Introduction. Despite reports of increased incidence of intrahepatic cholangiocarcinoma (iCCA) in the United States, the impact of age or influences of race and ethnicity are not clear. Disparities in iCCA outcomes across various population subgroups also are not readily recognized due to the rarity of this cancer. We examined ethnic, race, age, and gender variations in iCCA incidence and survival using data from the Surveillance, Epidemiology, and End Results Program (1995-2014).
Materials and methods. We assessed age-adjusted incidence rates, average annual percentage change in incidence, and hazard ratios (HRs) with 95% confidence intervals (CIs) for all-cause and iCCA-specific mortality.
Results. Overall, 11,127 cases of iCCA were identified, with an age-adjusted incidence rate of 0.92 per 100,000. The incidence rate increased twofold, from 0.49 per 100,000 in 1995 to 1.49 per 100,000 in 2014, with an average annual rate of increase of 5.49%. The iCCA incidence rate was higher among persons age 45 years or older than those younger than 45 years (1.71 vs. 0.07 per 100,000), among males than females (0.97 vs. 0.88 per 100,000) and among Hispanics than non-Hispanics (1.18 vs. 0.89 per 100,000). Compared to non-Hispanics, Hispanics had poorer 5-year all-cause mortality (HR = 1.11, 95%CI: 1.05-1.19) and poorer iCCA-specific mortality (HR = 1.15, 95%CI: 1.07-1.24). Survival rates were poor also for individuals age 45 years or older, men, Blacks, and American Indians/Alaska Natives.
Conclusion. The results demonstrate ethnic, race, age and gender disparities in iCCA incidence and survival, and confirm continued increase in iCCA incidence in the United States.
Intrahepatic cholangiocarcinoma (iCCA) is the second most common form of primary liver cancer, after hepatocellular carcinoma.1–4 These cancers arise from the intrahepatic biliary ducts or the intrahepatic ductules and typically present as mass lesions. There are major geographical differences in the incidence of iCCA, largely reflecting variations in the prevalence of risk factors.3,5,6 Although the etiology of iCCA is not completely understood, it has been association with cirrhosis, chronic viral hepatitis, and inflammatory cholangiopathies, such as primary sclerosing cholangitis and primary biliary cholangitis, are well-recognized.3,5,6 In the United States, iCCA is rare, with ~2,500 cases diagnosed annually.7 However, several studies have reported an increase in the incidence of iCCA in the United States over the last several decades.8–10 Epidemiologic studies have also shown an increasing incidence of iCCA in other parts of the world.11–15
Data from the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute (NCI) have provided insights into the epidemiology of iCCA in the United States.8–10 Using data from SEER, we first reported a fourfold increased incidence of iCCA over a 25 year period, from 1973 to 1997, with an annual percentage increase of 9.1%.8 We found also higher iCCA incidence rate and poorer survival for men and older individuals as compared to women and younger individuals. Moreover, despite that the rate of increase in iCCA incidence was lower in Blacks than Whites, all-cause mortality after diagnosis was similar among Blacks and Whites.8 A subsequent study examined racial and ethnic variation in iCCA incidence using SEER data from 1990 to 2001, and found higher incidence rate among American Indian/Alaska Natives (1.6 per 100,000), followed by Asians/ Pacific Islanders (1.2 per 100,000) and Hispanics (1.1 per 100,000), and lower incidence rates among Whites (0.8 per 100,000) and Blacks (0.3 per 100,000).9 More recently, Mosadeghi, et al. reported higher iCCA incidence rates among Asians (2.4 per 100,000) and Hispanics (2.2 per 100,000), than Blacks (1.6 per 100,000) and Whites (1.5 per 100,000).10 Despite these reports, racial and ethnic variations in survival outcomes remain unclear.
Knowledge of the impact of age and of racial and ethnic influences on the incidence of iCCA is necessary because it will guide both risk factor assessment and strategies for effective risk prevention in population groups at higher risk of the disease. A comprehensive and updated analysis is warranted for several additional reasons. First, differences in the reported incidence rates of iCCA may reflect variations in registration and coding within cancer registries, or differences in the diagnostic criteria. Recent iterations of staging systems have clearly defined three distinct types of cholangiocarcinoma, with the recognition of hilar cholangiocarcinoma as a distinct entity, which has resulted in a greater accuracy of diagnosis of iCCA. Moreover, practice trends towards a greater use of liver biopsy for liver masses may have contributed to more precise histological diagnosis. Given that iCCA typically occurs in older age groups, analyses of the impact of age on incidence and survival outcomes is necessary to determine whether the risk factors for iCCA in younger individuals differ from those for older individuals. Thus, we sought to reappraise the incidence of iCCA in the United States, and to examine long-term survival outcomes in racial and ethnic groups, and across age and gender groups. A primary purpose of this study was to identify population subgroups at increased risk of developing iCCA for targeted screening and timely clinical intervention, and thereby improve survival from this deadly malignancy.
Materials and MethodsData source and study populationData were obtained from the NCI’s SEER Program for the years 1995 to 2014.16 The SEER Program has been described in detail;16 a brief description follows. SEER collects cancer incidence and survival data from population-based cancer registries that cover 18 geographic regions: Atlanta, Georgia; Connecticut; Detroit, Michigan; Hawaii; Iowa; New Mexico; San Francisco-Oakland, California; Seattle-Puget Sound, Seattle Washington; Utah; Los Angeles, California; San Jose-Monterey, California; Rural Georgia; Alaska; Greater California; Greater Georgia; Kentucky; Louisiana; and New Jersey. The areas covered by SEER represent 28% of the United States population based on the 2010 census information and is reflective of sociodemographic patterns in the United States.16 Individual level data collected by SEER includes patients’ age at diagnosis, gender, race, ethnicity, primary tumor type, tumor morphology, and vital status. Data from all 18 population-based SEER registries were used in the present study. The study was exempt from review by the Mayo Clinic Institutional Review Board because of the use of pre-existing data that are devoid of patient identifiers.
The International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) code C22.1 was used to identify all patients diagnosed with iCCA and reported in SEER between 1995 and 2014. In SEER, race is categorized as White, Black, American Indian/Alaska Native (AI), Asian/Pacific Islander (API), or unknown. Ethnicity is classified in SEER as Spanish-Hispanic-Latino (hereafter referred as “Hispanic”) or non-Spanish-Hispanic-Latino (referred as “non-Hispanic”). These categories were retained for the analyses. Data on cancer stage at diagnosis (the SEER historic stage A variable) are categorized in SEER as localized, regional, distant and unstaged and was used in multivariable-adjusted survival analyses to account for differences in disease severity and the potential impact of cancer-directed therapy on survival. Data on age at diagnosis were categorized as 18-44, 45-54, 55-64, 65-74, and 75+ years for estimation of age-adjusted incidence rates and further categorized into less than 45 years vs. 45 years or older for subgroup analyses.
Statistical analysesDemographic characteristics were compared among the iCCA patients based on frequencies and proportions. Age-adjusted incidence rates for iCCA were calculated per 100,000 persons based on the 2010 standard US population proportions in five age groups (18-44, 45-54, 55-64, 65-74, and 75+ years), as described in detail.17 The age-adjusted incidence rates were calculated for the 20-year study period (1995-2014) and then stratified by the earlier decade (1995-2004) vs. the latter decade (2005-2014). Age-adjusted incidence rates were calculated also for subgroups defined by gender, race (White, Black, API, and AI), and ethnicity (Hispanic vs. non-Hispanic) based on corresponding proportions in the 2010 United States census population provided by SEER.17 Crude incidence rates were calculated for age group-specific analyses. In addition, we calculated average annual percentage change (AAPC) in incidence rates using log-linear modeling to estimate the average annual rate of change in iCCA incidence over a specified time (i.e., the entire 20-year period, earlier 10 years, and the latter 10 years)18 and for each of the subgroups described above.
Cox proportional hazards models19 were used to calculate hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) to examine the relative risk of death from any cause (all-cause mortality) and death from iCCA after 1 and 5 years of diagnosis of iCCA. Before fitting the hazard models, the proportional hazards assumption was examined based on visual inspection of log(-log) survival curves,19 and none of the study variables violated the proportional hazards assumption. The HRs were calculated in age-adjusted models and in multivariable-adjusted models, with additional adjustments for gender, race (White, Black, API, and AI), and cancer stage at diagnosis (localized, regional, distant, and unstaged). No adjustment for race was done in the models that examined ethnic variation in survival outcomes. Kaplan-Meier survival curves were used to describe 5-year survival patterns for the entire cohort and for subgroups defined by age (< 45 years vs. ≥ 45 years), gender, and ethnicity (Hispanic vs. non-Hispanic). All statistical tests were two-sided and a p-value lower than 0.05 was considered statistically significant. Statistical analyses were performed in SAS® version 9.4 (SAS Inc., Cary, NC, USA).
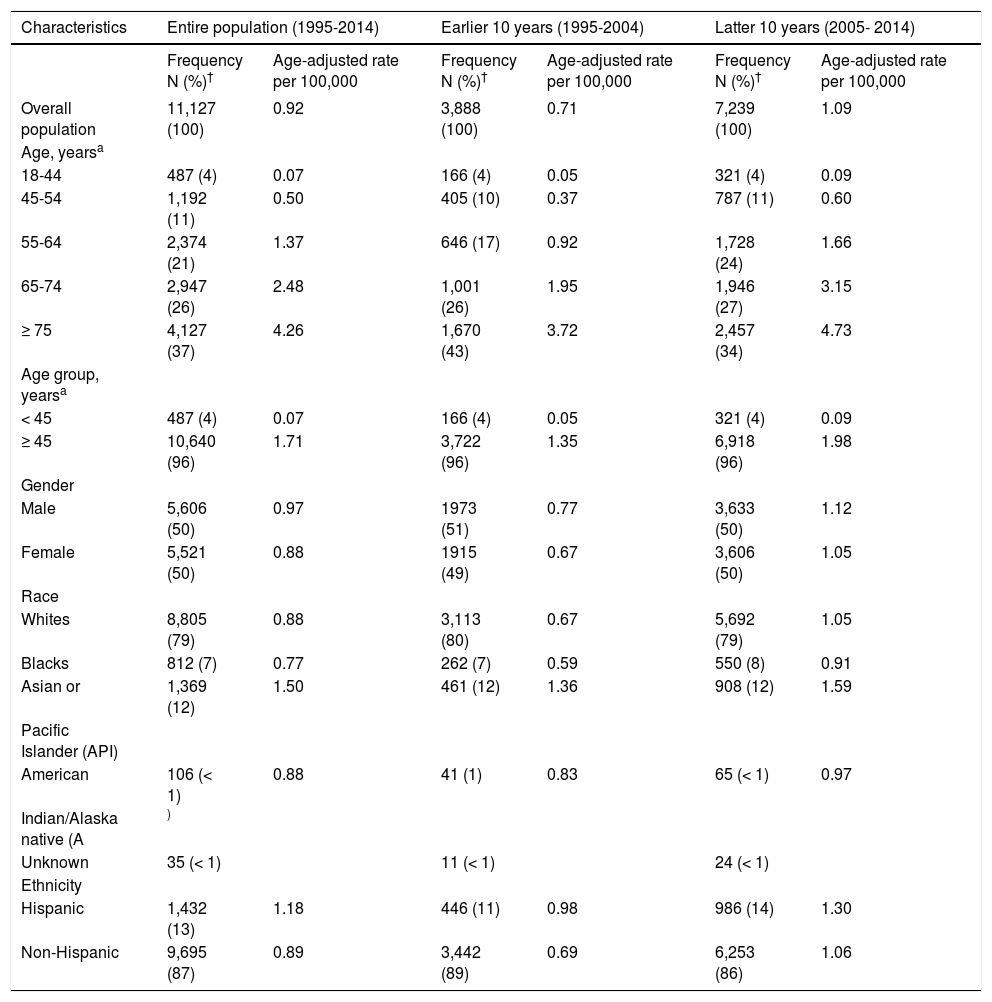
ResultsCharacteristics of the 11,127 incident, histopathologically confirmed, iCCA cases reported in SEER between 1995 and 2014 are presented in table 1. The majority of the patients were 45 years or older (96%), and there were equal proportions of males and females (50% each). Most of the patients were White (79%); few were API (12%), Black (7%), or AI (< 1%). Hispanic patients were fewer (13%) than non-Hispanic patients (87%).
Descriptive statistics and age-adjusted incidence rates for intrahepatic cholangiocarcinoma (iCCA); SEER, 1995-2014.
| Characteristics | Entire population (1995-2014) | Earlier 10 years (1995-2004) | Latter 10 years (2005- 2014) | |||
|---|---|---|---|---|---|---|
| Frequency N (%)† | Age-adjusted rate per 100,000 | Frequency N (%)† | Age-adjusted rate per 100,000 | Frequency N (%)† | Age-adjusted rate per 100,000 | |
| Overall population | 11,127 (100) | 0.92 | 3,888 (100) | 0.71 | 7,239 (100) | 1.09 |
| Age, yearsa | ||||||
| 18-44 | 487 (4) | 0.07 | 166 (4) | 0.05 | 321 (4) | 0.09 |
| 45-54 | 1,192 (11) | 0.50 | 405 (10) | 0.37 | 787 (11) | 0.60 |
| 55-64 | 2,374 (21) | 1.37 | 646 (17) | 0.92 | 1,728 (24) | 1.66 |
| 65-74 | 2,947 (26) | 2.48 | 1,001 (26) | 1.95 | 1,946 (27) | 3.15 |
| ≥ 75 | 4,127 (37) | 4.26 | 1,670 (43) | 3.72 | 2,457 (34) | 4.73 |
| Age group, yearsa | ||||||
| < 45 | 487 (4) | 0.07 | 166 (4) | 0.05 | 321 (4) | 0.09 |
| ≥ 45 | 10,640 (96) | 1.71 | 3,722 (96) | 1.35 | 6,918 (96) | 1.98 |
| Gender | ||||||
| Male | 5,606 (50) | 0.97 | 1973 (51) | 0.77 | 3,633 (50) | 1.12 |
| Female | 5,521 (50) | 0.88 | 1915 (49) | 0.67 | 3,606 (50) | 1.05 |
| Race | ||||||
| Whites | 8,805 (79) | 0.88 | 3,113 (80) | 0.67 | 5,692 (79) | 1.05 |
| Blacks | 812 (7) | 0.77 | 262 (7) | 0.59 | 550 (8) | 0.91 |
| Asian or | 1,369 (12) | 1.50 | 461 (12) | 1.36 | 908 (12) | 1.59 |
| Pacific Islander (API) | ||||||
| American | 106 (< 1) | 0.88 | 41 (1) | 0.83 | 65 (< 1) | 0.97 |
| Indian/Alaska native (A | ) | |||||
| Unknown | 35 (< 1) | 11 (< 1) | 24 (< 1) | |||
| Ethnicity | ||||||
| Hispanic | 1,432 (13) | 1.18 | 446 (11) | 0.98 | 986 (14) | 1.30 |
| Non-Hispanic | 9,695 (87) | 0.89 | 3,442 (89) | 0.69 | 6,253 (86) | 1.06 |
The overall age-adjusted incidence rate for iCCA between 1995 and 2014 was 0.92 per 100,000 (Table 1). The incidence rate was 53% higher in the latter 10 years (1.09 per 100,000; 2005-2014) compared to the earlier 10 years (0.71 per 100,000; 1995-2004). Over the 20-year study period, we observed a linear increase in iCCA incidence with increasing age, such that the per 100,000 incidence rates for the age groups 18-44, 45-54, 55-64, 65-74, and 75+ years were 0.07, 0.50, 1.37, 2.48 and 4.26, respectively. We also found slightly higher age-adjusted incidence rate for males (0.97 per 100,000) than females (0.88 per 100,000), and higher rate for APIs (1.50 per 100,000) than Whites (0.88 per 100,000), AIs (0.88 per 100,000), or Blacks (0.77 per 100,000). Hispanics had higher iCCA incidence rate (1.18 per 100,000) than non-Hispanics (0.89 per 100,000) over the 20-year period. These differences were observed also in both the analyses stratified by earlier 10 years versus the latter 10 years, although the incidence rates were much higher in the latter 10 years in all subgroups (Table 1).
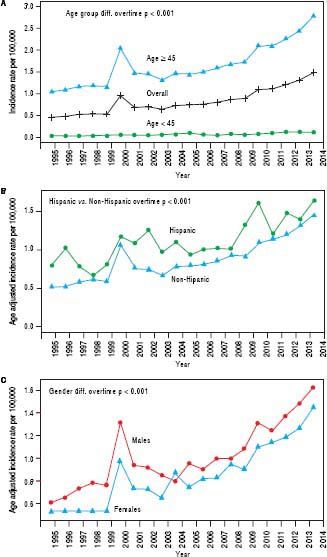
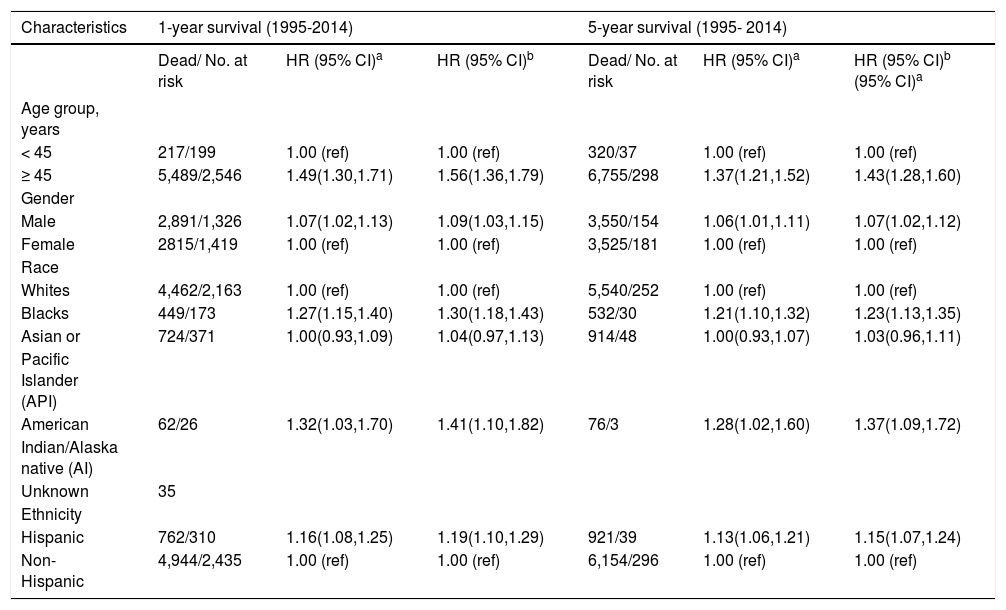
Trend analyses over the 20-year study period show an overall increasing incidence of iCCA across time, starting from 0.49 per 100,000 in 1995 to 1.49 per 100,000 in 2014 (Figure 1A); a twofold increase in the iCCA incidence rate. The trend plot shows a transient peak in iCCA incidence in 2000 (0.91 per 100,000) before declining in 2001 (0.73 per 100,000) and then gradually rising to 1.49 per 100,000 in 2014. Individuals of age 45 years or older had continuously higher incidence over time compared to those younger than 45 years (Figure 1A). Hispanics also had a higher incidence over time compared to non-Hispanics (Figure 1B) as did males compared to females, except for a brief flip in the incidence rate in 2004 (Figure 1C) and there was a greater increase in iCCA incidence in AIPs compared with other race groups.
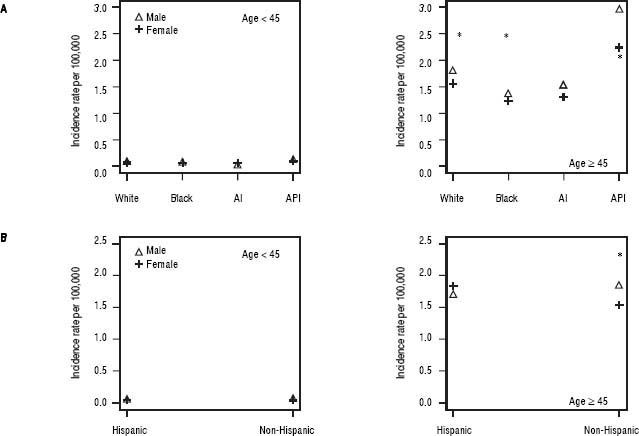
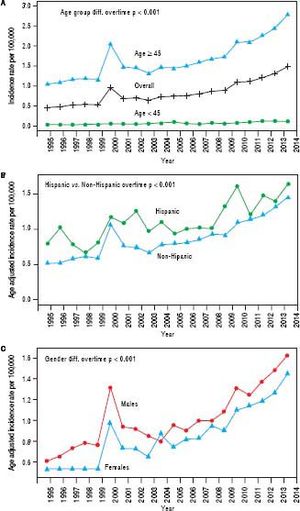
We further examined age-adjusted incidence rates between males and females in each race and in each ethnicity, stratified by age (Figures 2A and 2B). Among individuals younger than 45 years, no differences were observed in iCCA incidence rates between males and females in any of the race groups. However, among those 45 years or older, males had significantly higher iCCA incidence than females in nearly all of the race groups, except among AIs (Figure 2A). Similarly, no differences in incidence were observed between males and females among Hispanics and non-Hispanics who were younger than 45 years. Among those 45 years or older, higher iCCA incidence was observed among non-Hispanic males compared to non-Hispanic females, but Hispanic males and females had an equally high iCCA incidence rate (Figure 2B).
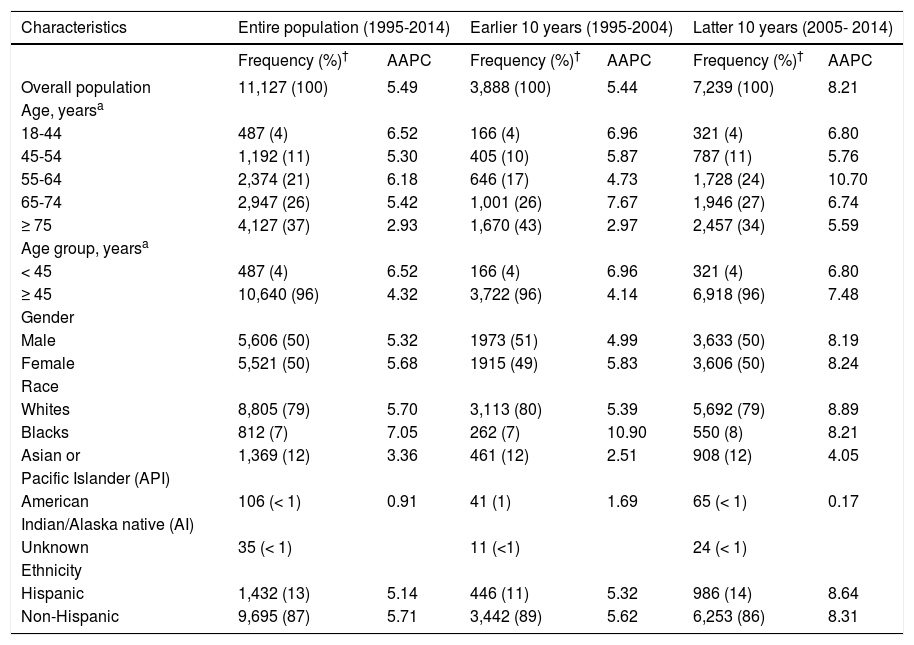
We next calculated AAPC in age-adjusted iCCA incidence rate from 1995 to 2014 and found a 5.49 average rate of increase in incidence over the 20-year period (Table 2).
Average Annual Percentage Change (AAPC) in age-adjusted intrahepatic cholangiocarcinoma (iCCA) incidence in SEER from 1995 to 2014.
| Characteristics | Entire population (1995-2014) | Earlier 10 years (1995-2004) | Latter 10 years (2005- 2014) | |||
|---|---|---|---|---|---|---|
| Frequency (%)† | AAPC | Frequency (%)† | AAPC | Frequency (%)† | AAPC | |
| Overall population | 11,127 (100) | 5.49 | 3,888 (100) | 5.44 | 7,239 (100) | 8.21 |
| Age, yearsa | ||||||
| 18-44 | 487 (4) | 6.52 | 166 (4) | 6.96 | 321 (4) | 6.80 |
| 45-54 | 1,192 (11) | 5.30 | 405 (10) | 5.87 | 787 (11) | 5.76 |
| 55-64 | 2,374 (21) | 6.18 | 646 (17) | 4.73 | 1,728 (24) | 10.70 |
| 65-74 | 2,947 (26) | 5.42 | 1,001 (26) | 7.67 | 1,946 (27) | 6.74 |
| ≥ 75 | 4,127 (37) | 2.93 | 1,670 (43) | 2.97 | 2,457 (34) | 5.59 |
| Age group, yearsa | ||||||
| < 45 | 487 (4) | 6.52 | 166 (4) | 6.96 | 321 (4) | 6.80 |
| ≥ 45 | 10,640 (96) | 4.32 | 3,722 (96) | 4.14 | 6,918 (96) | 7.48 |
| Gender | ||||||
| Male | 5,606 (50) | 5.32 | 1973 (51) | 4.99 | 3,633 (50) | 8.19 |
| Female | 5,521 (50) | 5.68 | 1915 (49) | 5.83 | 3,606 (50) | 8.24 |
| Race | ||||||
| Whites | 8,805 (79) | 5.70 | 3,113 (80) | 5.39 | 5,692 (79) | 8.89 |
| Blacks | 812 (7) | 7.05 | 262 (7) | 10.90 | 550 (8) | 8.21 |
| Asian or | 1,369 (12) | 3.36 | 461 (12) | 2.51 | 908 (12) | 4.05 |
| Pacific Islander (API) | ||||||
| American | 106 (< 1) | 0.91 | 41 (1) | 1.69 | 65 (< 1) | 0.17 |
| Indian/Alaska native (AI) | ||||||
| Unknown | 35 (< 1) | 11 (<1) | 24 (< 1) | |||
| Ethnicity | ||||||
| Hispanic | 1,432 (13) | 5.14 | 446 (11) | 5.32 | 986 (14) | 8.64 |
| Non-Hispanic | 9,695 (87) | 5.71 | 3,442 (89) | 5.62 | 6,253 (86) | 8.31 |
The rate of increase in iCCA incidence was higher in the latter 10 years (8.21%) compared to the preceding 10 years (5.44%). There also was evidence of increasing iCCA incidence in all subgroups, with a consistently higher AAPC in the latter 10 years compared to the earlier 10 years.
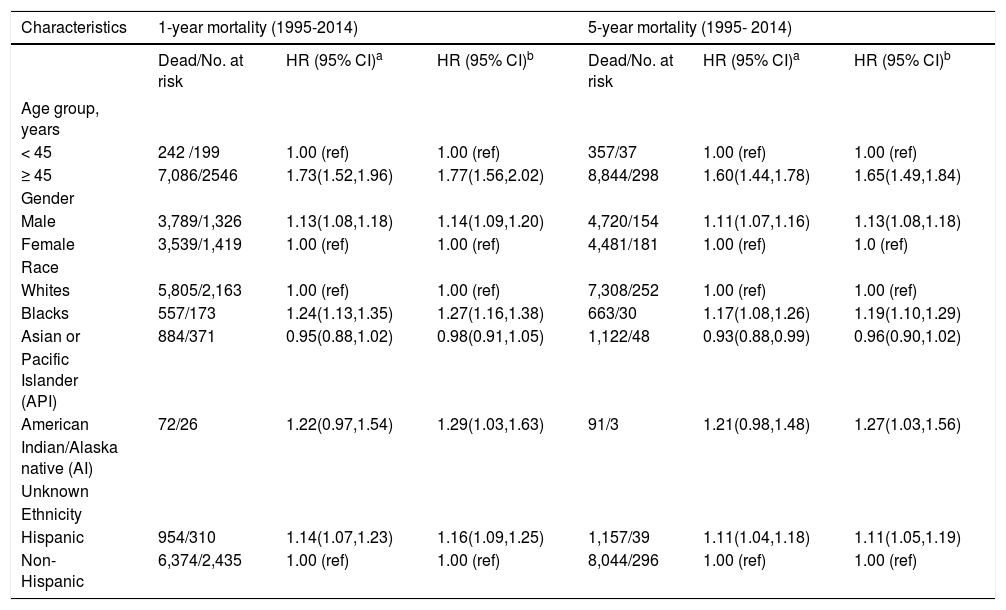
Results from analyses of all-cause mortality after 1 and 5 years of diagnosis of iCCA are shown in table 3. In the multivariable-adjusted models, HRs for risk of all-cause mortality for individuals of age 45 years or older after 1 and 5 years of diagnosis were 1.77 (95% CI: 1.56-2.02) and 1.65 (95% CI: 1.49-1.84), respectively, compared with those younger than 45 years. Hispanic patients also had higher all-cause mortality compared to non-Hispanic patients, with HRs after 1 and 5 years of diagnosis of 1.16 (95% CI: 1.09-1.25) and 1.11 (95% CI: 1.05-1.19), respectively. Higher risk of all-cause mortality was observed also among males (5-year HR = 1.13, 95% CI: 1.08-1.18) compared to females and among Blacks (5-year HR = 1.19, 95% CI: 1.10-1.29) and AIs (5-year HR = 1.27, 95% CI: 1.03-1.56) compared to Whites.
Hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause mortality among patients with intrahepatic cholangiocarcinoma; SEER, 1995-2014.
| Characteristics | 1-year mortality (1995-2014) | 5-year mortality (1995- 2014) | ||||
|---|---|---|---|---|---|---|
| Dead/No. at risk | HR (95% CI)a | HR (95% CI)b | Dead/No. at risk | HR (95% CI)a | HR (95% CI)b | |
| Age group, years | ||||||
| < 45 | 242 /199 | 1.00 (ref) | 1.00 (ref) | 357/37 | 1.00 (ref) | 1.00 (ref) |
| ≥ 45 | 7,086/2546 | 1.73(1.52,1.96) | 1.77(1.56,2.02) | 8,844/298 | 1.60(1.44,1.78) | 1.65(1.49,1.84) |
| Gender | ||||||
| Male | 3,789/1,326 | 1.13(1.08,1.18) | 1.14(1.09,1.20) | 4,720/154 | 1.11(1.07,1.16) | 1.13(1.08,1.18) |
| Female | 3,539/1,419 | 1.00 (ref) | 1.00 (ref) | 4,481/181 | 1.00 (ref) | 1.0 (ref) |
| Race | ||||||
| Whites | 5,805/2,163 | 1.00 (ref) | 1.00 (ref) | 7,308/252 | 1.00 (ref) | 1.00 (ref) |
| Blacks | 557/173 | 1.24(1.13,1.35) | 1.27(1.16,1.38) | 663/30 | 1.17(1.08,1.26) | 1.19(1.10,1.29) |
| Asian or | 884/371 | 0.95(0.88,1.02) | 0.98(0.91,1.05) | 1,122/48 | 0.93(0.88,0.99) | 0.96(0.90,1.02) |
| Pacific Islander (API) | ||||||
| American | 72/26 | 1.22(0.97,1.54) | 1.29(1.03,1.63) | 91/3 | 1.21(0.98,1.48) | 1.27(1.03,1.56) |
| Indian/Alaska native (AI) | ||||||
| Unknown | ||||||
| Ethnicity | ||||||
| Hispanic | 954/310 | 1.14(1.07,1.23) | 1.16(1.09,1.25) | 1,157/39 | 1.11(1.04,1.18) | 1.11(1.05,1.19) |
| Non-Hispanic | 6,374/2,435 | 1.00 (ref) | 1.00 (ref) | 8,044/296 | 1.00 (ref) | 1.00 (ref) |
AI: American Indian/Alaska native. API: Asian or Pacific Islander. SEER: Surveillance Epidemiology and End RESULTS Program of the National Cancer Institute.
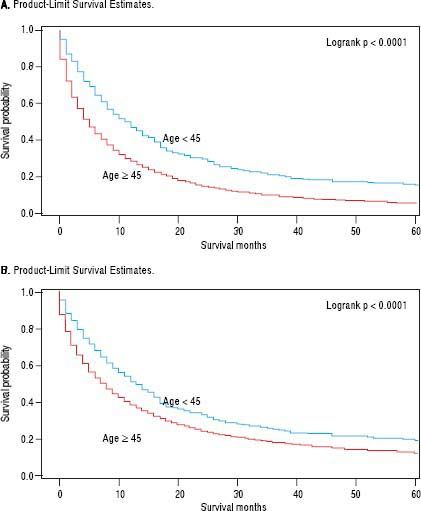
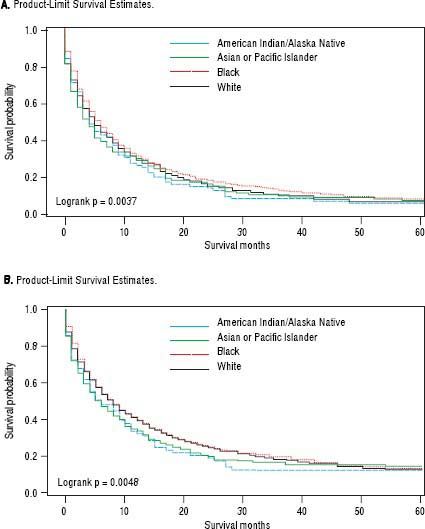
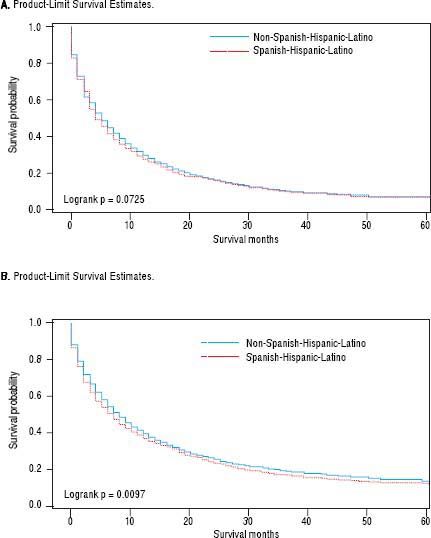
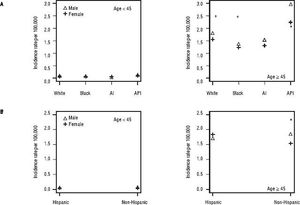
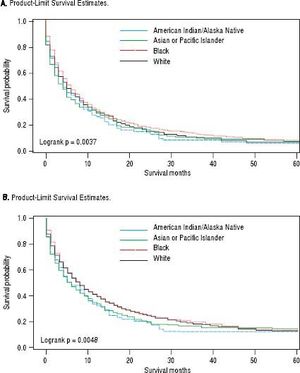
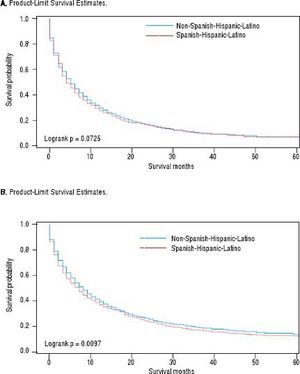
For iCCA-specific mortality (Table 4), Hispanics had a higher risk of death from iCCA after 1-year of diagnosis (HR = 1.19, 95% CI: 1.10-1.29) and after 5 years of diagnosis (HR = 1.16, 95% CI: 1.08-1.25) compared with nonHispanics. Higher iCCA-specific mortality after 1 and 5 years were observed also among individuals age 45 years or older compared to those younger than 45 years, among males compared to females, and among Blacks and AIs compared to Whites (Table 4). The Kaplan-Meier survival curves also show poorer 5-year overall and iCCA-specific survival among individuals younger than age 45 years compared to those 45 years of older (Figure 3), poorer 5-year survival among AIs and Blacks compared with Whites (Figure 4), and among Hispanics compared with non-Hispanics (Figure 5).
Hazard ratios (HRs) and 95% confidence intervals (CIs) for intrahepatic cholangiocarcinoma (iCCA)-specific mortality; SEER, 1995-2014.
| Characteristics | 1-year survival (1995-2014) | 5-year survival (1995- 2014) | ||||
|---|---|---|---|---|---|---|
| Dead/ No. at risk | HR (95% CI)a | HR (95% CI)b | Dead/ No. at risk | HR (95% CI)a | HR (95% CI)b (95% CI)a | |
| Age group, years | ||||||
| < 45 | 217/199 | 1.00 (ref) | 1.00 (ref) | 320/37 | 1.00 (ref) | 1.00 (ref) |
| ≥ 45 | 5,489/2,546 | 1.49(1.30,1.71) | 1.56(1.36,1.79) | 6,755/298 | 1.37(1.21,1.52) | 1.43(1.28,1.60) |
| Gender | ||||||
| Male | 2,891/1,326 | 1.07(1.02,1.13) | 1.09(1.03,1.15) | 3,550/154 | 1.06(1.01,1.11) | 1.07(1.02,1.12) |
| Female | 2815/1,419 | 1.00 (ref) | 1.00 (ref) | 3,525/181 | 1.00 (ref) | 1.00 (ref) |
| Race | ||||||
| Whites | 4,462/2,163 | 1.00 (ref) | 1.00 (ref) | 5,540/252 | 1.00 (ref) | 1.00 (ref) |
| Blacks | 449/173 | 1.27(1.15,1.40) | 1.30(1.18,1.43) | 532/30 | 1.21(1.10,1.32) | 1.23(1.13,1.35) |
| Asian or | 724/371 | 1.00(0.93,1.09) | 1.04(0.97,1.13) | 914/48 | 1.00(0.93,1.07) | 1.03(0.96,1.11) |
| Pacific Islander (API) | ||||||
| American | 62/26 | 1.32(1.03,1.70) | 1.41(1.10,1.82) | 76/3 | 1.28(1.02,1.60) | 1.37(1.09,1.72) |
| Indian/Alaska native (AI) | ||||||
| Unknown | 35 | |||||
| Ethnicity | ||||||
| Hispanic | 762/310 | 1.16(1.08,1.25) | 1.19(1.10,1.29) | 921/39 | 1.13(1.06,1.21) | 1.15(1.07,1.24) |
| Non-Hispanic | 4,944/2,435 | 1.00 (ref) | 1.00 (ref) | 6,154/296 | 1.00 (ref) | 1.00 (ref) |
AI: American Indian/Alaska native. API: Asian or Pacific Islander. SEER: Surveillance Epidemiology and End RESULTS Program of the National Cancer Institute.
In this sample of the United States population drawn from 18 population-based cancer registries, we observed a rising incidence of iCCA from 1995 to 2014, with an overall age-adjusted incidence rate of 0.92 per 100,000 persons. The incidence rose twofold over two decades, from 0.49 per 100,000 in 1995 to 1.49 per 100,000 in 2014. Compared to the preceding 10 year period from 1995-2004, the incidence rate was 53% higher in 10 year period from 20052014. Over the 20-year study period, the annual rate of increase was 5.49%. These findings are consistent with results from previous studies.8,10,20–28 A fourfold increase in incidence of iCCA in the United States was reported between 1973 and 1997, from 0.13 per 100,000 in 1973 to 0.67 per 100,000 in 1997, with an estimated annual percentage increase of 9.1%.8
This study highlighted a higher incidence of iCCA amongst Hispanics as well as poorer all-cause mortality and iCCA-specific mortality among Hispanics compared to non-Hispanics. The incidence rate among Hispanics APIs was higher than for other racial groups in the United States. Although Blacks had a lower incidence of iCCA than Whites, Black patients had higher mortality (both all-cause and iCCA-specific mortality) compared to Whites. Similar observations regarding the mortality risk in Blacks have been made by other groups,8,21 although these studies did not specifically evaluate ethnic variations in iCCA incidence or survival.
It is worth noting that the magnitude of the iCCA incidence rate observed in our study (0.92 per 100,000; 19952014) is lower than that reported by Mosadeghi, et al. (1.6 per 100,000; 2000-2011),10 even when compared to the incidence rate we observed in the latter 10-year period (1.09 per 100,00; 2005-2014). This may result from the use of different classification systems. The present study used the ICD-10-CM code C22.1 whereas the study by Mosadeghi, et al. used an unspecified ICD-O-3 code.10 The use of ICD-10-CM codes is less prone to misclassification of Klatskin tumors as iCCA.29 Concerns about coding misclassification of iCCA and its influence on reporting of incidence rates for iCCA have been discussed in detail.7,29,30 Notably, Tyson, et al. identified iCCA cases in SEER based on ICD-O3 codes and determined histology and topography codes and found that between 2001 and 2007, 45% of Klatskin tumors were misclassified as iCCA based on the ICD-O-3 coding system.30 In our study, the analysis of a broader time period using a more precise identification of iCCA cases, and analysis of trends in both incidence and survival, provide a better understanding of temporal changes in incidence and survival iCCA in the United States across racial, ethnic, and other sociodemographic groups.
The incidence of iCCA was higher in individuals of age 45 years or older compared with those younger than 45 years. Shaib, et al. also reported a 165% increase in iCCA incidence in the United States from 1975 to 1999, with a higher incidence rate among individuals of age 45 years or older compared to those younger 45 years.21 There is a potential for germline mutations to contribute to iCCA occurring in the young, and studies to evaluate this are warranted. Although we observed a higher incidence of iCCA in males compared to females, previous studies have not identified any differences between males and females.8
The increasing incidence of iCCA in the United States could reflect the rising prevalence of predisposing factors, such as cirrhosis, chronic viral hepatitis B or C and metabolic syndrome, or aging of the 1945-1965 birth cohort known to have high prevalence of hepatitis C infection.3,5.6.9.10,31–37 Changes in prevalence of fibropolycystic liver disease, primary sclerosing cholangitis, or intrahepatic biliary stones are also possible although these have not been documented. Differential prevalence of these risk factors would account for the racial, ethnic, age, and gender differences in iCCA incidence.33,38–40 The poorer survival outcomes after a diagnosis of iCCA observed among Hispanics and Blacks, in particular, is of significant public health concern giving that in the United Sates, Hispanics and Blacks are the fastest growing population and the largest ethnic minority group, respectively.41,42 Because the poor survival outcomes among Blacks and Hispanics may reflect limited access to care, intervention strategies aimed at eliminating health disparities in cancer outcome may be most effective if targeted at these populations.
Our study has several strengths and limitations. The strengths of the study include its large sample size, the assessment of both incidence and survival outcomes over a 20-year period, and investigation of iCCA outcomes across various population groups. Limitations include the limited depth of the SEER data which does not include data on symptoms or underlying liver cirrhosis and had limited data on cancer treatment (more than 30% of patients had missing data on treatment). This could have confounded the results of the survival analyses. However, we adjusted for cancer stage at diagnosis using the SEER historic stage A variable. Because treatment is dependent on initial cancer stage at diagnosis, the adjustment for stage at diagnosis would minimize the potential impact of differential treatment on survival. We were unable to characterize predisposing factors for iCCA in the various population subgroups or assess the determinant of poor survival among Hispanics, Blacks, and males because of data limitations. Furthermore, there is a potential for misclassification errors because SEER data abstractions are done from patient medical records by multiple people at different institutions and therefore may not be done in a consistent manner across all 18 SEER registries.
An abrupt peak in the incidence of iCCA was noted in the year 2000 and the reasons for this are not entirely clear. While this could be due to systematic surveillance bias or data input error, these factors are unlikely to completely explain the increased incidence of iCCA observed in the study. The increasing incidence of iCCA is also unlikely to be completely accounted for by improvements in iCCA diagnosis over time because in this case, the incidence rates would have been expected to plateau after sometime when the number of prevalent cases would have decreased substantially.29 Rather, we found increasing incidence rates across the follow-up time, an indication of true increase in the iCCA incidence rates.
In summary, this study shows an on-going increase in iCCA incidence in the United States between 1995 and 2014, with an estimated average annual percentage increase of 5.49%. These findings further demonstrate substantial ethnic, racial, gender, and age disparities in iCCA incidence and survival. Hispanics had both higher incidence and shorter survival after diagnosis with iCCA compared to non-Hispanics, while Blacks had a lower incidence but shorter survival compared to Whites. APIs had a higher iCCA incidence rate than any other racial group in the United States.These cancers have a higher incidence and poorer survival in individuals of age 45 years or older and in men compared to those younger than 45 years and women, respectively. Studies focused on delineating the factors responsible for the rising incidence of iCCA in the United States and identifying the determinants of poor survival among Hispanic and Black patients, in particular, would inform intervention strategies.
Abbreviations- •
AAPC: Average annual percentage change.
- •
AI: American Indian/Alaska native.
- •
API: Asian or Pacific Islander.
- •
API: Asian or Pacific Islander.
- •
iCCA: Intrahepatic cholangiocarcinoma.
- •
ICD-10-CM: International Classification of Diseases, 10th Revision, Clinical Modification code.
- •
ICD-O3: International Classification of Disease for Oncology, 3rd edition.
- •
SEER: Surveillance, Epidemiology, and End Results Program.
None.
Financial DisclosuresNone.