The clinical impact of relative adrenal insufficiency (AI) on patients with stable decompensated cirrhosis (DeCi) has not been yet elucidated.
AimExplore the association between AI and outcome [death or liver transplantation (LT)] in patients with DeCi.
Material and methodsPatients with DeCi presenting no active complication have been included. Clinical and laboratory data, including serum levels of corticosteroid-binding globulin (CBG), interleukin (IL)-1b, IL-6 and tumor necrosis factor (TNFa) were recorded in each participant. Salivary cortisol (SC) and serum total cortisol (STC) were assessed at (T0) and 1 h (T60) after intravenous injection of 250 |ig corticotropin.
Results113 consecutive patients were totally tested. Median SC was 3.9 ng/mL and 15.5 ng/mL and median STC was 10.7 ng/dL and 22.7 ng/dL at T0 and T60 respectively. The patients with AI [group 1, n = 34 (30%)] had significantly lower systolic blood pressure (106 ± 12 vs. 113 ± 13 mmHg, p = 0.05), serum sodium (133 ± 7 vs. 137 ± 12 mEq/ L, p = 0.04), HDL (29.9 ± 14 vs. 38.6 ± 18 mg/dL, p = 0.034) and albumin (2.7 ± 0.5 vs. 3.1 ± 0.5 g/dL, p = 0.002), but higher direct bilirubin (median: 1.6 vs. 0.8 mg/dL, p = 0.029) compared to those without AI [group 2, n = 79 (70%)]. Moreover, group 1 patients presented more frequently past history of spontaneous bacterial peritonitis (SBP) [10/34 (29.4%) vs. 6/79 (7.5%), p = 0.002]. AI was significantly associated with death [HR = 2.65, 95% C.I.: 1.55 - 4.52, p = 0.003 over a follow up period of 12 (6-48) months.]
ConclusionsThe presence of AI in patients with stable DeCi predispose to obvious clinical implications since it is associated with circulatory dysfunction, previous history of SBP and worse survival.
Over the last decades decompensated cirrhosis (DeCi) has been accepted as a chronic disease affecting, except for the liver itself, many extra-hepatic organ systems.1 In view of that, additional relative adrenal insufficiency diagnosis among patients with DeCi (i.e. hepato-adrenal syndrome) perplex management and prognosis of this patient population and trigger further research in this field.2 Although AI was firstly described in patients with liver diseases several decades ago,3 the evidence on its exact pathogenetic mechanisms among cirrhotic patients are not satisfactory. While several lines of evidence exist for cirrhotic patients with sepsis or acute complication of cirrhosis4,5 minimal detailed studies have been published for clinically stable patients with DeCi.6
The most crucial issue requiring further exploration, is the clinical impact of AI on the prognosis of stable cirrhotic patients. So far, AI in critically ill cirrhotic patients (with septic shock or variceal bleeding) has been found to correlate with higher mortality, with the corticosteroid administration not to account for survival improvement in all cases.2 In regards to non-critically ill cirrhotic patients, the published studies remain controversial. While a recent study showed that AI was associated with worse outcome in patients with DeCi7 after their complication [with at least one episode of ascites, hepatic encephalopathy, bacterial infection, variceal bleeding or hepatorenal syndrome (HRS)] this was not confirmed in other two studies.8,9 As demonstrable causes for this contrast is the lack of consensus for the appropriate definition of adrenal function evaluation (e.g. using total serum vs saliva cortisol) and the various cortisol cut offs which result in fluctuations on prevalence and clinical significance of AI.2 The aim of the present study was to evaluate the prevalence of AI -by measuring the total and salivary cortisol using the short synacthen test (i.e. before and after the administration of 250 μg corticotrophin intravenously)- and the impact of AI on patients with clinically stable DeCi.
Material and MethodsAll the consecutive adult patients with stable DeCi, admitted for liver transplant (LT) evaluation to our Department from March 2011 till December 2013 were studied prospectively. DeCi diagnosis was based on laboratory and/or ultrasound evidences. All the patients suffering from hypothalamus-pituitary-adrenal axis diseases, who had been receiving steroids or other immunosuppressive medications over the last 6 months before admission and had hepatocellular carcinoma (or other types of cancer), variceal bleeding, encephalopathy or infection, such as spontaneous bacterial peritonitis (SBP), during the last month before admission were excluded. Furthermore, detailed clinical evaluation, laboratory measurements (white blood cells, Creactive protein, procalcitonin, blood cultures and ascitic fluid paracentesis) and radiological exams (chest x-ray, upper abdominal ultrasound) if necessary, were performed to exclude active infection on admission.
Demographic details registration included the cause and duration of liver disease, the past complications of DeCi [i.e. variceal bleeding, hepatic encephalopathy, ascites, SBP], the medication dosage and duration and the vital signs (blood pressure, pulse rate, body temperature) on admission. The short synacthen test (SST) was performed after an overnight bed rest. In details, an intravenous catheter was inserted from the previous night and strict instructions to avoid tooth-brushing, eating or drinking 4 h prior to AI evaluation were given to all patients. Basal total serum cortisol was taken at 08:00 am (time T0, baseline) through the intravenous catheter almost in parallel with the salivary cortisol evaluation performed through a specific oral cotton (Plain Salivette; Sarstedt, Newton, North Carolina, USA) insertion. The cotton was left for 2-3 min in the oral cavity following prompt test for blood contamination.
Subsequently, 250 μg corticotrophin (Synacthen, Novartis Pharma, Basel, Switzerland) was given bolus intravenously and the patients remained recumbent for an hour until a second blood sample obtained, for the (T60, 09:00 am) serum total cortisol level, and a suitable cotton placed in the oral cavity, for the (T60, 09:00 am) salivary cortisol evaluation. Each cotton was collected in a plastic tube and the saliva was separated by centrifugation according to the manufacturer instructions. Basic (T0) and T60 salivary cortisol values were estimated by using an electrochemiluminescence immunoassay (Roche Diagnostics Ltd, Rotkreuz, Switzerland). AI was defined when serum total cortisol at T60 minus serum total cortisol at T0 was < 9 (Xg/dL providing that serum total cortisol at T0 was < 35 (Xg/dL.7 In addition, based on salivary cortisol levels, AI was defined as a value of T0 < 1.8 ng/mL or < 12.7 ng/mL at T60 or an increase between T0 and T60 less than 3 ng/ mL. Serum levels of cortisol binding globulin (CBG) (competitive radioimmunoassay; Biosource, Belgium) were measured and used for calculation of free cortisol index (FCI) at T0 and T60, which stands for the ratio between serum total cortisol and CBG.
At the same day, a full biochemical and lipid profile, blood count, coagulation and proinflammatory cytokines including serum interleukin (IL)-1b, IL-6 and tumor necrosis factor (TNF)-oc were all prospectively recorded. 24-hour urine collections for urine sodium excretion (UNa24h) and urine samples for random “spot” sodium and potassium in order to calculate UNa/K ratio were obtained before or after the completion of the urine collection. The severity of liver disease was estimated by the Child-Pugh (CTP) and the model for end stage liver disease (MELD) scores.
Renal function was assessed by estimated glomerular filtration rate (eGFR) using CKD-EPI (chronic kidney disease-epidemiology) creatinine-based equation.10“True” GFR was evaluated with 51Chromium-EDTA (51Chr-EDTA) using the slope-intercept technique, correcting for body surface area, and the fast exponential curve recommended by the British Nuclear Medicine Society guidelines11 after intravenous injection of a tracer, at 2, 4, and 6 h. Finally, cardiac echo was performed to search for diastolic dysfunction, which was classified into three categories depending on severity (Grade I, II, III) and current guidelines.12 The study protocol was approved by our Institutional Review Board and conformed to the ethical guidelines of the 2013 Declaration of Helsinki.
Statistical analysisAll data were analysed by using the statistical package SPSS (version 22.0 SPSS Inc, Chicago, IL) and MedCalc for Windows (MedCalc Software, Mariakerke, Belgium). The normally distributed quantitative variables were expressed as mean values ± standard deviation (SD) and the non-normally distributed variables as median values (range). Student's t-test or Mann-Whitney U test was used for comparison of continuous parameters between patients with and without AI and %2 test for categorical variables.
Multivariable analysis was performed using backward selection of variables, starting with all variables with p < 0.1 in univariate analysis to find the independent factors significantly associated with the presence of AI. The discriminative ability of the independent variables to predict the presence of AI in patients with DeCi was evaluated by using the area under a receiver operating characteristic curve (AUC). At the best cut off point (in which the sum of sensitivity plus specificity is maximal), sensitivity, specificity, positive (PPV) and negative (NPV) predictive values were calculated. Cox proportional hazards regression analysis was used to identify the factors significantly associated with the outcome. The patients’ survival according to the presence of AI was calculated using Kaplan-Meier analysis and compared with the log rank sum test, while competing risk analysis was performed with the R project (version 3.2.2). A p value < 0.05 was considered statistically significant.
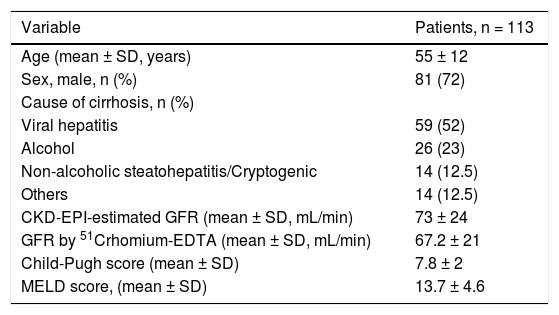
ResultsPatients’ characteristicsOne hundred and thirteen patients with stable DeCi were included in the study (Table 1). The mean CTP and MELD scores were 7.8 ± 2 and 13.7 ± 4.6, respectively. The mean total serum cortisol was 10.7 ± 4.8 (Xg/dL and 22.7 ± 7 (Xg/dL at T0 and T60, respectively. The median salivary cortisol was 3.9 ng/mL (range: 1.6-17.8) and 15.5 ng/mL (range: 7.1-47.7) at T0 and T60, respectively. Free cortisol index (FCI) was 9.1 (range: 1.2-67) and 19.1 (range: 5.2-139) (Xg/dL at T0 and T60, respectively. CBG plasma concentrations were 38.7 ± 11.5 (Xg/mL and they were higher in patients with Child-Pugh class A patients, compared to those with Child-Pugh class B or C (42.6 ± 12.2 (xg/mL vs. 31.4 ± 9.3 (xg/mL, p = 0.02). The CBG and albumin plasma concentrations had poor correlation (Spearman r2: 0.063, p = 0.65).
Baseline clinical and laboratory characteristics of patients with decompensated cirrhosis in our cohort.
| Variable | Patients, n = 113 |
|---|---|
| Age (mean ± SD, years) | 55 ± 12 |
| Sex, male, n (%) | 81 (72) |
| Cause of cirrhosis, n (%) | |
| Viral hepatitis | 59 (52) |
| Alcohol | 26 (23) |
| Non-alcoholic steatohepatitis/Cryptogenic | 14 (12.5) |
| Others | 14 (12.5) |
| CKD-EPI-estimated GFR (mean ± SD, mL/min) | 73 ± 24 |
| GFR by 51Crhomium-EDTA (mean ± SD, mL/min) | 67.2 ± 21 |
| Child-Pugh score (mean ± SD) | 7.8 ± 2 |
| MELD score, (mean ± SD) | 13.7 ± 4.6 |
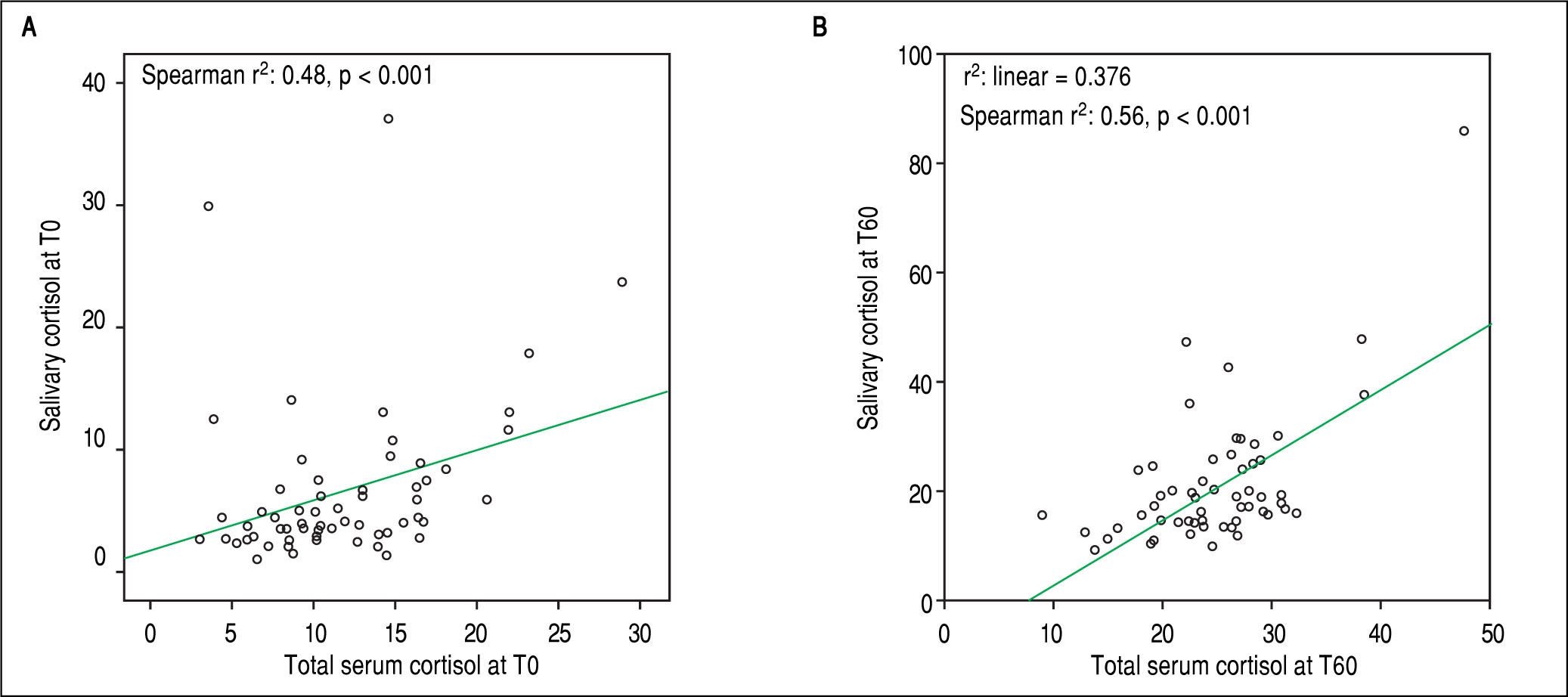
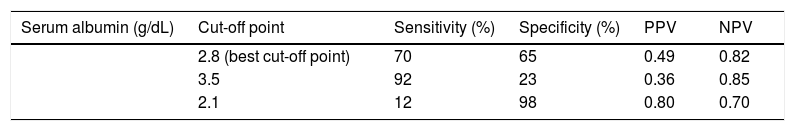
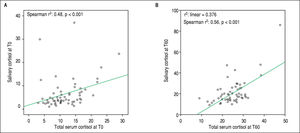
Salivary and serum cortisol were significantly correlated at T0 (Spearman r2: 0.48, p < 0.001) and T60 (Spearman r2: 0.56, p < 0.001) in patients with serum albumin > 2.5 mg/dL (Figure 1 A and 1B). No similar correlation was observed at both time points among patients with serum albumin ≤ 2.5 mg/dL (T0 and T60: Spearman r2: 0.012 and 0.12, respectively, p > 0.05). Based on total serum cortisol levels 34 (30%) patients presented AI (group 1) and 79 (70%) patients had not AI (group 2). When AI was defined by salivary cortisol, 25 (22%) had AI and 88 (78%) of 113 patients had not AI. Patients (n = 82) with concordant serum and salivary assays had statistically significant lower triglyceride levels compared to those with discordant tests (n = 31) (58 ± 10 mg/dL vs. 87 ± 22 mg/dL, p = 0.022).
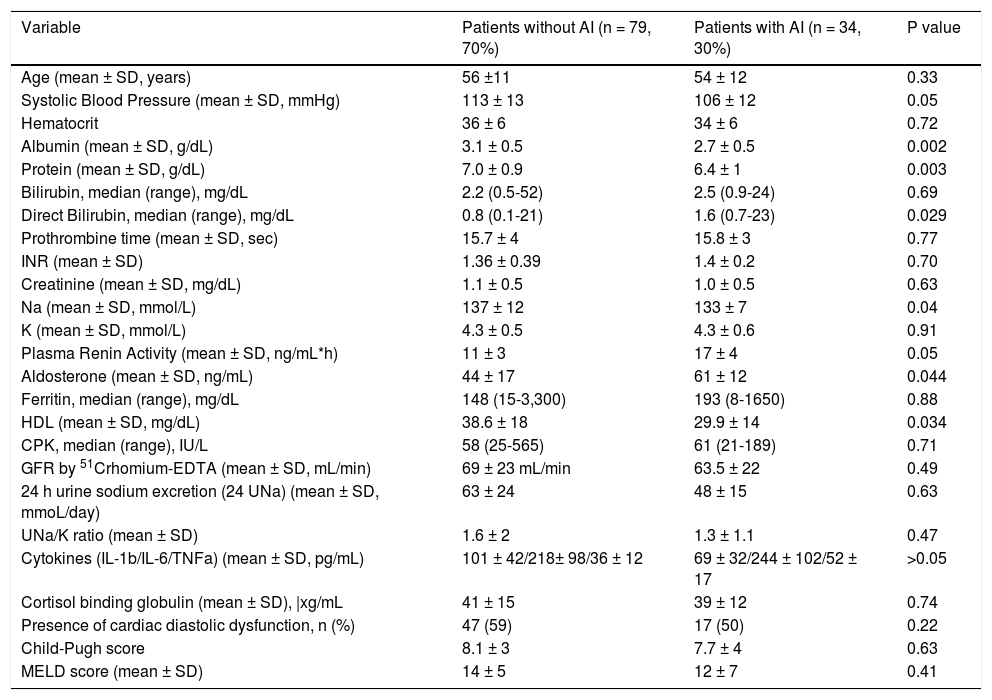
Characteristics of patients with or without AI based on total serum cortisolBased on total serum cortisol levels, the patients with AI (n = 34, group 1) presented similar incidence of previous cirrhotic complications compared to those (n = 79, group 2) without AI: moderate to severe ascites (22% vs. 18%, respectively, p = 0.87), encephalopathy (27% vs. 28%, respectively, p = 0.98) or variceal bleeding (23.5% vs. 24% respectively, p = 0.96) except for previous history of SBP which was more frequent in group 1 patients [10/34 (29.4%) vs. 6/79 (7.5%), p = 0.002]. The prevalence of AI was similar across different Child-Pugh classes: 21% in Child-Pugh class A, 25% in class B and 28% in class C patients (p > 0.05). Group 1 patients had significantly lower systolic blood pressure (106 ± 12 vs. 113 ± 13 mmHg, p = 0.05), serum sodium levels (133 ± 7 vs. 137 ± 12 mEq/L, p = 0.04), HDL levels (29.9 ± 14 vs. 38.6 ± 18 mg/dL, p = 0.034), as well as albumin (2.7 ± 0.5 vs. 3.1 ± 0.5 g/dL, p = 0.002) and protein levels (6.4 ± 1 vs. 7 ± 0.9, p = 0.003) compared to group 2 patients (Table 2). However, direct bilirubin [median: 1.6 (0.7-23) vs. 0.8 (0.1-21) mg/dL, p = 0.029] (but with no difference in total bilirubin levels), plasma rennin activity (17 ± 4 vs. 11 ± 3 ng/mL/h, p = 0.05) and aldosterone (61 ± 12 vs. 44 ± 17 ng/mL, p = 0.044) were higher in group 1 patients indicating higher circulatory dysfunction and activation of renin-angiotensin system (Table 2). IL-1b, IL-6 and TNF-α and cardiac diastolic dysfunction were not significantly different between the two groups (p > 0.05) (Table 2). Finally, estimated GFR (GFR-CKD-EPI: 71 ± 25 vs. 75 ± 26 mL/min, p = 0.76) and “true” GFR (63.5 ± 22 vs. 69 ± 23mL/min, p = 0.49) were similar among the two groups.
Clinical and laboratory characteristics of the patients with decompensated cirrhosis and with or without relative adrenal insufficiency (AI) based on total serum cortisol levels.
| Variable | Patients without AI (n = 79, 70%) | Patients with AI (n = 34, 30%) | P value |
|---|---|---|---|
| Age (mean ± SD, years) | 56 ±11 | 54 ± 12 | 0.33 |
| Systolic Blood Pressure (mean ± SD, mmHg) | 113 ± 13 | 106 ± 12 | 0.05 |
| Hematocrit | 36 ± 6 | 34 ± 6 | 0.72 |
| Albumin (mean ± SD, g/dL) | 3.1 ± 0.5 | 2.7 ± 0.5 | 0.002 |
| Protein (mean ± SD, g/dL) | 7.0 ± 0.9 | 6.4 ± 1 | 0.003 |
| Bilirubin, median (range), mg/dL | 2.2 (0.5-52) | 2.5 (0.9-24) | 0.69 |
| Direct Bilirubin, median (range), mg/dL | 0.8 (0.1-21) | 1.6 (0.7-23) | 0.029 |
| Prothrombine time (mean ± SD, sec) | 15.7 ± 4 | 15.8 ± 3 | 0.77 |
| INR (mean ± SD) | 1.36 ± 0.39 | 1.4 ± 0.2 | 0.70 |
| Creatinine (mean ± SD, mg/dL) | 1.1 ± 0.5 | 1.0 ± 0.5 | 0.63 |
| Na (mean ± SD, mmol/L) | 137 ± 12 | 133 ± 7 | 0.04 |
| K (mean ± SD, mmol/L) | 4.3 ± 0.5 | 4.3 ± 0.6 | 0.91 |
| Plasma Renin Activity (mean ± SD, ng/mL*h) | 11 ± 3 | 17 ± 4 | 0.05 |
| Aldosterone (mean ± SD, ng/mL) | 44 ± 17 | 61 ± 12 | 0.044 |
| Ferritin, median (range), mg/dL | 148 (15-3,300) | 193 (8-1650) | 0.88 |
| HDL (mean ± SD, mg/dL) | 38.6 ± 18 | 29.9 ± 14 | 0.034 |
| CPK, median (range), IU/L | 58 (25-565) | 61 (21-189) | 0.71 |
| GFR by 51Crhomium-EDTA (mean ± SD, mL/min) | 69 ± 23 mL/min | 63.5 ± 22 | 0.49 |
| 24 h urine sodium excretion (24 UNa) (mean ± SD, mmoL/day) | 63 ± 24 | 48 ± 15 | 0.63 |
| UNa/K ratio (mean ± SD) | 1.6 ± 2 | 1.3 ± 1.1 | 0.47 |
| Cytokines (IL-1b/IL-6/TNFa) (mean ± SD, pg/mL) | 101 ± 42/218± 98/36 ± 12 | 69 ± 32/244 ± 102/52 ± 17 | >0.05 |
| Cortisol binding globulin (mean ± SD), |xg/mL | 41 ± 15 | 39 ± 12 | 0.74 |
| Presence of cardiac diastolic dysfunction, n (%) | 47 (59) | 17 (50) | 0.22 |
| Child-Pugh score | 8.1 ± 3 | 7.7 ± 4 | 0.63 |
| MELD score (mean ± SD) | 14 ± 5 | 12 ± 7 | 0.41 |
All variables with p < 0.1 in the univariate statistics were included in multivariable regression analysis. Only serum albumin (OR: 4.5, 95%C.I.: 1.1-15.1, p = 0.032) was independently associated with the presence of AI (Table 3). Based on the area under the ROC curve, serum albumin had relatively low discriminative ability for the presence of AI (AUC = 0.68, 95% C.I.: 0.59 to 0.77). The best cut off point for serum albumin was < 2.8 g/dL giving a sensitivity 70%, specificity 65%, PPV 49% and NPV 82%. Interestingly, for albumin < 3.5 g/dL sensitivity, specificity, PPV and NPV were 92%, 23%, 36% and 85%, respectively, while for albumin < 2.1g/dL sensitivity, specificity, PPV and NPV were 12%, 98%, 80% and 70%, respectively (Table 4).
Prediction of relative adrenal insufficiency using serum albumin in 113 consecutive patients with stable decompensated cirrhosis.
| Serum albumin (g/dL) | Cut-off point | Sensitivity (%) | Specificity (%) | PPV | NPV |
|---|---|---|---|---|---|
| 2.8 (best cut-off point) | 70 | 65 | 0.49 | 0.82 | |
| 3.5 | 92 | 23 | 0.36 | 0.85 | |
| 2.1 | 12 | 98 | 0.80 | 0.70 |
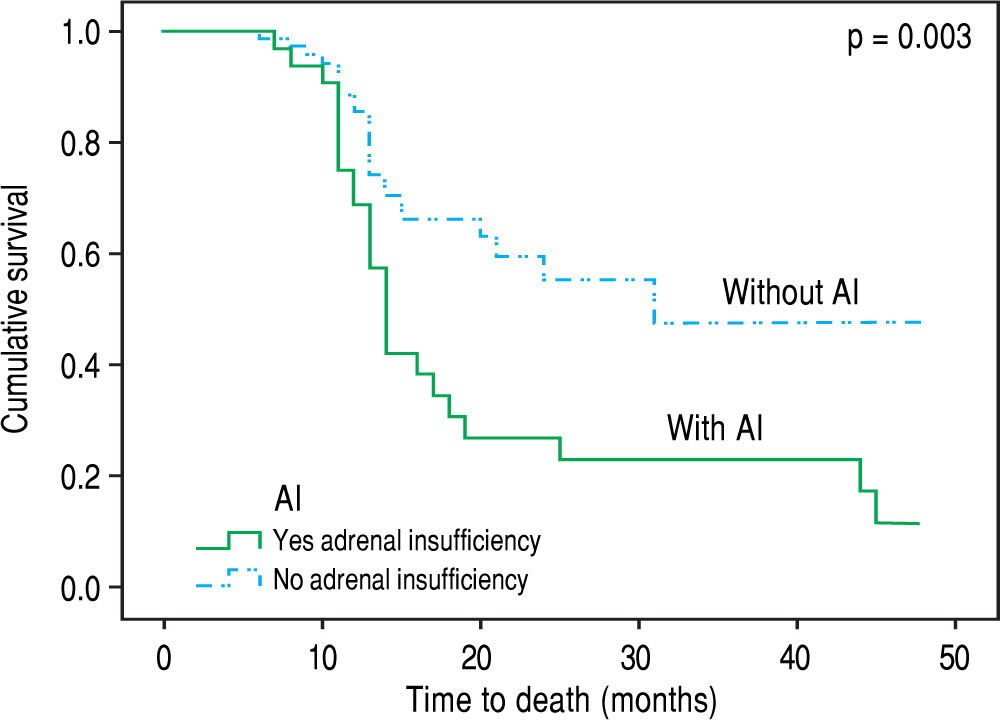
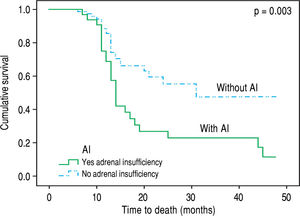
Over the follow up period [median 12 (6-48) months], 83 patients died or underwent LT and 30 remained alive without LT. All deaths (n = 48) were liver related (liver/ multiorgan failure: 29, encephalopathy: 8, variceal bleeding/liver failure = 11) and no significant association was found between causes of death and AI. In univariate analysis, patients who died or underwent LT had lower “true” GFR (mean: 64 ± 25 mL/min vs. 81 ± 19 mL/min, p = 0.023), but higher serum urea [median: 38 mg/dL (11-130) vs. 34 (14-93), p = 0.041] and MELD score (18.5 ± 6 vs. 12 ± 3, p = 0.03), while they presented more frequently AI at baseline [χ2 test: 36% (30/83) vs. 13% (4/30), p = 0.02] compared to those who were alive without LT. “True” GFR was independently associated with the outcome (death or LT) (HR: 0.96, 95%CI: 0.94-0.99, p = 0.05) in multivariable Cox proportional hazards regression model (the covariates included were “true” GFR, urea, AI and MELD score). Based on competing risk analysis we found that AI was significantly associated with death (using liver transplantation as a competing risk) (HR = 2.65, 95%C.I.: 1.55 - 4.52, p = 0.003) (Figure 2). Finally, the patients with AI, based on salivary cortisol, had similar mortality with those without AI [in the total cohort: log rank p = 0.94; excluding the patients who underwent LT: log rank p = 0.45].
DiscussionDuring the last decade there is an increased interest regarding the presence of AI, which is found not only in critically ill cirrhotic patients but also in cirrhotics with stable cirrhosis indicating that AI is a feature of liver dysfunction per se.2 So far, there is no consensus regarding the appropriate definition of AI in cirrhosis; total serum cortisol measurements are considered to overestimate AI as the low serum albumin and CBG -which are both common findings in cirrhotic patients- are not taken into account.13 This study demonstrates that the prevalence of AI in cirrhotics is greater when serum total cortisol-based definition is used compared with salivary cortisol-based definition (30% vs. 22%) and the correlation between salivary (a surrogate marker of free serum cortisol) and total serum cortisol is significant only in patients with albumin concentrations > 2.5 mg/dL (Figures 1A and 1B) which is seemingly consistent with the results of earlier studies.9,14 Although principal findings emerge from our study, their clinical impact remain un-known, and thus, total serum cortisol is still the mainstay for assessment of AI.2
Based on the same criteria applied in the study of Acevedo, et al.7 the diagnosis of AI in our study was 30% (34/113) in patients with stable DeCi. Despite the initial correlation of AI with several factors in the univariate analysis, only serum albumin was independently associated with the presence of AI in the multivariable model (OR: 4.5, 95%C.I.: 1.1-15.1, p = 0.032) (Table 3); showing though relatively low discriminative ability (AUC = 0.68, 95% C.I.: 0.59 to 0.77). The sensitivity if serum albumin < 3.5 g/dL was 92%, and the specificity if serum albumin < 2.1 g/dL was 98% (Table 4).
The exact pathogenetic mechanisms implicated in the development of AI in patients with cirrhosis have not been fully elucidated. Since cirrhotic patients have low levels of lipids, AI could be attributed to cortisol production impairment at the level of adrenal glands particularly under conditions of stress.15 Moreover, the high levels of circulating cytokines, such as TNF-α, IL-6, IL-1b which is a common finding in cirrhosis, may have a negative impact on normal adrenal function directly or indirectly via reduced lipid delivery to adrenal glands.16 Nevertheless, no correlation between AI and serum levels of IL-1b, IL-6 and TNFα was confirmed in our study, similarly to previous studies.7 Concomitantly, no association between cirrhotic cardiomyopathy and development of AI was detected, as generally has been hypothesized, since diastolic dysfunction has not been found more commonly in patients with AI.17 However, patients who developed AI presented greater circulatory dysfunction and activation of renin-angiotensin system than those without AI as is verified by the lower systolic blood pressure (106 ± 12 vs. 113 ± 13 mmHg, p = 0.05), and the higher plasma renin activity (17 ± 4 vs. 11 ± 3 ng/mL/h, p = 0.05) and aldosterone (61 ± 12 vs. 44 ± 17 ng/mL, p = 0.044).
Contrary to previous studies,18 we found that the presence of AI was not associated with the severity of liver disease (based on Child-Pugh and MELD scores). Furthermore, the patients with AI presented significantly lower serum sodium (133 ± 7 vs. 137 ± 12 mEq/L, p = 0.04), lower albumin (2.7 ± 0.5 vs. 3.1 ± 0.5 g/dL, p = 0.002) and greater direct bilirubin (median: 1.6 vs. 0.8 mg/ dL, p = 0.029) compared to patients without AI (Table 2) as it has been highlighted in earlier literature data.19 Notably patients with AI suffered more often from SBP in the past compared to the group without AI [10/34 (29.4%) vs. 6/79 (7.5%), p = 0.002], which could indicate the association of AI with increased susceptibility in bacterial infection. Indeed, Acevedo, et al.7 found that AI was linked with the development of infection/severe sepsis and hepatorenal syndrome during the follow up period possibly due to the compensatory increase of sympathetic tone which is translated to increased translocation of bacterial products from the intestinal lumen to the systemic circulation.20 However, this is not confirmed in our study, as no association between AI and complications of cirrhosis (including SBP) or causes of death (including sepsis or hepatorenal syndrome) was demonstrated. In the same direction, only “true” GFR was independently associated with the outcome (death or LT) in our study during the follow up period. Nevertheless, as compared with those with normal adrenal function, patients with AI had higher mortality based on competing risk analysis [HR = 2.65, 95%C.I.: 1.55-4.52, p = 0.003) (Figure 2).
The results of the study should be viewed in the light of some limitations. The present data used a single centre design, including mainly patients with viral-related decompensated cirrhosis, did not evaluate the free serum cortisol, which is the optimal for AI evaluation and did not assess any therapeutic manipulation of AI such as cortisol substitution. Despite these limitations, our study is the first to our knowledge which evaluated AI in patients with well-established stable decompensated cirrhosis by using both serum and salivary cortisol and in parallel assessed the impact of AI on the outcome.
In conclusion, the present study demonstrates relatively high prevalence of AI in patients with DeCi accounting for essential clinical implication as it is associated with circulatory impairment, previous history of SBP and worse outcome. Subsequently the study emphasizes on the need of AI assessment in patients with stable DeCi on daily clinical basis and decision on the appropriate therapeutic management.
Abbreviations- •
AI: relative adrenal insufficiency.
- •
aPTT: activated partial thromboplastin time.
- •
AUC: receiver operating characteristic curve.
- •
CBG: corticosteroid-binding globulin.
- •
CKD-EPI: Chronic Kidney Disease-Epidemiology.
- •
CTP: child-Turcotte-Pugh.
- •
DeCi: decompensated cirrhosis.
- •
eGFR: estimated GFR.
- •
GFR: glomerular filtration rate.
- •
HRS: hepatorenal syndrome.
- •
IL: interleukin.
- •
INR: International Bormalised Ratio
- •
LT: liver transplantation.
- •
MELD: Model for End-stage Liver Disease.
- •
PPV and NPV: positive and negative predictive values.
- •
PT: prothrombin time.
- •
SBP: spontaneous bacterial peritonitis.
- •
SC: salivary cortisol.
- •
SST: short synacthen test.
- •
STC: serum total.
- •
TNF: tumor necrosis factor.
- •
UNa/K: “spot” sodium and potassium ratio.
- •
UNa24h: urine sodium excretion.
Nothing to disclose.
Conflicts of InterestNone.