Sleep disturbance and excessive daytime sleepiness (EDS) have been reported in patients with hepatic cirrhosis with no hepatic encephalopathy (HE). The objective of this study was to evaluate daytime sleepiness and risk of obstructive sleep apnea (OSA) among liver cirrhosis patients.
Material and methodsA cross-sectional study was conducted at King Abdulaziz Medical City (KAMC)-Riyadh over a period of six months, using a structured questionnaire that investigated: 1) Sleep patterns and daytime sleepiness using the Epworth Sleeping Scale (ESS), and 2) The risk for sleep apnea using the Berlin Questionnaire (BQ). We enrolled patients with a confirmed diagnosis of liver cirrhosis who were being followed at the hepatology and preliver transplant clinics. Results. We enrolled 200 patients with liver cirrhosis, 57.5% of whom were male. The mean age was 60 (± SD 12.2). The reported prevalence of EDS, OSA, and both EDS and OSA were 29.5%, 42.9%, and 13.6%, respectively. The prevalence of EDS was higher in patients with Hepatitis-C and patients with DM, who experienced short sleep duration. We did not find any association between the severity of liver disease and EDS or OSA as measured by Child-Pugh scores (CPS). Conclusions. The risk of OSA and EDS is high among liver cirrhosis patients. Those patients with cirrhosis secondary to Hepatitis C are at higher risk of EDS and OSA. Both EDS and OSA affect patients designated as CPS Class A more frequently than patients designated as CPS Class B.
Obstructive sleep apnea (OSA) is a common disorder that affects 4-24% of males, 2-9% of females, and exceeds 30-50% in obese individuals.1-5 OSA is characterized by re-current upper-airway collapse during sleep, resulting in recurrent oxygen desaturations, increased sleep arousal, fragmentation of sleep, and excessive daytime somnolence.6 Chronic intermittent hypoxia sequentially may cause hepatic ischemia-reperfusion injury.7-13 The patho-physiology of sleep disturbances in cirrhosis remains un-clear.14 Although clinical reports vary, one possible explanation for sleep dysfunction in patients is a disruption in melatonin circadian rhythms.15 Studies reported elimination of OSA after ascites treatment, raising the possibility of the mechanical effect of ascites on diaphragmatic movement and reduction of the lung volume as a cause of OSA. Another possibility is due to upper airway edema in liver cirrhosis patients.16,17 Hypocapnia secondary to hormonal and chemical change in liver cirrhosis may cause sleep apnea, although this is considered controversial.18 Excessive day time sleepiness (EDS) appears to be attributable to a dysfunction of the neural circuit responsible for the maintenance of wakefulness and sleep states. High levels of ammonia can reduce serotonin and no-radrenaline levels in the central nervous system, resulting in low alertness and attention-associated sleep complaints, including difficulty falling asleep and EDS.14,19,20 Studies in patients with compensated cirrhosis have illustrated that alterations in melatonin, cortical, and ghrelin secretion rhythms are the reasons for poor sleep architecture; however, this requires further study in cirrhotic patients with concomitant OSA.14,20,21 Sleep disturbance, including sleep apnea, is playing a major role in impairment of the quality of life in patients with cirrhosis and in the worsening of cirrhosis.16,22,23 Frequently, sequences of sleep disturbance are overlooked, and multiple other factors are blamed, such as fatigue, HE, and the underlying etiology of liver disease.23,24 Studies show OSA is associated with elevated alanine aminotransferase levels and a trend toward histologic evidence of progressive liver disease.7-10,13,25-29 OSA is also associated with markedly increased mortality and morbidity due to the metabolic and cardiovascular risks.30-33 While OSA is an important sleep disorder that can coexist with liver cirrhosis, treatment with continuous positive airway pressure (CPAP) improves quality of life and prevents worsening of liver cirrhosis.34,35
There are few studies assessing the sleep patterns in patients with liver cirrhosis without overt HE.15,36
This study aimed to estimate the risk of OSA, as evaluated by the Berlin questionnaires (BQ),37 the presence of EDS assessed by the Epworth sleepiness scale (ESS),38 among liver cirrhosis patients without overt HE. Also, we recognized the need to study the association between severity of liver cirrhosis as assessed by Child-Pugh Scores (CPS) and the risk of OSA.
Material and MethodsThis was a cross-sectional study conducted at King Abdulaziz Medical City (KAMC)-Riyadh over a period of six months, between January 2012 and July 2012. The Institutional Review Board (IRB) at King Abdullah International Medical Research Center (KAIMRC), Riyadh, approved this study.
Data collection was carried out by personal professional interviews using structured sleep questionnaires. These questionnaire were adopted from validated international questionnaires, validated in the Arabic language, and used previously among hemodialysis patients and general Saudi population, this including Berlin Questionnaires (BQ) to estimate the risk for sleep apnea, Epworth Sleepiness Scale (ESS) to assess EDS and International Classification of Sleep Disorders-2 (ICSD-2), which defines insomnia.39-43 We enrolled all stable patients with a confirmed diagnosis of liver cirrhosis who were being followed at the hepatology and pre-liver transplant clinics. We excluded patients with chronic pulmonary diseases, congestive heart failure, and patients with hepatic encephalopathy.
The consultant hepatologist identified all patients with a confirmed diagnosis of liver cirrhosis, and classified them according to the severity of the liver cirrhosis based on the CPS.44 The diagnosis of liver cirrhosis was based on liver radiological studies, liver biopsy when available, and compatible clinical data as per the diagnosis of the hepatologist who referred the case for study. The patients who agreed to participate were introduced by the primary physician to the study co-investigator, who interviewed the patients, obtained the consent, and reviewed all the questionnaires with the participant.
We used the Arabic version of the BQ to assess the presence of risk of OSA among participants, since the Arabic BQ is a reliable and valid scale in screening patients for OSA risk among Arabic-speaking nations.45 The BQ consisted of 10 items related to sleep apnea risk that include: snoring behavior, wake time, sleepiness or fatigue, history of obesity or hypertension, and body mass index (BMI). According to BMI values, respondents were classified as underweight (< 18.5); normal weight (18.5-24.9); overweight (25-29.9); and obese (> 30). Participants were classified as having a high risk of OSA if there were two or more categories where the score was positive. Participants were classified as low risk of OSA if there was only one or no category where the score was positive.37,46 We also used the ESS Arabic version to assess EDS.47 A score of 11 or more (i.e., ESS > 11) is considered EDS. We also used the International Classification of Sleep Disorders-2 (ICSD-2), which defines insomnia as difficulty in falling asleep, waking up too early, frequent awakening with difficulty in falling asleep again, and secondary daytime impairment related to nighttime sleep difficulties.48 In addition, we gathered demographic data and information pertinent to liver cirrhosis such as the underlying cause of liver cirrhosis, and the severity of liver cirrhosis based on CPS.44,49 We collected data on neck size as measured in cm, large neck size classified as having (≥ 38 cm for female and ≥ 40 for male) and small neck size classified as having (< 38 cm for female and < 40 for male).
Statistical analysisThe collected data were transferred and analyzed using SAS version 9.2 (SAS Institute Inc., Cary, NC).
- •
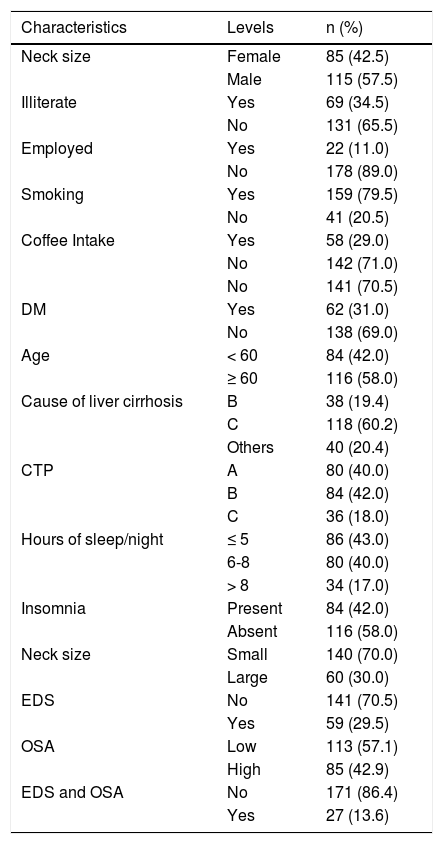
Univariate analysis. The mean and standard deviation was used to summarize age. Percentages were used to summarize the demographic and clinical characteristics such as gender, marital status, occupation, smoking status, depression, EDS, and OSA (Table 1Table 1).
Table 1.Demographic/clinical characteristics of patients with liver cirrhosis.
Characteristics Levels n (%) Neck size Female 85 (42.5) Male 115 (57.5) Illiterate Yes 69 (34.5) No 131 (65.5) Employed Yes 22 (11.0) No 178 (89.0) Smoking Yes 159 (79.5) No 41 (20.5) Coffee Intake Yes 58 (29.0) No 142 (71.0) No 141 (70.5) DM Yes 62 (31.0) No 138 (69.0) Age < 60 84 (42.0) ≥ 60 116 (58.0) Cause of liver cirrhosis B 38 (19.4) C 118 (60.2) Others 40 (20.4) CTP A 80 (40.0) B 84 (42.0) C 36 (18.0) Hours of sleep/night ≤ 5 86 (43.0) 6-8 80 (40.0) > 8 34 (17.0) Insomnia Present 84 (42.0) Absent 116 (58.0) Neck size Small 140 (70.0) Large 60 (30.0) EDS No 141 (70.5) Yes 59 (29.5) OSA Low 113 (57.1) High 85 (42.9) EDS and OSA No 171 (86.4) Yes 27 (13.6) - •
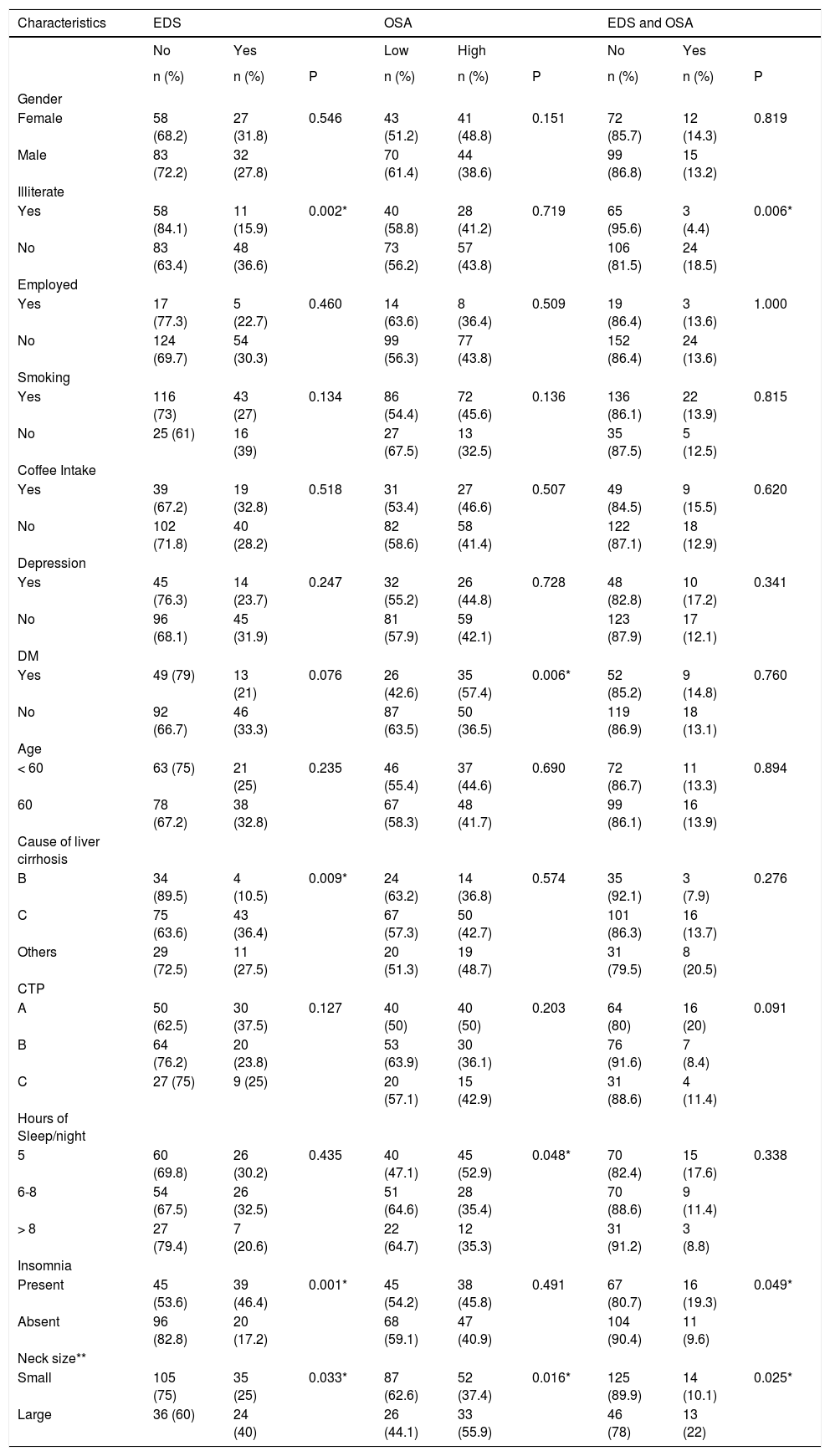
Bivariate analysis. χ2 tests were used to test the associations between the demographic/clinical characteristics across EDS, OSA, and both EDS and OSA in patients with liver cirrhosis (Table 2Table 2).
Table 2.Characteristics Factors associated with EDS, OSA, or EDS and OSA in patients with liver cirrhosis.
Characteristics EDS OSA EDS and OSA No Yes Low High No Yes n (%) n (%) P n (%) n (%) P n (%) n (%) P Gender Female 58 (68.2) 27 (31.8) 0.546 43 (51.2) 41 (48.8) 0.151 72 (85.7) 12 (14.3) 0.819 Male 83 (72.2) 32 (27.8) 70 (61.4) 44 (38.6) 99 (86.8) 15 (13.2) Illiterate Yes 58 (84.1) 11 (15.9) 0.002* 40 (58.8) 28 (41.2) 0.719 65 (95.6) 3 (4.4) 0.006* No 83 (63.4) 48 (36.6) 73 (56.2) 57 (43.8) 106 (81.5) 24 (18.5) Employed Yes 17 (77.3) 5 (22.7) 0.460 14 (63.6) 8 (36.4) 0.509 19 (86.4) 3 (13.6) 1.000 No 124 (69.7) 54 (30.3) 99 (56.3) 77 (43.8) 152 (86.4) 24 (13.6) Smoking Yes 116 (73) 43 (27) 0.134 86 (54.4) 72 (45.6) 0.136 136 (86.1) 22 (13.9) 0.815 No 25 (61) 16 (39) 27 (67.5) 13 (32.5) 35 (87.5) 5 (12.5) Coffee Intake Yes 39 (67.2) 19 (32.8) 0.518 31 (53.4) 27 (46.6) 0.507 49 (84.5) 9 (15.5) 0.620 No 102 (71.8) 40 (28.2) 82 (58.6) 58 (41.4) 122 (87.1) 18 (12.9) Depression Yes 45 (76.3) 14 (23.7) 0.247 32 (55.2) 26 (44.8) 0.728 48 (82.8) 10 (17.2) 0.341 No 96 (68.1) 45 (31.9) 81 (57.9) 59 (42.1) 123 (87.9) 17 (12.1) DM Yes 49 (79) 13 (21) 0.076 26 (42.6) 35 (57.4) 0.006* 52 (85.2) 9 (14.8) 0.760 No 92 (66.7) 46 (33.3) 87 (63.5) 50 (36.5) 119 (86.9) 18 (13.1) Age < 60 63 (75) 21 (25) 0.235 46 (55.4) 37 (44.6) 0.690 72 (86.7) 11 (13.3) 0.894 60 78 (67.2) 38 (32.8) 67 (58.3) 48 (41.7) 99 (86.1) 16 (13.9) Cause of liver cirrhosis B 34 (89.5) 4 (10.5) 0.009* 24 (63.2) 14 (36.8) 0.574 35 (92.1) 3 (7.9) 0.276 C 75 (63.6) 43 (36.4) 67 (57.3) 50 (42.7) 101 (86.3) 16 (13.7) Others 29 (72.5) 11 (27.5) 20 (51.3) 19 (48.7) 31 (79.5) 8 (20.5) CTP A 50 (62.5) 30 (37.5) 0.127 40 (50) 40 (50) 0.203 64 (80) 16 (20) 0.091 B 64 (76.2) 20 (23.8) 53 (63.9) 30 (36.1) 76 (91.6) 7 (8.4) C 27 (75) 9 (25) 20 (57.1) 15 (42.9) 31 (88.6) 4 (11.4) Hours of Sleep/night 5 60 (69.8) 26 (30.2) 0.435 40 (47.1) 45 (52.9) 0.048* 70 (82.4) 15 (17.6) 0.338 6-8 54 (67.5) 26 (32.5) 51 (64.6) 28 (35.4) 70 (88.6) 9 (11.4) > 8 27 (79.4) 7 (20.6) 22 (64.7) 12 (35.3) 31 (91.2) 3 (8.8) Insomnia Present 45 (53.6) 39 (46.4) 0.001* 45 (54.2) 38 (45.8) 0.491 67 (80.7) 16 (19.3) 0.049* Absent 96 (82.8) 20 (17.2) 68 (59.1) 47 (40.9) 104 (90.4) 11 (9.6) Neck size** Small 105 (75) 35 (25) 0.033* 87 (62.6) 52 (37.4) 0.016* 125 (89.9) 14 (10.1) 0.025* Large 36 (60) 24 (40) 26 (44.1) 33 (55.9) 46 (78) 13 (22) *x2 test is significant ata = 0.05. ** Large neck size (> 38 cm for female and> 40 for male) and small neck size (< 38 cm for female and < 40 for male). - •
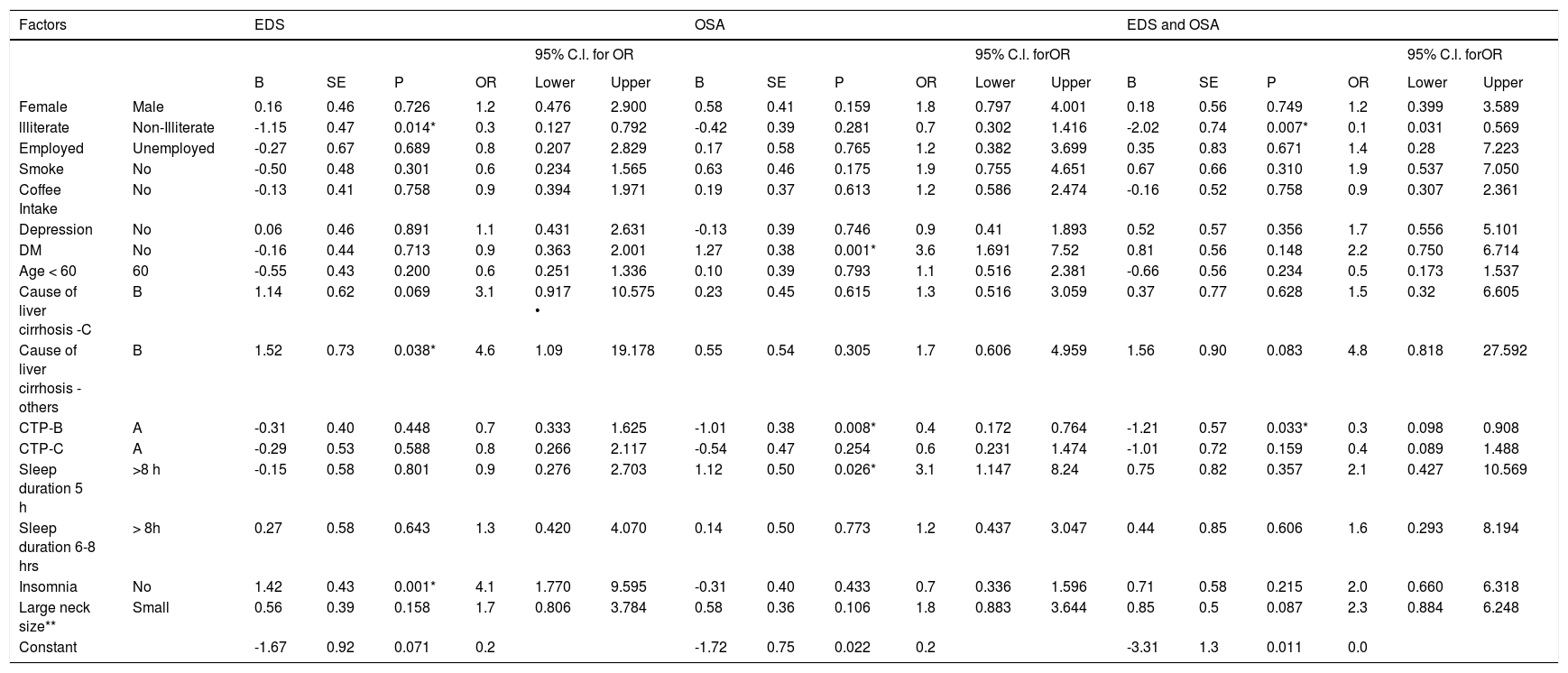
Multivariate analysis. Multivariate logistic regression models were used to determine the association between demographic and clinical characteristics and the presence EDS, OSA, and both EDS and OSA in patients with liver cirrhosis (Table 3Table 3). P values (P) < 0.05 were considered significant.
Table 3.Multivariate factors associated with EDS, OSA, or EDS and OSA in patients with liver cirrhosis.
Factors EDS OSA EDS and OSA 95% C.l. for OR 95% C.l. forOR 95% C.l. forOR B SE P OR Lower Upper B SE P OR Lower Upper B SE P OR Lower Upper Female Male 0.16 0.46 0.726 1.2 0.476 2.900 0.58 0.41 0.159 1.8 0.797 4.001 0.18 0.56 0.749 1.2 0.399 3.589 llliterate Non-Illiterate -1.15 0.47 0.014* 0.3 0.127 0.792 -0.42 0.39 0.281 0.7 0.302 1.416 -2.02 0.74 0.007* 0.1 0.031 0.569 Employed Unemployed -0.27 0.67 0.689 0.8 0.207 2.829 0.17 0.58 0.765 1.2 0.382 3.699 0.35 0.83 0.671 1.4 0.28 7.223 Smoke No -0.50 0.48 0.301 0.6 0.234 1.565 0.63 0.46 0.175 1.9 0.755 4.651 0.67 0.66 0.310 1.9 0.537 7.050 Coffee Intake No -0.13 0.41 0.758 0.9 0.394 1.971 0.19 0.37 0.613 1.2 0.586 2.474 -0.16 0.52 0.758 0.9 0.307 2.361 Depression No 0.06 0.46 0.891 1.1 0.431 2.631 -0.13 0.39 0.746 0.9 0.41 1.893 0.52 0.57 0.356 1.7 0.556 5.101 DM No -0.16 0.44 0.713 0.9 0.363 2.001 1.27 0.38 0.001* 3.6 1.691 7.52 0.81 0.56 0.148 2.2 0.750 6.714 Age < 60 60 -0.55 0.43 0.200 0.6 0.251 1.336 0.10 0.39 0.793 1.1 0.516 2.381 -0.66 0.56 0.234 0.5 0.173 1.537 Cause of liver cirrhosis -C B 1.14 0.62 0.069 3.1 0.917 • 10.575 0.23 0.45 0.615 1.3 0.516 3.059 0.37 0.77 0.628 1.5 0.32 6.605 Cause of liver cirrhosis - others B 1.52 0.73 0.038* 4.6 1.09 19.178 0.55 0.54 0.305 1.7 0.606 4.959 1.56 0.90 0.083 4.8 0.818 27.592 CTP-B A -0.31 0.40 0.448 0.7 0.333 1.625 -1.01 0.38 0.008* 0.4 0.172 0.764 -1.21 0.57 0.033* 0.3 0.098 0.908 CTP-C A -0.29 0.53 0.588 0.8 0.266 2.117 -0.54 0.47 0.254 0.6 0.231 1.474 -1.01 0.72 0.159 0.4 0.089 1.488 Sleep duration 5 h >8 h -0.15 0.58 0.801 0.9 0.276 2.703 1.12 0.50 0.026* 3.1 1.147 8.24 0.75 0.82 0.357 2.1 0.427 10.569 Sleep duration 6-8 hrs > 8h 0.27 0.58 0.643 1.3 0.420 4.070 0.14 0.50 0.773 1.2 0.437 3.047 0.44 0.85 0.606 1.6 0.293 8.194 Insomnia No 1.42 0.43 0.001* 4.1 1.770 9.595 -0.31 0.40 0.433 0.7 0.336 1.596 0.71 0.58 0.215 2.0 0.660 6.318 Large neck size** Small 0.56 0.39 0.158 1.7 0.806 3.784 0.58 0.36 0.106 1.8 0.883 3.644 0.85 0.5 0.087 2.3 0.884 6.248 Constant -1.67 0.92 0.071 0.2 -1.72 0.75 0.022 0.2 -3.31 1.3 0.011 0.0 * x2 fest ‘s significant at cc = 0.05. ** Large neck size ( > 38 cm for female and > 40 for male) and small neck size (< 38 cm for femaie and < 40 for male).
We enrolled 200 patients with liver cirrhosis, 57.5% were male, the mean age was 60 (± SD 12.2), and other sample characteristics are shown in table 1. Hepatitis C was the most common cause of liver cirrhosis, 118 (60.2%). EDS was reported by 59/200 (29.5%) (95% confidence limit: 23.3%-36.3%). High risk for OSA was reported by 85/198 (42.9%) (95% confidence limit: 35.9%-50.1%). The presence of both EDS and OSA was reported by 27/198 (13.6%) (95% confidence limit: 9.2%-19.2%). The bivariate analysis in table 2 shows patients with liver cirrhosis due to hepatitis C were significantly more likely to report EDS symptoms (36.4%) than hepatitis B patients (10.5%), or other factors (20.4%) (P = 0.009). EDS was reported more in the non-illiterate than in the illiterate (36.6% vs. 15.9%, P = 0.002). EDS was more frequent among liver cirrhosis patients with insomnia than among those without it (46.4% vs. 17.2%, P = 0.001). EDS was reported more in liver cirrhosis patients with large neck size (≥ 38 cm for female and ≥ 40 for male) compared to liver cirrhosis patients with small neck size (< 38 cm for female and < 40 for male) (40% vs. 25%, P = 0.033).
High risk for OSA was reported more in liver cirrhosis patients with large neck size than liver cirrhosis patients with small neck size (55.9% vs. 37.4%, P = 0.016). Liver cirrhosis patients with short sleep duration were more likely to report high risk for OSA than patients who reported sleeping 6-8 h and more than 8 h (52.9% vs. 35.4% and 35.3%, respectively, P = 0.048). Liver cirrhosis patients with DM were significantly more likely to report high risk for OSA than those without DM (57.4% vs. 36.5%, P = 0.006).
The presence of both EDS and high risk for OSA were reported more in liver cirrhosis patients with large neck size than f liver cirrhosis patients with small neck size (22% vs. 10.1%, P = 0.025). The presence of both EDS and high risk for OSA were associated with insomnia (19.3% vs. 9.6%, P = 0.049). The presence of both EDS and high risk for OSA was noted more frequently in the non-illiterate than in the illiterate (18.5% vs. 4.4%, P = 0.006).
In multivariate models (Table 3), insomnia (adjusted odds ratio [aOR] = 4.1; 95% CI: 1.770-9.595) and other causes of liver cirrhosis (aOR = 4.6; 95% CI: 1.090-19.178) were associated with the presence of EDS. The adjusted odds of EDS decreased by 70% in illiterate (aOR = 0.3; 95% CI: 0.127-0.792) as compared to non-illiterate. Compared with having no diabetes, those with diabetes had greater odds of high risk for OSA (aOR = 3.6; 95% CI: 1.691-7.520). Compared with CPS Class A, CPS Class B had 60% less odds of high risk for OSA (aOR = 0.40; 95% CI: 0.172-0.764). The odds of OSA were 3.1 times high in liver cirrhosis patients with short sleep duration (aOR = 3.1; 95% CI: 1.147-8.240). Liver cirrhosis patients with CPS Class B were 70% less likely to report both EDS and high risk for OSA compared with liver cirrhosis patients with CPS Class A (aOR = 0.30; 95% CI: 0.098-0.908). Lack of formal education was associated with less presence of both EDS and high risk for OSA (aOR = 0.10; 95% CI: 0.031-0.569).
DiscussionThis study was designed to predict the prevalence of EDS and high risk for OSA as determined in a series of patients diagnosed with liver cirrhosis. Our study confirms that patients with cirrhosis frequently have EDS at 29.5%, high risk of OSA at 42%, and a combination of both symptoms at 13.6%. Previous studies reported prevalence of EDS between 18.5%-38% in patients with liver cirrhosis.50-53 Previously we reported the prevalence of EDS, high risk for OSA, or both among hemodialysis Saudi patients39 and healthy Saudis using the same questionnaires, ESS and BQ.41,43 The prevalence of EDS, OSA, or both among healthy studies was 20.5%, 31.9%, and 7.9 respectively.43 When we compared the prevalence of EDS, high risk for OSA, or both to healthy Saudis, the prevalence for all variables was higher than among those with liver cirrhosis. Furthermore, risk for OSA in this study was much lower than the risk for OSA reported among Saudi dialysis patients which was 70.9%.39 Pulixi, et al. used the ESS as a measurement for EDS and the BQ to estimate risk for sleep apnea, and found ESD at 5%, risk for OSA at 25% and both at 8%.54 The age of the participants in our study is much higher than the study reported by Pulixi, et al. among nonalcoholic fatty liver disease at 60 ± 12 vs. 51.8 ± 12 respectively, and the weight was similar: the mean was 27.7.54 The increasing age and obesity, among others, are major risks for OSA and other sleep disorders.55,56 In our study, almost one-third of our patients were obese > 35%, and almost 50% of the participants were 60 years or older, which partially explains the higher prevalence in our study. Also 43% of our patient reported short sleep duration less than 5 h which may contribute to EDS. The etiologies of sleep problems in cirrhotic patients are likely multifactorial, and include enlarged abdominal diameter due to ascites compromising lung volume and upper airway edema, in addition to other hormonal changes and comorbid conditions such as obesity, DM, and changes in the autonomic nervous activities.16,17,36,57,58
The association between liver cirrhosis severity or its underlying cause and sleep apnea is controversial, partially due to the small number of studies.
Ogata, et al.36 reported an association between severe liver cirrhosis CPS-C compared to CPS-A and B, and they attribute this to changes in the autonomic nervous activities. Other studies also reported an association between liver disease severity and OSA.17,50,54 Studies reported that OSA is related to disease severity, more in CPS-C than in A.16,17,36 In our study there is no statistical difference between the severity of liver cirrhosis and OSA and both (EDS and OSA), all at P < 0.05. This is probably so because our patients had other comorbid conditions such as obesity where the prevalence was 33.9% and being older in age, in this study population. We did not adjust for obesity because it is one of the criteria used in BQ to classify participants as being at high risk for OSA, but DM was one of the independent risk factors for sleep apnea. Even if the risk of OSA was linked to BMI, the association of high risk for OSAS and EDS with liver damage was independent of the BMI and of the other major clinical confounders, such as age, gender, the presence of hyperglycemia, and ALT levels.12 The relationship between high risk for OSA and liver damage is independent of advanced liver disease, or presence of other comorbidities such as obesity, insulin resistance, or hyperglycemia.12 Indeed, ALT levels and other noninvasive biomarkers, which were correlated with OSA in previous studies as indices of liver damage,11 are insufficient as accurate biomarkers of the severity of liver disease.60
We found that hepatitis C infection was the most common cause of liver cirrhosis in our study population and similar to other studies, it was, from a statistical perspective, significantly associated with EDS and sleep disturbances. To the best of our knowledge this is the only study from the region which reports the association between liver cirrhosis and EDS and OSA. The number is reasonably large, and included 200 patients using validated questionnaires and personal interviews that document the association between liver disease and OSA. This is extremely significant, as the OSA by itself is a highly documented cause of the worsening of liver cirrhosis and a risk factor for further liver injury and increased liver enzymes, independent of other risk factors.810,bib006513, bib0120,bib0130,61 Furthermore, identification and treatment of OSA may ameliorate the progression of liver injury and progression to cirrhosis.34,35 Knowing the negative impact of OSA in liver cirrhosis patients and the benefit of identifying and treating patients at risk for OSA is crucial for physicians taking care of these patients. There are several limitations to our study:
- •
It was based on questionnaires which are less specific than formal polysomnographic studies.
- •
We did not correlate with the criteria of other laboratories, and
- •
We did not use non-liver cirrhosis as a control group.
EDS and OSA are common in Saudi liver cirrhosis patients, and both affect patients with CPS Class A more than those with CPS Class B. Physicians should be aware of such association knowing that OSA has a deleterious effect on the liver. OSA should be considered in the differential diagnosis of deteriorating liver function and progressive liver cirrhosis. Controlled prospective studies are warranted in studying the effect of OSA on sleep in patients with cirrhosis.
Abbreviations- •
aOR: adjusted odds ratio.
- •
BQ: berlin questionnaire.
- •
CI: confidence interval.
- •
CPS: child-Pugh scores.
- •
DM: diabetes mellitus.
- •
EDS: excessive daytime sleepiness.
- •
ESS: epworth sleepiness scale.
- •
HE: hepatic encephalopathy.
- •
NAFLD: nonalcoholic fatty liver disease.
- •
OSA: obstructive sleep apnea.
We declare the absence of any conflicts of interest of each author, and any off-label or investigational use. This research is supported by King Abdullah International Medical Research Center (KAIMRC).