Introduction. Adiponectin and resistin levels are increased in patients with cirrhosis, but it prognostic significance is unknown. We sought to investigate the factors associated with adiponectin and resistin levels and its clinical significance in patients with cirrhosis.
Materials and methods. This was a prospective cohort study that included 122 subjects with cirrhosis who attended an outpatient clinic and were initially evaluated in 2012. Serum adiponectin and resistin levels were measured in samples collected in 2012 (adiponectin and resistin) and 2014 (adiponectin). Thirty healthy subjects served as a control group.
Results. Higher adiponectin (21.59 μg/mL vs. 12.52 μg/mL, P < 0.001) and resistin levels (3.83 ng/mL vs. 2.66 ng/mL, P < 0.001) were observed among patients with cirrhosis compared to controls. Patients classified as Child-Pugh B/C had higher adiponectin levels in relation to ChildPugh A patients. At second measurement, adiponectin levels increased significantly in non-transplant patients and decreased in liver transplant recipients. Univariate Cox analysis showed that among patients with alcoholic liver disease, adiponectin levels were associated with lower transplant-free survival (HR = 1.034, 95% CI 1.006 - 1.062, P = 0.016). The transplant-free survival was significantly lower among patients with alcoholic liver disease and adiponectin ≥ 17 μg/mL (26.55 months, 95% CI 21.40-31.70) as compared to those with levels < 17 μg/mL (33.76 months, 95% CI 30.70-36.82) (P = 0.045). No relationship was found between the levels of resistin and survival.
Conclusion. Adiponectin but not resistin levels were associated with intensity of liver dysfunction and worse prognosis in patients with alcoholic liver disease, suggesting a potential as a prognostic biomarker.
The liver is primarily a metabolic organ that orchestrates a complex order of biochemical and physiological processes, including regulation of protein and energy metabolismo.1 Consequently, and not surprisingly, patients with advanced liver disease suffer from malnutrition.1 Loss of body fat involves a series of metabolic changes as the fat is not simply a deposit of lipids but is also recognized as an important source of hormones that influence body adiposity, glucose homeostasis, inflammation, and cardiovascular disease.2 The adipocyte secretes various adipokines, and changes in body composition observed in cirrhotic individuals are associated with various metabolic effects that are possibly mediated by disturbances in the balance of production and/or action of adipokines.
Among the adipokines, adiponectin and resistin have been highlighted in the context of liver disease. Adiponectin is a 28 kDa protein composed of 274 amino acids whose AdipoQ gene is encoded in the long arm of chromosome 3, locus 3q27. Adiponectin exists in circulation in two isoforms: as an intact molecule (fAd) and as a globular fragment (cleaved proteolytic fragment consisting of gAd).3 The intact molecule has the ability to group three globular domain oligomeric isoforms: trimeric, hexameric and multimeric forms.3 Each oligomeric form has distinct biological properties and activates different cellular signaling pathways in several tissues.3 Since its discovery, adiponectin has proved to be a key component in the relationship between adiposity and insulin resistance (IR).3 Resistin is an adipocytederived hormone with 12.5 kDa that forms multimeric complexes characterized by the presence of cysteine residues.4 It is involved in the regulation of glucose homeostasis, inflammation, and adipogenesis and thus influences the development of insulin resistance, obesity, and type 2 diabetes.5,6
The hepatoprotective role of adiponectin in liver diseases has been described in various experimental and clinical studies, including antiesteatotic, antiinflammatory, and antifibrogenic effects.7 Low levels of adiponectin were related to various liver diseases including non-alcoholic fatty liver disease (NAFLD) and hepatitis B and C.7 Paradoxically, previous studies have observed a significant increase in serum levels of these adipokines in patients with advanced cirrhosis, regardless of etiology.8 Moreover, a positive correlation between adiponectin levels and hepatic fibrosis markers have been demonstrated.9 Few studies have investigated the relevance of resistin in hepatic diseases. The resistin expression in the human liver is increased in various liver diseases,10-13 and the positive correlation between resistin and inflammation and hepatic fibrosis, which suggests the involvement of this adipokine in the pathophysiology of liver fibrosis, has been demonstrated in alcoholic hepatitis and NAFLD.10,13 In liver cirrhosis, resistin levels are also significantly higher and have been associated with increased disease severity, suggesting potential as a prognostic biomarker.14 Thus, the aims of this study were to investigate the factors associated with adiponectin and resistin levels and its prognostic significance in patients with liver cirrhosis.
Material and MethodsPatientsThis was a prospective cohort study that included consecutive adult subjects (> 18 years of age) attending the outpatient clinic at the University Hospital of the Federal University of Santa Catarina, Brazil. The diagnosis of cirrhosis was established histologically (when available) or by the combination of clinical, imaging, and laboratory findings in patients with evidence of portal hypertension. Patients in the following situations were excluded: hepatocellular carcinoma; insulin or thiazolidinedione therapy; interferon-based therapy over the last 30 days; or refusal or inability of the patient to understand the informed consent. Thirty sex- and age-matched healthy subjects evaluated during routine laboratory tests served as a control group. A sample power of 91% was obtained for comparing adiponectin means between cases and controls using a 1-sided test.
Informed consent in writing was obtained from each participant (i.e., patients and healthy volunteers), and the study protocol conformed to the ethical guidelines of the 1975 Helsinki Declaration and was approved by our institutional review board.
MethodsPatients were evaluated at the outpatient clinic of the Gastroenterology Division, and the following clinical variables were collected: age, gender, race, alcohol abuse and smoking history, etiology of cirrhosis, history of previous descompensation and hospitalization, previous diagnosis of diabetes mellitus, dyslipidemia and systemic arterial hypertension, diagnosis of esophageal varices, presence of ascites, encephalopathy and peripheral edema. All subjects underwent laboratory evaluation on the same day as clinical evaluation and the following tests were performed for this study: hepatic enzymes, albumin, total and conjugated bilirubin, international normalized ratio (INR), creatinine, sodium, cholesterol, triglycerides, fasting blood glucose, glycated hemoglobin and insulin, and C-reactive protein (CRP). Insulin resistance was evaluated by the homeostasis model assessment-insulin resistance (HOMA-IR) test.15 Patients that had used at least one cigarette in the past 30 days were considered to be currently smoking.16 Current significant alcohol intake was defined as an average overall consumption of 21 or more drinks per week for men and 14 or more drinks per week for women during the 4 weeks before enrolment (one standard drink is equal to 12 g absolute alcohol).17 The same criterion was used to define previous alcohol abuse, considering the habitual alcohol intake pattern before the last four weeks. Child-Pugh classification18 and the MELD (Model for End-Stage Liver Disease) score19 were used to assess the severity of the hepatic disease. Major cardiovascular events were defined as myocardial infarction, acute coronary syndrome, cerebrovascular accident or transient ischemic attack.
Nutritional assessment and anthropometric parametersAll patients were submitted to a general nutritional assessment procedure that was proposed and validated for use in cirrhotic patients (Royal Free Hospital Global Assessment - RFH-GA).20 This evaluation includes the following parameters: body mass index (BMI) based on estimated dry weight; mid-arm muscle circumference (MAMC); estimated daily caloric intake; and clinical data (gastrointestinal symptoms, recent history of infections, renal failure, hepatic encephalopathy, gastrointestinal bleeding, weight variation, physical activity and fatigue). According to the proposed algorithm, patients were divided into adequately nourished, moderately malnourished (or suspected to be), and severely malnourished. The MAMC measurements were expressed in relation to the 5th percentile for age and gender.21 The triceps skinfold thickness (TSF) was measured with a Lange Skinfold Caliper as an estimate of fat mass.
Follow-up of included patientsThe entire cohort was initially evaluated from June to October 2012. Patients underwent clinical and laboratory reevaluation in 2014. The development of complications, mortality, or liver transplantation was assessed by periodic phone calls and during outpatient visits. The final reevaluation was performed between June and October 2015. The occurrence of major cardiovascular events was evaluated only in the second reevaluation, performed in 2014.
Serum adiponectin and resistin levelsThe tests were performed in serum samples collected after clinical evaluation and stored at -80 °C. Serum adiponectin levels were measured by ELISA in samples collected at baseline (2012) and in the first reevaluation (2014) using a commercially available assay (Human Adiponectin ELISA Kit, EMD Millipore - Missouri USA). Serum resistin was also measured by ELISA only in baseline samples using the Human Resistin ELISA kit (BioVendor, Brno-Czech Republic). The maximum length of storage was 12 months for adiponectin and 24 months for resistin, and all samples were not previously thawed.
Statistical analysisThe normality of the variable distribution was determined by the one-sample Kolmogorov-Smirnov test. The correlation between the numerical variables was evaluated using Spearman’s correlation coefficient. Continuous variables were compared using the MannWhitney test. Univariate Cox regression analysis was used to investigate the association between the adipokines levels and transplant-free survival. The Kaplan-Meier curve was used to illustrate transplant-free survival according to two strata, which were defined by the cutoff of selected adipokine. Survival differences between groups were compared using the log-rank test. Survival analysis initially included all patients, and thereafter, subanalysis including specific etiologies of cirrhosis was conducted. The Wilcoxon signed rank-test was used to compare adi-ponectin levels at two times. A p value < 0.05 was considered statistically significant. All tests were two-tailed and were performed by the SPSS software, version 17.0 (SPSS Inc. Released 2008).
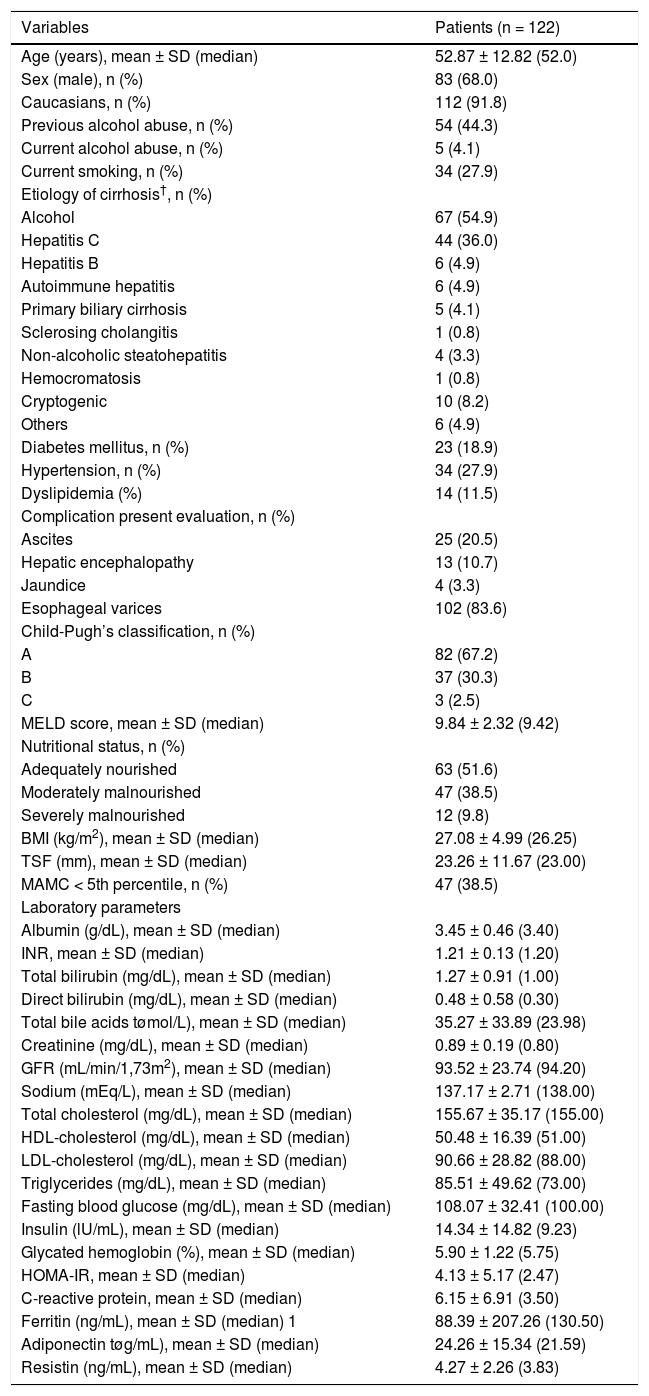
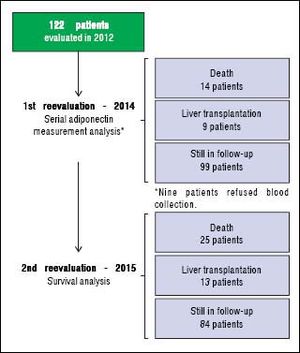
RESULTSCharacteristics of the patientsThe characteristics of the 122 subjects included in this study are summarized in table 1. The mean age was 52.8 ± 12.8 years, and there was a predominance of males (68.0%). The diagnosis of cirrhosis was defined by liver biopsy in 22 patients (18%) and by other criteria in the remaining subjects. The most common cause of cirrhosis was alcohol abuse (54.9%) followed by chronic viral hepatitis (HBV infection in 4.9% and HCV in 36.0%). Current alcohol abuse was observed in five patients, and all of them were diagnosed with alcoholic cirrhosis. At the time of initial evaluation, 67.2% were classified as Child-Pugh A, 30.3% as B, and 2.5% as C. The mean MELD score was 9.84 ± 2.32 (median 9.42). According to the RFH-GA, malnutrition was present in 48.4% of patients and was classified as moderate in 38.5% and severe in 9.8% of cases. None of the patients were undergoing renal replacement therapy at this time.
Characteristics of included patients.
| Variables | Patients (n = 122) |
|---|---|
| Age (years), mean ± SD (median) | 52.87 ± 12.82 (52.0) |
| Sex (male), n (%) | 83 (68.0) |
| Caucasians, n (%) | 112 (91.8) |
| Previous alcohol abuse, n (%) | 54 (44.3) |
| Current alcohol abuse, n (%) | 5 (4.1) |
| Current smoking, n (%) | 34 (27.9) |
| Etiology of cirrhosis†, n (%) | |
| Alcohol | 67 (54.9) |
| Hepatitis C | 44 (36.0) |
| Hepatitis B | 6 (4.9) |
| Autoimmune hepatitis | 6 (4.9) |
| Primary biliary cirrhosis | 5 (4.1) |
| Sclerosing cholangitis | 1 (0.8) |
| Non-alcoholic steatohepatitis | 4 (3.3) |
| Hemocromatosis | 1 (0.8) |
| Cryptogenic | 10 (8.2) |
| Others | 6 (4.9) |
| Diabetes mellitus, n (%) | 23 (18.9) |
| Hypertension, n (%) | 34 (27.9) |
| Dyslipidemia (%) | 14 (11.5) |
| Complication present evaluation, n (%) | |
| Ascites | 25 (20.5) |
| Hepatic encephalopathy | 13 (10.7) |
| Jaundice | 4 (3.3) |
| Esophageal varices | 102 (83.6) |
| Child-Pugh’s classification, n (%) | |
| A | 82 (67.2) |
| B | 37 (30.3) |
| C | 3 (2.5) |
| MELD score, mean ± SD (median) | 9.84 ± 2.32 (9.42) |
| Nutritional status, n (%) | |
| Adequately nourished | 63 (51.6) |
| Moderately malnourished | 47 (38.5) |
| Severely malnourished | 12 (9.8) |
| BMI (kg/m2), mean ± SD (median) | 27.08 ± 4.99 (26.25) |
| TSF (mm), mean ± SD (median) | 23.26 ± 11.67 (23.00) |
| MAMC < 5th percentile, n (%) | 47 (38.5) |
| Laboratory parameters | |
| Albumin (g/dL), mean ± SD (median) | 3.45 ± 0.46 (3.40) |
| INR, mean ± SD (median) | 1.21 ± 0.13 (1.20) |
| Total bilirubin (mg/dL), mean ± SD (median) | 1.27 ± 0.91 (1.00) |
| Direct bilirubin (mg/dL), mean ± SD (median) | 0.48 ± 0.58 (0.30) |
| Total bile acids tømol/L), mean ± SD (median) | 35.27 ± 33.89 (23.98) |
| Creatinine (mg/dL), mean ± SD (median) | 0.89 ± 0.19 (0.80) |
| GFR (mL/min/1,73m2), mean ± SD (median) | 93.52 ± 23.74 (94.20) |
| Sodium (mEq/L), mean ± SD (median) | 137.17 ± 2.71 (138.00) |
| Total cholesterol (mg/dL), mean ± SD (median) | 155.67 ± 35.17 (155.00) |
| HDL-cholesterol (mg/dL), mean ± SD (median) | 50.48 ± 16.39 (51.00) |
| LDL-cholesterol (mg/dL), mean ± SD (median) | 90.66 ± 28.82 (88.00) |
| Triglycerides (mg/dL), mean ± SD (median) | 85.51 ± 49.62 (73.00) |
| Fasting blood glucose (mg/dL), mean ± SD (median) | 108.07 ± 32.41 (100.00) |
| Insulin (lU/mL), mean ± SD (median) | 14.34 ± 14.82 (9.23) |
| Glycated hemoglobin (%), mean ± SD (median) | 5.90 ± 1.22 (5.75) |
| HOMA-IR, mean ± SD (median) | 4.13 ± 5.17 (2.47) |
| C-reactive protein, mean ± SD (median) | 6.15 ± 6.91 (3.50) |
| Ferritin (ng/mL), mean ± SD (median) 1 | 88.39 ± 207.26 (130.50) |
| Adiponectin tøg/mL), mean ± SD (median) | 24.26 ± 15.34 (21.59) |
| Resistin (ng/mL), mean ± SD (median) | 4.27 ± 2.26 (3.83) |
SD: Standard deviation. MELD: Model for End-Stage Liver Disease. BMI: Body mass index based on estimated dry weight TSF: Triceps skin-fold thickness. MAMC: Mid-arm muscle circumference. INR: International normalized ratio. GFR: Estimated glomerular filtration rate. HOMA-IR: Homeostasis model assessment-insulin resistance. The authors give permission for use from publisher on Annals of Hepatology to any borrowed or modified tables from the author and copyright holder.
Significantly higher levels of adiponectin (21.59 µg/mL vs. 12.52 µg/mL, P < 0.001) and resistin (3.83 ng/mL vs. 2.66 ng/mL, P < 0.001) were observed among patients with cirrhosis compared to healthy controls.
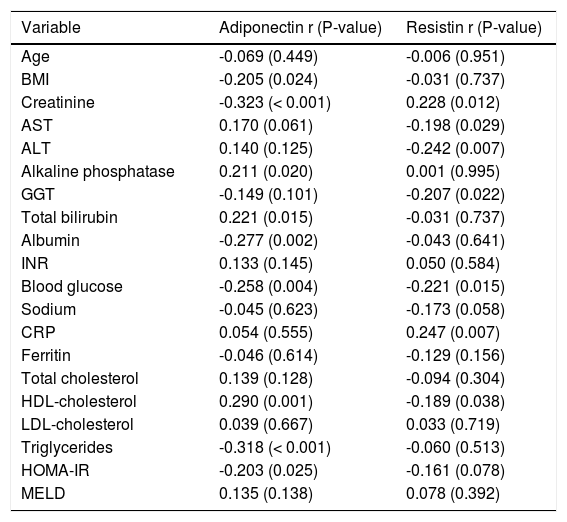
Table 2 shows the Spearman’s correlation analysis between adipokines and other numerical variables. Among cirrhotic patients, adiponectin levels were positively correlated with total bilirubin (P = 0.015) and HDL-cholesterol (P = 0.001) and negatively correlated with BMI (P = 0.024), creatinine (P < 0.001), albumin (P = 0.002), blood glucose (P = 0.004), triglycerides (P < 0.001), and HOMA-IR (P = 0.025). The resistin levels directly correlated to creatinine (P = 0.012) and CRP (P = 0.007) and inversely to AST (P = 0.029), ALT (P = 0.007), GGT (P = 0.022), fasting glucose (P = 0.015), and HDL cholesterol (P = 0.038).
Spearman’s correlation analysis between adipokines and demographic, anthropometric and laboratory variables.
| Variable | Adiponectin r (P-value) | Resistin r (P-value) |
|---|---|---|
| Age | -0.069 (0.449) | -0.006 (0.951) |
| BMI | -0.205 (0.024) | -0.031 (0.737) |
| Creatinine | -0.323 (< 0.001) | 0.228 (0.012) |
| AST | 0.170 (0.061) | -0.198 (0.029) |
| ALT | 0.140 (0.125) | -0.242 (0.007) |
| Alkaline phosphatase | 0.211 (0.020) | 0.001 (0.995) |
| GGT | -0.149 (0.101) | -0.207 (0.022) |
| Total bilirubin | 0.221 (0.015) | -0.031 (0.737) |
| Albumin | -0.277 (0.002) | -0.043 (0.641) |
| INR | 0.133 (0.145) | 0.050 (0.584) |
| Blood glucose | -0.258 (0.004) | -0.221 (0.015) |
| Sodium | -0.045 (0.623) | -0.173 (0.058) |
| CRP | 0.054 (0.555) | 0.247 (0.007) |
| Ferritin | -0.046 (0.614) | -0.129 (0.156) |
| Total cholesterol | 0.139 (0.128) | -0.094 (0.304) |
| HDL-cholesterol | 0.290 (0.001) | -0.189 (0.038) |
| LDL-cholesterol | 0.039 (0.667) | 0.033 (0.719) |
| Triglycerides | -0.318 (< 0.001) | -0.060 (0.513) |
| HOMA-IR | -0.203 (0.025) | -0.161 (0.078) |
| MELD | 0.135 (0.138) | 0.078 (0.392) |
BMI: Body mass index based on estimated dry weight. AST: Aspartate aminotransferase. ALT: Alanine aminotransferase. GGT: Gamma-glutamyl-transferase. INR: International normalized ratio. CRP: C-reactive protein. HOMA-IR: Homeostasis model assessment-insulin resistance. MELD: Model for End-Stage Liver Disease. *The authors give permission for use from publisher on Annals of Hepatology to any borrowed or modified tables from the author and copyright holder.
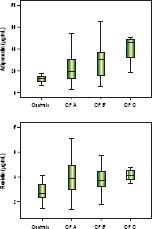
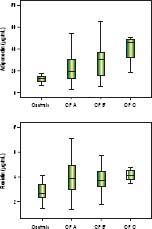
Significantly lower median adiponectin was observed among men (18.42 µg/mL vs. 27.18 µg/mL, P = 0.002) and in those with alcoholic etiology (16.83 µg/mL vs. 27.40 mg/ mL, P = 0.001). Figure 1 shows the adiponectin (Figure 1A) and resistin levels (Figure 1B) in the control group and according to the Child-Pugh classification. Patients classified as Child-Pugh B/C had significantly higher levels of adiponectin in relation to Child-Pugh A patients (30.47 µg/mL vs. 17.04 µg/mL, P = 0.008). Similarly, higher adiponectin levels were observed in patients with MELD > 10 as compared to those with lower MELD scores (25.67 µg/mL vs. 19.07 µg/mL, P = 0.017). There were no differences in adiponectin levels regarding to other variables, including nutritional status and current alcohol consumption. Higher median resistin levels were observed in patients with a history of hepatic encephalopathy (4.34 ng/mL vs. 3.63 ng/mL, P = 0.003). There was a trend towards higher values resistin among individuals with a history of cirrhosis decompensation (4.06 ng/mL vs. 3.38 ng/mL, P = 0.064). No differences were observed in relation to other variables, including etiology of cirrhosis, Child-Pugh classification, MELD score, and nutritional status.
Box-plots of adiponectin (A) and resistin (B) levels among control group and patients with cirrhosis divided according to the Child-Pugh (CP) Classifcation. Significantly higher levels of adiponectin (21.59 µ/mL vs. 12.52 µ/mL, P < 0.001) and resistin (3.83 ng/mL vs. 2.66 ng/mL, P < 0.001) were observed among patients with cirrhosis compared to healthy controls.
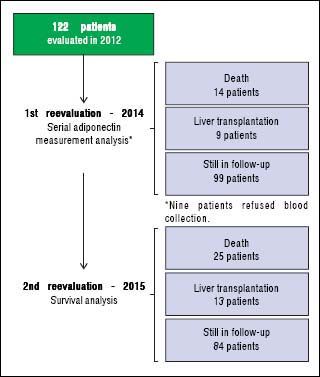
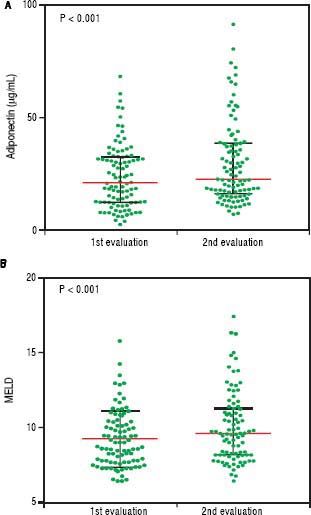
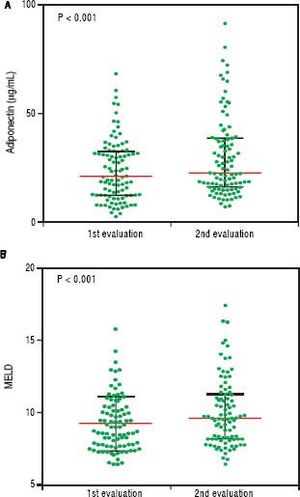
An initial clinical and laboratory reassessment was conducted in 2014; then, the patients were followed for another year for survival evaluation in 2015 (Figure 2). At the 2014 evaluation, 14 patients (11.5%) had died, nine patients (7.4%) underwent liver transplantation, and the remaining 99 patients were still in follow-up and were reassessed (81.1%). When considering only those who did not undergo liver transplantation, 7 patients progressed from Child-Pugh A to B, 1 patient from Child-Pugh A to C, and 1 from Child-Pugh B to C. Major cardiovascular events in this reevaluation were observed in 4 patients, 3 cases of acute coronary syndrome and one death due to acute myocardial infarction. Given the relatively low number of cardiovascular events during follow-up, no statistical analysis could be performed to investigate its relationship with adipokines levels. Nine patients refused blood collection (one liver transplant recipient and eight cirrhotics). After analyzing the adipokines results at baseline, adiponectin was chosen as a potential prognostic biomarker, and it was measured again at the second evaluation. Figures 3A and 3B exhibit the adi-ponectin and MELD results at the first and second evaluations.
In non-transplant patients, the median adiponectin increased significantly in the second evaluation (22.49 µg/ mL vs. 19.13 µg/mL, P < 0.001). Similarly, the median MELD score was significantly higher at the second evaluation (9.22 ± 1.89 vs. 10.01 ± 2.36, P < 0.001). When stratified accordingly to the Child-Pugh classification, median adiponectin levels were 18.65 µg/mL in 2012 and 21.96 µg/mL in 2014 among Child-Pugh A subjects (P < 0.001). In Child-Pugh B/C patients, median adiponectin increased from 24.54 µg/mL in 2012 to 34.93 µg/mL in 2014 (P = 0.003). Among the eight liver transplant recipients, the median adiponectin levels numerically decreased from 38.24 µg/mL in 2012 to 24.08 µg/mL in 2014; although without statistical significance (P = 0.123).
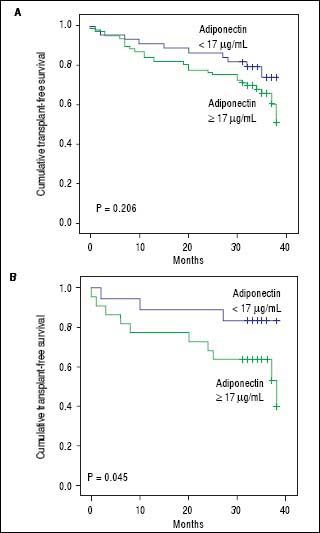
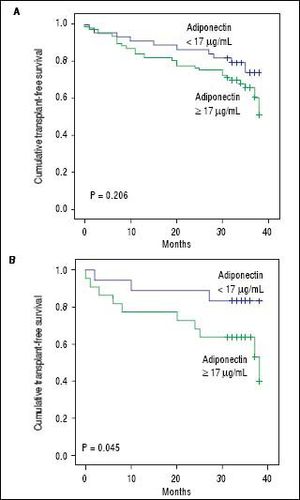
Adipokines and prognosis in liver cirrhosisPatients were followed for a median of 34 months. During follow-up, 25 patients (20.5%) died, and 13 (10.7%) underwent liver transplantation. Univariate Cox analysis showed a trend toward association between adiponectin and overall transplant-free survival (HR = 1.019, 95% CI 0.999 - 1.038, P = 0.057). No relationship was found between the levels of resistin and transplantfree survival (HR = 0.950, 95% CI 0.801-1.126, P = 0.554). A subsequent analysis was performed that evaluated the relationship between adiponectin and survival according to the etiology of the liver disease (i.e., viral and alcoholic). Twenty-five patients had both alcoholism and chronic viral hepatitis (HCV in 23 and HBV in 2) and were included in both analyses. Less common etiologies were not evaluated in this subanalysis due to the low number of subjects. Of the patients with alcoholic liver disease, 19 patients died, and 9 underwent transplants during follow-up. Univariate Cox analysis showed that among patients with alcoholic liver disease (as either a single etiology or a co-factor), adiponectin levels were significantly associated with lower transplant-free survival (HR = 1.034, 95% CI 1.006-1.062, P = 0.016). Figure 4 shows Kaplan-Meier survival curves giving to adiponectin levels dichotomized according to the median (17 µg/mL) in the entire cohort (Figure 4A) and in patients with alcoholic liver disease (Figure 4B). In the entire cohort, no differences were observed when patients with adiponectin > 17 µg/mL were compared to those with adiponectin < 17 µg/mL (P = 0.494, log-rank test). The transplant-free survival was significantly lower among alcoholic liver disease patients with adiponectin > 17 ¿Ag/mL (26.55 months, 95% CI 21.40-31.70) as compared to those with levels < 17 µg/mL (33.76 months, 95% CI 30.70 to 36.82) (P = 0.045, log-rank test). No differences were observed in the Kaplan-Meier survival analysis for the selected adiponectin cutoff in cases of viral etiology or when grouping other less common etiologies of cirrhosis.
Kaplan-Meier transplant-free survival of the entire cohort (A) and of patients with alcoholic liveoase (B) stratified according to the adiponectin cutoff level of 17 µg/mL. No signifcant differences were noted for the entire cohort (P = 0.206, log-rank test). Transplant-free survival was significantly lower among patients with adiponectin = 17 µg/mL (26.55 months, 95% CI 21.40 to 31.70) as compared to those with levels < 17µg/mL (33.76 months, 95% CI 30.70 to 36.82) (P = 0.045).
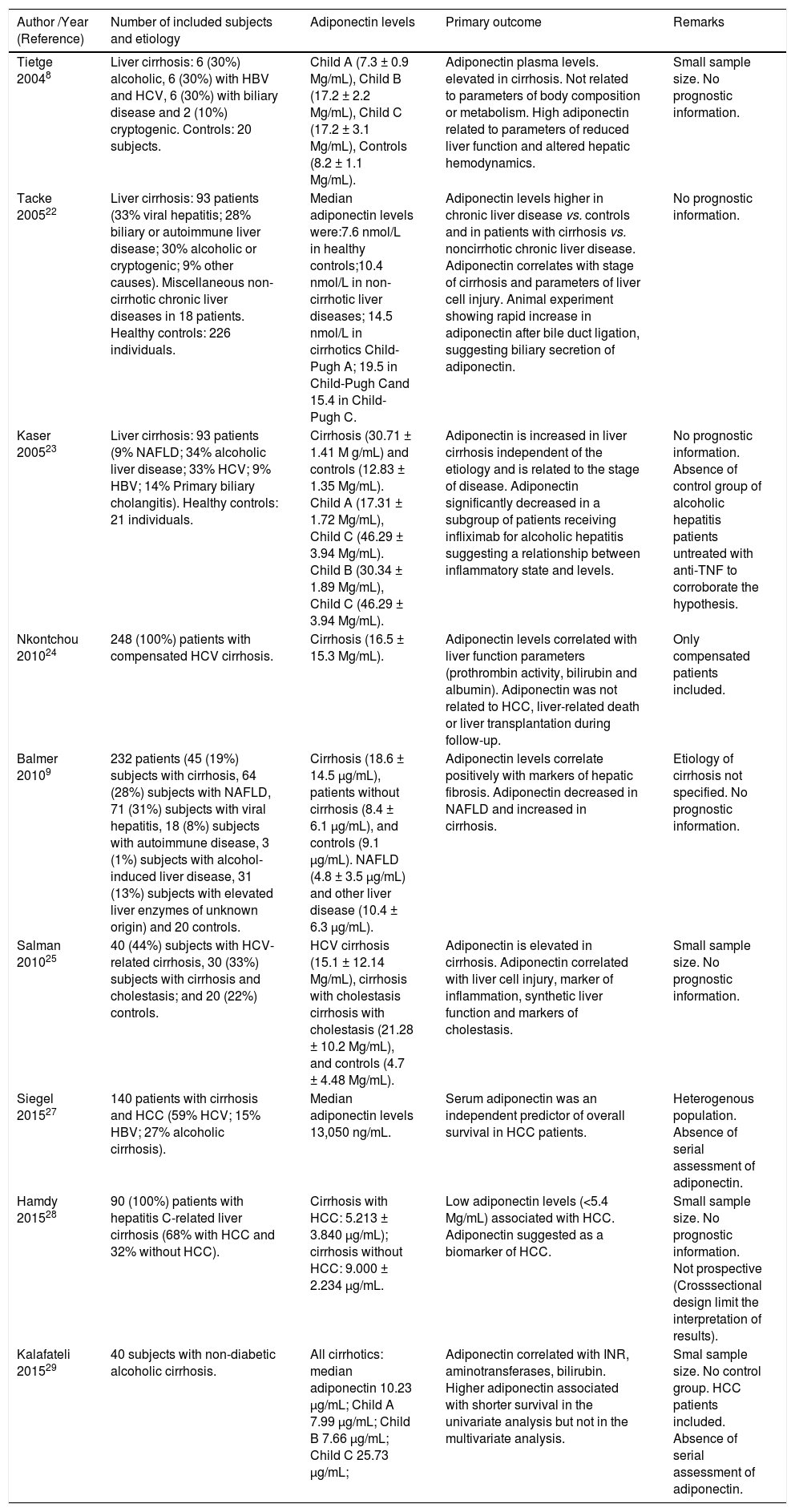
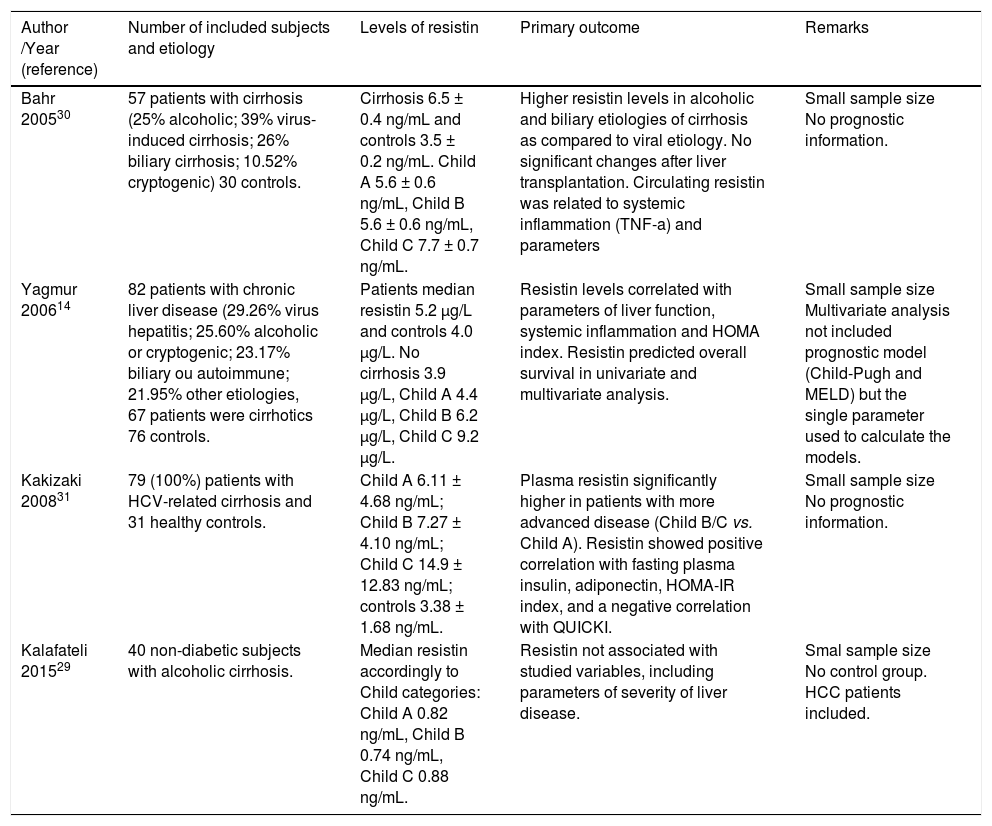
Patients with liver cirrhosis have a wide spectrum of metabolic disorders, and the interaction between the different aspects of these changes is not yet fully established. Multiple factors such as insulin resistance, changes in body composition and nutritional status as well as liver dysfunction can affect the levels of adiponectin and resistin in patients with cirrhosis. Tables 3 and 4 summarize the major human studies that investigated the relationship between adiponectin (Table 3), resistin (Table 4), and cirrhosis.
Human studies that investigated the relationship between adiponectin and cirrhosis.
| Author /Year (Reference) | Number of included subjects and etiology | Adiponectin levels | Primary outcome | Remarks |
|---|---|---|---|---|
| Tietge 20048 | Liver cirrhosis: 6 (30%) alcoholic, 6 (30%) with HBV and HCV, 6 (30%) with biliary disease and 2 (10%) cryptogenic. Controls: 20 subjects. | Child A (7.3 ± 0.9 Mg/mL), Child B (17.2 ± 2.2 Mg/mL), Child C (17.2 ± 3.1 Mg/mL), Controls (8.2 ± 1.1 Mg/mL). | Adiponectin plasma levels. elevated in cirrhosis. Not related to parameters of body composition or metabolism. High adiponectin related to parameters of reduced liver function and altered hepatic hemodynamics. | Small sample size. No prognostic information. |
| Tacke 200522 | Liver cirrhosis: 93 patients (33% viral hepatitis; 28% biliary or autoimmune liver disease; 30% alcoholic or cryptogenic; 9% other causes). Miscellaneous non-cirrhotic chronic liver diseases in 18 patients. Healthy controls: 226 individuals. | Median adiponectin levels were:7.6 nmol/L in healthy controls;10.4 nmol/L in non-cirrhotic liver diseases; 14.5 nmol/L in cirrhotics Child-Pugh A; 19.5 in Child-Pugh Cand 15.4 in Child-Pugh C. | Adiponectin levels higher in chronic liver disease vs. controls and in patients with cirrhosis vs. noncirrhotic chronic liver disease. Adiponectin correlates with stage of cirrhosis and parameters of liver cell injury. Animal experiment showing rapid increase in adiponectin after bile duct ligation, suggesting biliary secretion of adiponectin. | No prognostic information. |
| Kaser 200523 | Liver cirrhosis: 93 patients (9% NAFLD; 34% alcoholic liver disease; 33% HCV; 9% HBV; 14% Primary biliary cholangitis). Healthy controls: 21 individuals. | Cirrhosis (30.71 ± 1.41 M g/mL) and controls (12.83 ± 1.35 Mg/mL). Child A (17.31 ± 1.72 Mg/mL), Child C (46.29 ± 3.94 Mg/mL). Child B (30.34 ± 1.89 Mg/mL), Child C (46.29 ± 3.94 Mg/mL). | Adiponectin is increased in liver cirrhosis independent of the etiology and is related to the stage of disease. Adiponectin significantly decreased in a subgroup of patients receiving infliximab for alcoholic hepatitis suggesting a relationship between inflammatory state and levels. | No prognostic information. Absence of control group of alcoholic hepatitis patients untreated with anti-TNF to corroborate the hypothesis. |
| Nkontchou 201024 | 248 (100%) patients with compensated HCV cirrhosis. | Cirrhosis (16.5 ± 15.3 Mg/mL). | Adiponectin levels correlated with liver function parameters (prothrombin activity, bilirubin and albumin). Adiponectin was not related to HCC, liver-related death or liver transplantation during follow-up. | Only compensated patients included. |
| Balmer 20109 | 232 patients (45 (19%) subjects with cirrhosis, 64 (28%) subjects with NAFLD, 71 (31%) subjects with viral hepatitis, 18 (8%) subjects with autoimmune disease, 3 (1%) subjects with alcohol-induced liver disease, 31 (13%) subjects with elevated liver enzymes of unknown origin) and 20 controls. | Cirrhosis (18.6 ± 14.5 µg/mL), patients without cirrhosis (8.4 ± 6.1 µg/mL), and controls (9.1 µg/mL). NAFLD (4.8 ± 3.5 µg/mL) and other liver disease (10.4 ± 6.3 µg/mL). | Adiponectin levels correlate positively with markers of hepatic fibrosis. Adiponectin decreased in NAFLD and increased in cirrhosis. | Etiology of cirrhosis not specified. No prognostic information. |
| Salman 201025 | 40 (44%) subjects with HCV-related cirrhosis, 30 (33%) subjects with cirrhosis and cholestasis; and 20 (22%) controls. | HCV cirrhosis (15.1 ± 12.14 Mg/mL), cirrhosis with cholestasis cirrhosis with cholestasis (21.28 ± 10.2 Mg/mL), and controls (4.7 ± 4.48 Mg/mL). | Adiponectin is elevated in cirrhosis. Adiponectin correlated with liver cell injury, marker of inflammation, synthetic liver function and markers of cholestasis. | Small sample size. No prognostic information. |
| Siegel 201527 | 140 patients with cirrhosis and HCC (59% HCV; 15% HBV; 27% alcoholic cirrhosis). | Median adiponectin levels 13,050 ng/mL. | Serum adiponectin was an independent predictor of overall survival in HCC patients. | Heterogenous population. Absence of serial assessment of adiponectin. |
| Hamdy 201528 | 90 (100%) patients with hepatitis C-related liver cirrhosis (68% with HCC and 32% without HCC). | Cirrhosis with HCC: 5.213 ± 3.840 µg/mL); cirrhosis without HCC: 9.000 ± 2.234 µg/mL. | Low adiponectin levels (<5.4 Mg/mL) associated with HCC. Adiponectin suggested as a biomarker of HCC. | Small sample size. No prognostic information. Not prospective (Crosssectional design limit the interpretation of results). |
| Kalafateli 201529 | 40 subjects with non-diabetic alcoholic cirrhosis. | All cirrhotics: median adiponectin 10.23 µg/mL; Child A 7.99 µg/mL; Child B 7.66 µg/mL; Child C 25.73 µg/mL; | Adiponectin correlated with INR, aminotransferases, bilirubin. Higher adiponectin associated with shorter survival in the univariate analysis but not in the multivariate analysis. | Smal sample size. No control group. HCC patients included. Absence of serial assessment of adiponectin. |
HBV: Hepatitis B virus. HCV: Hepatitis C virus. NAFLD: Non-Alcoholic Fatty Liver Disease. TNF: Tumour necrosis factor. HCC: Hepatocellular carcinoma. The authors give permission for use from publisher on Annals of Hepatology to any borrowed or modified tables from the author and copyright holder.
Human studies that investigated the relationship between resistin and cirrhosis.
| Author /Year (reference) | Number of included subjects and etiology | Levels of resistin | Primary outcome | Remarks |
|---|---|---|---|---|
| Bahr 200530 | 57 patients with cirrhosis (25% alcoholic; 39% virus-induced cirrhosis; 26% biliary cirrhosis; 10.52% cryptogenic) 30 controls. | Cirrhosis 6.5 ± 0.4 ng/mL and controls 3.5 ± 0.2 ng/mL. Child A 5.6 ± 0.6 ng/mL, Child B 5.6 ± 0.6 ng/mL, Child C 7.7 ± 0.7 ng/mL. | Higher resistin levels in alcoholic and biliary etiologies of cirrhosis as compared to viral etiology. No significant changes after liver transplantation. Circulating resistin was related to systemic inflammation (TNF-a) and parameters | Small sample size No prognostic information. |
| Yagmur 200614 | 82 patients with chronic liver disease (29.26% virus hepatitis; 25.60% alcoholic or cryptogenic; 23.17% biliary ou autoimmune; 21.95% other etiologies, 67 patients were cirrhotics 76 controls. | Patients median resistin 5.2 µg/L and controls 4.0 µg/L. No cirrhosis 3.9 µg/L, Child A 4.4 µg/L, Child B 6.2 µg/L, Child C 9.2 µg/L. | Resistin levels correlated with parameters of liver function, systemic inflammation and HOMA index. Resistin predicted overall survival in univariate and multivariate analysis. | Small sample size Multivariate analysis not included prognostic model (Child-Pugh and MELD) but the single parameter used to calculate the models. |
| Kakizaki 200831 | 79 (100%) patients with HCV-related cirrhosis and 31 healthy controls. | Child A 6.11 ± 4.68 ng/mL; Child B 7.27 ± 4.10 ng/mL; Child C 14.9 ± 12.83 ng/mL; controls 3.38 ± 1.68 ng/mL. | Plasma resistin significantly higher in patients with more advanced disease (Child B/C vs. Child A). Resistin showed positive correlation with fasting plasma insulin, adiponectin, HOMA-IR index, and a negative correlation with QUICKI. | Small sample size No prognostic information. |
| Kalafateli 201529 | 40 non-diabetic subjects with alcoholic cirrhosis. | Median resistin accordingly to Child categories: Child A 0.82 ng/mL, Child B 0.74 ng/mL, Child C 0.88 ng/mL. | Resistin not associated with studied variables, including parameters of severity of liver disease. | Smal sample size No control group. HCC patients included. |
HOMA-IR: Homeostasis model assessment-insulin resistance. MELD: Model for End-Stage Liver Disease. HCC: Hepatocellular carcinoma. *The authors give permission for use from publisher on Annals of Hepatology to any borrowed or modified tables from the author and copyright holder.
In the present study, cirrhosis patients had significantly higher levels of adiponectin and resistin than healthy controls. These findings are consistent with previous research that demonstrated consistently higher levels of these adipokines in patients with advanced liver disease.8,14,29–31 Some mechanisms have been proposed to explain the increase in adiponectin levels in cirrhosis, such as the effect on the inflammatory response,23 reduction in biliary excretion,22 or an imbalance between adiponectin production and its hepatic excretion.32 Adiponectin may act to reverse HSC activation, maintain HSC quiescence, or significantly, may have important therapeutic implications in liver fibrosis.33
Resistin, in turn, is involved in inducing inflammatory response, and it is likely that higher levels in cirrhosis patients merely reflect the chronic inflammatory status seen in these patients as a result either of baseline disease or of the typical immune disorders observed in advanced liver disease.34,35
Adiponectin levels were positively correlated with HDL-cholesterol and negatively correlated with triglycerides, blood glucose, HOMA-IR, and BMI. Adiponectin is considered to be a modulator of glucose and lipid metabolism with antiatherogenic, antidiabetic, and anti-inflammatory properties. It has an important role in regulating insulin sensitivity and inflammatory response36 and acts as an important mediator in the pathogenesis of metabolic diseases.3 Previous studies in non-cirrhotic patients found results similar to those described here.37,38 In non-cirrhotic patients, adiponectin secretion is inversely related to BMI,39 and the inverse association between adiponectin and insulin resistance is well known. Its plasma levels negatively correlate with adiposity, IR, and metabolic syndrome and positively correlate with insulin sensitivity.37 In the present study, adiponectin inversely correlated with HOMA-IR.
These findings contradict the majority of studies, which indicate that increased adiponectin levels in cirrhosis occur independently of body composition and metabolic parameters, including HOMA-IR,8,22,25,32 although some studies demonstrate this association.27 In patients with chronic liver disease, a progressive reduction in insulin sensitivity in parallel with disease progression has been described,40 and it is probably a result of chronic hyperinsulinemia due to decreased hepatic extraction, enhanced insulin secretion, and presence of portosystemic shunts.41 The association between high adiponectin levels and lower blood glucose and HOMA-IR might indicate a role of insulin resistance as a determinant of adiponectinemia in cirrhotics as well - at least in those with less advanced disease.
The inverse relationship between adiponectin and triglycerides has been previously demonstrated in other studies9,42,43 as well as the positive correlation with HDL-cholesterol.42,44 Adiponectin may reduce the accumulation of triglycerides and fatty acid concentrations in skeletal muscle through oxidation of fatty acids by the activation of acetylcoenzyme A oxidase, carnitine palmitoyl-transferase-1, and adenosine monophosphate-activated protein kinase (AMPK). Adiponectin may also indirectly stimulate lipoprotein lipase,45 the enzyme that catabolizes VLDL, by increasing expression in Peroxisome Proliferator Activated Alpha Receptor (PPARa) in the liver and adipocytes.46 It also plays a role in the apolipoprotein B VLDL catabolism, which is independent of insulin resist-ance,47 suggesting that reduced levels of adiponectin may play a role in hyperlipidemia.48 However, in the majority of studies in patients with cirrhosis, the increased adi-ponectin levels observed were not related to metabolic and anthropometric parameters.8,22,25,28,32 Possible explanations for these discrepancies include the small number of patients evaluated in the majority of previous studies and the inclusion of patients with less advanced diseases in the present analysis. In fact, the severity of liver disease appears to influence the circulating adiponectin levels in cirrhotic patients also in the present study since its levels were negatively correlated with albumin and positively correlated with total bilirubin. In addition, significantly higher adiponectin levels were observed among Child-Pugh B/C patients in relation to Child-Pugh A ones and in those with MELD scores > 10. Previous series reported similar results.8,33 Furthermore, in this study, low levels of adiponectin have been observed in patients with alcoholic liver disease. Chronic exposure to ethanol is related to direct inhibition of gene expression and adiponectin secretion in adipocytes,49-51 which alter lipid metabolism mediated by regulatory systems as SIRT1, AMPK, PPARγ, PPARα, and SREBP-1, leading to excessive fat accumulation in the liver.52 This dysregulation of adiponectin and its receptors mediated by ethanol is probably a key mechanism for the development of alcoholic fatty liver disease.52
The resistin levels were positively correlated with creatinine and CRP and negatively correlated with AST, ALT, GGT, glucose, and HDL-cholesterol. Resistin may play an important role in the regulation of glucose homeostasis and adipogenesis,5,6 thereby influencing the development of insulin resistance, type 2 diabetes, and endothelial dysfunction, thrombosis, and angiogenesis.53–55 The inverse relation between resistin and HDL-cholesterol has been demonstrated in previous studies.56,57 In addition to the important role of resistin as an inflammatory mediator,34 these adipokines also have high levels in diseases associated with chronic liver inflammation, such as hepatitis C and NASH.58–60 The role of resistin in the inflammatory response as a proinflammatory cytokine has also been demonstrated, which explains many of the metabolic effects associated with high levels of resistin inducing IR and/or progression of atherosclerosis.61,62 Therefore, some studies suggest that resistin is a potential biomarker and mediator of cardiovascular disease.63 Resistin appears to be the link between inflammation and RI.64,65 These studies justify the observed correlation between resistin and CRP as well as with the markers of liver inflammation. This study also showed higher median resistin in patients with a history of hepatic encephalopathy and a trend toward higher resistin levels in individuals with previous report of hepatic decompensation. These findings are in agreement with previous studies that demonstrated significant effects of previous de-compensated cirrhosis on resistin levels.14 However, unlike observed here, most previous studies have noted increased levels of resistin in Child-Pugh B or C patients.14,28–30 It is probable that this difference is due to the lower proportion of patients with more severe liver diseases included in this study and also disparities in the etiology of cirrhosis across the studies.
During follow-up, a significant increase in adiponectin levels was observed in patients with cirrhosis. This rise was numerically more expressive in Child-Pugh B/C patients. There have been no previous studies with serial measurements of adiponectin in cirrhotic. It is likely that increased levels of this adipokine reflect the progressive deterioration of liver function, since the baseline values were associated with variables related to disease severity. Interestingly, a reduction in adiponectin levels was found after transplantation in six out of eight patients undergoing the procedure during the study period. These data are consistent with a study that assessed levels of adiponectin pre- and post-liver transplantation in 77 subjects, showing rapid reduction of these adipokines after transplantation.66 When considered together, these results reinforce the impact of cirrhosis on circulating levels of adiponectin.
A trend toward association between adiponectin and overall transplant-free survival was observed when the entire cohort was considered in the analysis. However, when patients were assessed according to the etiology of cirrhosis, adiponectin levels were associated with shorter transplant-free survival only in patients with alcoholic liver disease. There are few data regarding the relationship between adiponectin and prognosis in liver diseases. In patients with chronic hepatitis C with or without cirrhosis, higher adiponectin levels were associated with higher all-cause and liver-related mortality.67 Higher adiponectin levels were also associated with reduced survival in a study that included Child-Pugh A or B patients with hepatocellular carcinoma.26 In a recent small study that included 40 patients with alcoholic cirrhosis, higher adiponectin was associated with shorter survival.28 These findings suggest an association between adiponectin and prognosis in cirrhosis, although some factors, especially the underlying disease, appear to influence this relationship.
Resistin levels were not associated with survival in cirrhotic patients even when subanalysis were performed according to the etiology of cirrhosis. These results contrast with those observed by Yagmur, et al., where significantly higher resistin levels were observed in patients who died.14 However, this association was not observed in the most recent study that included only patients with alcoholic cirrhosis.28 It is important to note that the study of Yagmur, et al. included a greater proportion of individuals with biliary liver disease or autoimmune etiology and with much longer follow-up time than the present study.14
Some limitations of our analysis should be discussed. First, the small number of patients classified as Child-Pugh C may limit the ability to extrapolate the results. Although further studies with a greater number of ChildPugh C are desirable, the patients included in the present study probably reflect the characteristics of cirrhotic patients receiving outpatient treatment, which are mostly Child-Pugh A and B. Other limiting factors include the relatively short follow-up time and the measurement of total adiponectin only, which did not allow us to identify the clinical significance of other isoforms of these adipok-ine (trimeric, hexameric, and multimeric) in patients with cirrhosis. Lastly, given the relatively small number of patients, those with an alcoholic etiology were grouped with other causes of liver disease for the survival subanalysis. Validation of our results specifically in patients with alcoholic cirrhosis and no other major co-factors would be of great value, even though a complete exclusion of other factors that may eventually be related to chronic liver disease could be problematic.
It can be concluded that in outpatients with stable cirrhosis, adiponectin but not resistin levels were associated with the intensity of liver dysfunction. Adiponectin increased during follow-up and its levels at baseline were associated with worse prognosis in patients with alcoholic liver disease, suggesting a potential as a prognostic biomarker. The significance of elevated adiponectin levels and the effect of any interventions that influence this adipokine should be addressed in future studies.
ABBREVIATIONS- •
ALT: alanine aminotransferase.
- •
AST: aspartate aminotransferase.
- •
BMI: body mass index.
- •
CRP: C-reactive protein.
- •
GGT: gamma-glutamyl-transferase.
- •
HCV: hepatitis C virus.
- •
HBV: hepatitis B virus.
- •
HOMA-IR: homeostasis model assessment-insulin resistance.
- •
INR: international normalized ratio.
- •
IR: insulin resistance.
- •
MAMC: mid-arm muscle circumference.
- •
MELD: Model for End-Stage Liver Disease.
- •
NAFLD: Non-Alcoholic Fatty Liver Disease.
- •
NASH: non-alcoholic steatohepatitis.
- •
RPB4: retinol binding protein 4.
- •
RFH-GA: Royal Free Hospital Global Assessment.
- •
TNF-α: alpha- tumor necrosis factor.
- •
TSF: triceps skinfold thickness.