaries (micrograph labeled 1 min), suggesting endocytosis of MA by PECs. Note capillaries with red blood cells in the phase-contrast micrograph after 1 min. After 10 min, the presence of PIMs is evident, and the green fluorescence started to translocate from PECs to PIMs, showing an accumulation of MA in PIMs. The fluorescence accumulation was more significant after 20 min, when the green fluorescence accumulated within the PIMs, residing inside the capillaries (arrows). The arrows in the fuorescence micrographs corresponded with PIMs in the phase-contrast micrographs. Sonicated MDPPs were infused via left jugular vein. In fluorescence micrograph labeled MDPP, note co-localization after 60 min of orange fluorescence (MDPP) and green fluorescence (MA) in a PlM (vertical arrow). Horizontal arrow indicated a PIM without MDPP but with MA. Scale bars: 20 microm. MA: modified albumin. PECs: pulmonary capillary endothelial cells. PIMs: pulmonary intravascular macrophages. MDPPs: monodisperse polymer particles.
(0.07MB).Figure 5.Ultrastructural studies in lung cells following intravenous administration of macromolecule. Immuno-gold staining visualized both large gold particles (MA, horizontal arrows) and small gold particles (anti-angiotensin converting enzyme, vertical arrow) labeling the surface of PEC (< 10 min) (A). Of note, there was no accumulation of MA in the phagolysosomes of the PIM, and the PIM was not labeled with anti-angiotensin converting enzyme. There is a blood clot within the capillary. Beyond 10 min, MA however accumulated in the phagolysosomes of PIMs indicated by arrows (B). Greater magnification of a segment of B showed accumulation of MA within phagolysosomes more clearly (C). Scale bars. A. 2 microm. B. 1 microm. C. 500 nm. MA: modified albumin. PECs: pulmonary capillary endothelial cells. PIM: pulmonary intravascular macrophage.
(0.07MB). - •
Systemic inflammatory markers. Serum TNF-α was detectable (4.0 ± 2.7 pg/mL) 40 min after MA injection, i.e. 20 min after the translocation of MA from PECs to PIMs. After 60 min, TNF-α levels increased to 32.0 ± 7.3 pg/mL (PGT < 0.05). Even greater TNF-α levels were measured after injection of LPS; baseline TNF-α secretion was 0.0 ± 0.0 pg/ mL and after 60 min 411.8 ± 70.1 pg/mL (PGT < 0.05). In the control group, the TNF-α was undetectable after 60 min.
After 60 min, the white blood cell count (WBC x 1,000/ mm3) decreased from 18.6 to 8.3 in the LPS group, consistent with a leukopenia (PGT < 0.05). The same pattern was observed in the MA group, where WBC decreased from 19.1 to 14.1 (PGT < 0.05). In the control group, the WBC remained relatively unchanged from 18.1 to 19.7 after 60 min (PGT > 0.05).
Potential interfering factors associated with Study outline 2No serum TNF-α secretion was observed after a 3 mg/kg bolus of bovine serum albumin (i.e. no modification of albumin) administration, suggesting no cross-reaction between species. The blood drawn in the MA-group showed no pyrogenes as demonstrated with a limulus amebocyte lysate assay. Blood cultures after 60 min from Study outline 2 showed no bacterial growth.
DiscussionSignificance of lung uptake of soluble macromolecule in ALFIn healthy subjects, the soluble macromolecules investigated in this study are known to be endocytosed by the LSECs.9 It has been shown that serum levels of HA are significantly greater in paracetamol induced ALF among non-survivors vs. survivors.6 The soluble macromolecule t-PA is a major determinant of fibrinolytic activity in blood, cleaving plasminogen into plasmin and causing degradation of fibrin in blood clots, and potentially contributing to the bleeding diathesis observed in ALF. Increased serum levels of lysosomal enzymes in ALF, due to either decreased LSEC scavenger capacity, increased release, or a combination may add to the complications of ALF such as acute lung injury, renal failure and myocardial dysfunction.19 Moreover, a study revealed that ALF caused by paracetamol overdose, non-survivors had 10-fold greater levels of lysosomal enzymes in the blood compared with survivors.20
Chronic liver disease, on the other hand, is known to induce vascular clearance of particles by the lungs.10 However, there is a paucity of data on lung clearance in ALF. One study showed that the lungs took up radiolabeled E. coli permanently in a rodent ALF model with 90% hepatic resection.21 Chronic liver failure studies in rodents have shown increased lung uptake of lysosomal enzymes,22 radiolabeled E. coli.,23 and LPS.24 This is in line with our results where the lungs compensate for the loss of liver scavenger function by LSECs.
Proinflammatory effects of soluble macromoleculesOur study revealed that MA, which is a soluble probe that is taken up by the same receptor as HA,13 can elicit a similar inflammatory response as to the one observed with LPS. PIMs are known to secrete TNF-α upon stimulation, while depletion of PIMs reduce TNF-α secretion.25 Minute amounts of LPS are potent triggers of an inflammatory response, hence the 12-fold greater TNF-α release with LPS than with MA after 60 min (infused LPS amount was 600-fold less than the MA amount). This reflects normal homeostasis, where minute amounts of pro-inflammatory endogenous molecules in most mammals would be lethal. The concentrations of low molecular weight HA required to induce Toll-like receptor-4 mediated signaling in dendritic cells are several orders of magnitude greater than for LPS.26 A study performed showed a 1/3 increase of TNF-α secretion in the systemic circulation with proinflammatory heparan sulfate stimulation as compared with LPS stimulation in mice.27
Our findings suggest a role for PECs in vascular clearance of soluble macromolecules. Furthermore, our results showed that LPS is cleared both by the liver and lungs with the greatest fraction remaining in the blood most likely bound to LPS-binding proteins.28
ConclusionIn conclusion, this study shows for the first time that lung uptake of harmful soluble macromolecules occurs in ALF. The significant lung uptake of harmful soluble macromolecules compensated for the defect liver scavenger function in the ALF-group. Our findings suggest a role for PECs in vascular clearance of soluble macro-molecules. Moreover, PECs may induce an inflammatory response, similar to the effect of the known inflammatory mediator LPS. These observations suggest a potential mechanism that may contribute to lung damage secondary to liver disease.
Abbreviations- •
ALF: acute liver failure.
- •
HA: hyaluronic acid.
- •
LPS: lipopolysaccharide.
- •
LSECs: liver sinusoidal endothelial cells.
- •
MA: modified albumin.
- •
MDPP: monodisperse polymer particles.
- •
PECs: pulmonary endothelial cells.
- •
PIMs: pulmonary intravascular macrophages.
- •
SIRS: systemic inflammatory response syndrome.
None.
AWARDThis manuscript reports on the work that received a Travel Award for the Oral Presentation “Uptake of Soluble Macromolecules by Pulmonary Capillary Endothelial Cells - Implications for Lung Complications in Liver Disease” performed at the 18th International Liver Transplantation Society (2012 Travel Award - ILTS - San Francisco CA).
Financial SupportSupported by the Norwegian Research Council.
AuthorshipGIN: participated in study conception and design, experiment performances, acquisition of the research data, analysis and interpretation of data and writing of the manuscript. KE and MFC participated in study conception and design, analysis and interpretation of data and writing of the manuscript; LMY, CFR, SS, BS, RJ, and AR participated in study conception and design, analysis and interpretation of data, and critical revision of the manuscript.
AcknowledgementWe express our gratitude to Ellinor Hareide and Victoria Nilsen for excellent analytical service, Hege Hagerup for technical assistance, and Dr. Tom Wilsgaard for statistical advice.