Introduction and aim. Combined hepatocellular-cholangiocarcinoma (HCC-CCA) is a rare liver malignancy distinct from either hepatocellular carcinoma (HCC) or cholangiocarcinoma. Liver transplantation (LT) is not recommended for HCC-CCA because of suboptimal outcomes. Non-invasive diagnosis of HCC-CCA is extremely challenging; thus, some HCC-CCAs are presumed as HCC on imaging and listed for LT with the correct diagnosis ultimately made on explant pathology. We compared HCC-CCA with HCC to determine the utility of response to pre-transplant loco-regional therapy (LRT) in predicting outcomes for HCC-CCA after LT as a potential means of identifying appropriate HCC-CCA patients for LT.
Material and methods. Retrospective review of 19 patients with pathologically confirmed HCC-CCA were individually matched to 38 HCC patients (1:2) based on age, sex, and Milan criteria at listing was performed. The modified response evaluation criteria in solid tumors was used to categorize patients as responders or non-responders to pre-transplant LRT based on imaging performed before and after LRT. Overall survival (OS) and recurrence-free survival (RFS) were examined.
Results. OS at 3 years post-transplant was 74% for HCC-CCA and 87% for HCC. RFS at 3 years was 74% for HCC-CCA, and 87% for HCC. Among responders to LRT, the 3-year OS was 92% for HCC-CCA and 88% for HCC; among non-responders, 3-year OS was 43% for HCC-CCA and 83% for HCC. Higher 3-year OS was observed among HCC-CCA responders (77%) compared with HCC-CCA non-responders (23%).
Conclusions. OS was similarly high among responders to pre-transplant LRT irrespective of tumor type. Radiologic response to LRT could potentially be used to select appropriate HCC-CCA patients for LT if the findings are validated in independent studies.
Combined hepatocellular-cholangiocarcinoma (HCCCCA) is a rare primary liver cancer, with histopathological features that overlap both hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (iCCA).1 HCC-CCA accounts for some 1-5% of primary liver cancers and is distinct from HCC and iCCA, in that, it is more aggressive and associated with worse prognosis.1–6 The optimal management of HCC-CCA is not well established, due to the lack of specific and definitive non-invasive diagnostic criteria, and the rarity of this malignancy. There are limited data on the benefits of liver transplantation (LT) in HCC-CCA patients. At present, HCC-CCA is not an indication for LT due, in part, to the absence of data supporting improved prognosis following LT and aggressive disease course observed in practice.7,8
The clinical and radiological presentation of HCCCCA and HCC are very similar.1,9–11 Imaging features associated with HCC such as arterial phase enhancement, delayed phase washout and the presence of capsule are also present in HCC-CCA and may result in their misdiagnosis as HCC.2,9,12 Consequently, in the absence of direct pathological evidence, HCC-CCA may be misdiagnosed and managed as HCC. Due to the overlap in clinical and radiological features, some patients who undergo LT for HCC may have a precise diagnosis of HCC-CCA made only after histopathological evaluation of the explanted liver. Such incidentally identified HCC-CCA may occur in 1-2% of transplants for HCC.13,14 The pathogenesis of HCC-CCA is not completely understood, and tumors could arise from either dual differentiation along hepatocellular and cholangiocellular lineages or from malignant transformation of hepatic progenitor cells (HPC).15 The World Health Organization (WHO) classification divides HCC-CCA into two histologically distinguished groups, classical type and subtypes with stem cell features.16,17 A recent molecular profiling of HCC-CCA by Moeini, et al. showed that HCC-CCA is a heterogeneous disease with subtypes that include cholangiocellular carcinoma and a stem cell-derived subtype that is more aggressive and associated with worse prognosis.18
Co-presentation of HCC with iCCA on explant pathology can be categorized into three distinct types. Type I, or collision tumors, consist of contiguous or separate HCC and CCA that exist coincidentally. These tumors have mucin production and lack transitional zone between different tumor types. Type II tumors have a transitional area between HCC and CCA where a mixture of cells with hepatocellular and cholangiocellular differentiation are present. These tumors have bile and/or mucin secretion. Type III, or fibrolamellar tumors, have a combination and mingling of cellular features of hepatocellular and cholangiocellular differentiation. These tumors have mucin-producing pseudoglands and larger cells with eosinophilic granular cytoplasm. Compared to the other types, type III is more prevalent in younger patients but it has a longer survival after diagnosis if left untreated.19–22 Other histological classifications have been proposed but have not been shown to provide prognostic insight.23
Management of HCC-CCA patients include the use of Loco-regional therapy (LRT) with or without surgical resection.7,22,24 Patients with HCC awaiting LT may undergo LRT, such as trans-arterial chemoembolization and radiofrequency ablation to control tumor growth while awaiting a replacement organ.25 Pre-transplant response to LRT has been associated with a post-transplant survival benefit for HCC, and proposed as a proxy for tumor biology.26 Although response to LRT could be useful for identifying a subset of HCC-CCA patients who will have favorable outcomes from LT, the prognostic value of response to LRT for HCC-CCA is currently unknown.27 The purpose of this study was to investigate whether radiologic response to pre-transplant LRT could predict overall survival (OS) and recurrence-free survival (RFS) after LT for HCCCCA by comparing HCC-CCA patients with HCC patients.
Material and MethodsStudy subjectsFollowing approval by our Institutional Review Board, we identified patients who underwent orthotopic LT for presumed HCC at our institution, between January 01, 2001 and October 31, 2016, from a prospective database and reviewed their medical records. Patients were eligible for the study if they received neoadjuvant LRT and had magnetic resonance imaging (MRI) scan performed before and after LRT to assess treatment response. Pre-transplant diagnosis of HCC was determined by a dedicated team of oncologists, hepathologist, radiologists and liver transplant surgeons, following the American Association for the Study of Liver Diseases practice guidelines.8 Based on the medical records review, 19 incidentally transplanted HCC-CCA cases were identified from explant pathology reports. Each HCC-CCA case was matched to 2 pathologically confirmed HCC cases diagnosed at the same institution and over the same period, based on age (± 5 years), sex, and the Milan criteria used for selecting patients for orthotopic LT (i.e., 1 lesion ≤ 5 cm, or 2-3 lesions each ≤ 3 cm and no vascular invasion).28 Patients who did not have an imaging prior to and post LRT, did not undergo LT, or did not have a diagnosed hepatic mass were excludid from this study. The study population included 19 HCC-CCA cases and 38 HCC control patients. This study was approved by the Mayo Clinic Institutional Review Board (IRB 12-004111).
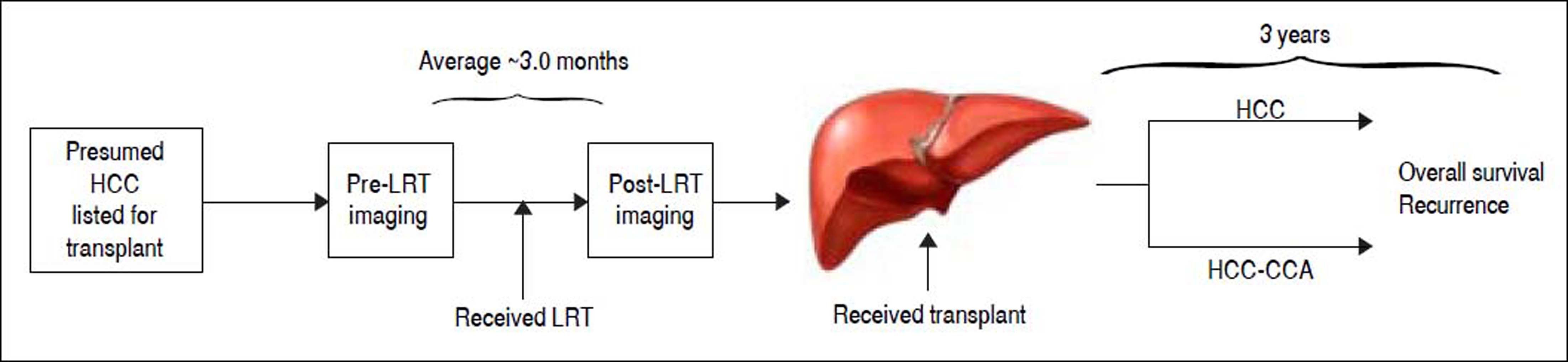
An overview of the study design is provided in figure 1. In brief, all patients had presumed HCC and were listed for LT. All patients received neoadjuvant LRT with an average 3-month interval between pre- and post-imaging LRT for assessment of treatment response. The patients then underwent LT and histopathological evaluation was performed on the explanted liver for definitive diagnosis. Although some patients had evidence of complete radiological response to pre-transplant LRT, none had a complete pathological response of target lesions where histological confirmation of tumor type could not be made. All patients were followed for up to 3 years for assessment of recurrence and vital status.
Study overview. Time intervals are reported as the average months among hepatocellular carcinoma (HCC) and combined hepatocellular-cholangiocarcinoma (HCC-CCA) patients. Al patients were presumed to have hepatocellular carcinoma (HCC) at diagnosis, received loco-regional therapy (LRT) with pre- and post-treatment magnetic resonance imaging (MRI) scan to access response to treatment, followed by liver transplantation. Patients were followed for up to 3 years.
Data abstracted from patients’ medical records included demographics (e.g., age and sex), MELD (model for end-stage liver disease) score at listing for LT including exception points, native MELD score without exception points, number and size of tumor lesions identified on imaging, tumor vascular invasion, type of LRT received, dates of pre- and post-LRT imaging used to assess treatment response, dates of LT, time between post-LRT imaging and LT, and vital and recurrence status at 1 and 3 years following LT. The number and size of hepatic lesions and evidence of tumor invasion or extrahepatic spread were used to determine whether or not a patient met the Milan criteria. Response to LRT was determined by hepatic MRI performed before and after LRT, but prior to LT. The modified response evaluation criteria in solid tumors (mRECIST) were used to assess treatment response and categorized as complete response, partial response, stable disease, or progressive disease.29 In mRECIST, complete response is defined as disappearance of all intratumoral arterial enhancements in all target lesions, partial response as at least a 30% decrease in the sum of the diameters of viable target lesions, stable disease as neither growing nor shrinking lesions, and progressive disease as an increase of 20% or more in diameter of target viable lesions.29,30 Multiple tumors were present in 26 patients (8 in HCC-CCA group, and 18 in the HCC groups). In such instances, the sum of the diameter of viable lesions assessed before and after LRT was averaged to one response category for each patient and classified as a complete radiologic response, partial radiologic response, stable disease, or progressive disease. To ensure more meaningful interpretation of the data given the small numbers, patients with complete or partial response to LRT were combined into a “responders” group, while patients with stable or progressive disease after LRT were combined into a “non-responders” group.
Data analysisMeans and proportions were used to compare demographic and clinical attributes between the HCC-CCA and the HCC only patients, using Students’ t-tests for continuous variable and chi-square tests for categorical variables. OS and RFS rates were assessed at 1-year and at 3-years following LT using Fisher’s exact tests to compare the HCC-CCA patients with the HCC patients. Separate analyses were performed among responders to pre-transplant LRT and non-responders assessing both OS and RFS by comparing rates between HCC-CCA and HCC patients in each of the treatment response groups. For the assessment of RFS, patients who died over the 3-year study period were censored at the time of death. Kaplan-Meier survival curves were used to describe OS and RFS patterns over the 3-year follow-up period, using log-rank tests to examine differences in survival patterns between the HCC-CCA and HCC patients, and stratified by the treatment response groups. We also compared OS and RFS between HCC-CCA responders versus HCC-CCA non-responders. In examining the survival rates and patterns, OS was defined by the length of time (months) from the date of LT to date of death or date of last follow-up, while RFS was defined by time from transplant date to date of recurrence, date of death, or date of the last follow-up, whichever occurred first. All statistical tests were two-sided, and a p-value < 0.05 was considered statistically significant. Given the rarity of HCC-CCA,1 reflected by our small sample size, we considered p-values lower than 0.15 as marginally significant. All analyses were performed in SAS® version 9.4 (SAS Institute, Cary, NC, USA).
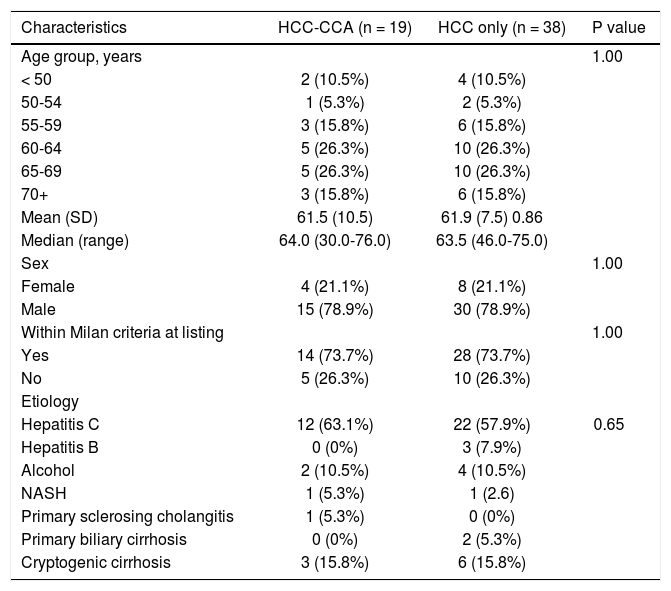
ResultsThe study comprised of 19 patients who on pathology were found to have HCC-CCA (4 females and 15 males). Six patients had Goodman type1 lesions and thirteen patients had Goodman type 2 lesions. Characteristics of the study groups are presented in table 1. The age at transplant for these patients ranged between 30-76 years; and for the 38 HCC patients (matched 1:2, HCC-CCA: HCC) eight were females and sixteen were males with age at transplant ranging from 46-75 years. All patients met radiological criteria for HCC, were presumed to have HCC at the time of listing for LT, and underwent LRT while awaiting transplantation. The primary underlying disease in each group is listed in table 1.
Descriptive characteristics of study participants (n = 57).
| Characteristics | HCC-CCA (n = 19) | HCC only (n = 38) | P value |
|---|---|---|---|
| Age group, years | 1.00 | ||
| < 50 | 2 (10.5%) | 4 (10.5%) | |
| 50-54 | 1 (5.3%) | 2 (5.3%) | |
| 55-59 | 3 (15.8%) | 6 (15.8%) | |
| 60-64 | 5 (26.3%) | 10 (26.3%) | |
| 65-69 | 5 (26.3%) | 10 (26.3%) | |
| 70+ | 3 (15.8%) | 6 (15.8%) | |
| Mean (SD) | 61.5 (10.5) | 61.9 (7.5) 0.86 | |
| Median (range) | 64.0 (30.0-76.0) | 63.5 (46.0-75.0) | |
| Sex | 1.00 | ||
| Female | 4 (21.1%) | 8 (21.1%) | |
| Male | 15 (78.9%) | 30 (78.9%) | |
| Within Milan criteria at listing | 1.00 | ||
| Yes | 14 (73.7%) | 28 (73.7%) | |
| No | 5 (26.3%) | 10 (26.3%) | |
| Etiology | |||
| Hepatitis C | 12 (63.1%) | 22 (57.9%) | 0.65 |
| Hepatitis B | 0 (0%) | 3 (7.9%) | |
| Alcohol | 2 (10.5%) | 4 (10.5%) | |
| NASH | 1 (5.3%) | 1 (2.6) | |
| Primary sclerosing cholangitis | 1 (5.3%) | 0 (0%) | |
| Primary biliary cirrhosis | 0 (0%) | 2 (5.3%) | |
| Cryptogenic cirrhosis | 3 (15.8%) | 6 (15.8%) |
HCC-CCA: combined hepatoceiiuiar-choiangiocarcinoma. HCC: hepatoceiiuiar carcinoma. NASH: non-aicohoiic steatohepatitis.
Because of the individual matching design, the HCCCCA and HCC patients did not differ in age, sex, mean tumor number from pre-LT imaging, mean tumor size from pre-LT imaging, or the proportion that were within the Milan criteria. Five HCC-CCA and 10 HCC patients were downstaged to within Milan criteria following LRT and before LT. Only one HCC-CCA patient received post-LT chemotherapy and none of the HCC patients received post-LT chemotherapy. The types of LRT received by the patients were transcatheter arterial chemoembolization (TACE), transarterial radioembolization (TARE), radiofrequency ablation (RFA), percutaneous ethanol ablation (PEI), and microicrowave ablation (MWA). Three HCC-CCA patients had a combination of TACE with TARE, while four HCC patients had combination LRT of TACE with RFA (two), TARE (one) or MWA (one) (Table 2). Among the 19 HCC-CCA patients, five had HCCCCA and concurrent HCC, six had CCA and HCC, seven had HCC-CCA only, and one had HCC-CCA and concurrent CCA pathologically identified in the explanted liver.
Tumor features and treatment response among study participants (n = 57).
| Characteristics | Combined HCC & CCA (n = 19) | HCC only (n = 38) | P value |
|---|---|---|---|
| Number of tumors on imaging | 0.23 | ||
| Mean (SD) | 2.00 (1.3) | 1.6 (0.9) | |
| Median (range) | 1.0 (1-5) | 1.0 (1-6) | |
| Tumor size on imaging, cm | 0.81 | ||
| Mean (SD) | 2.9 (1.9) | 2.8 (1.4) | |
| Median (range) | 2.4 (1.2-10.2) | 2.4 (0.8-8.0) | |
| MELD score at listing, before LRT | <0.001 | ||
| Mean (SD) | 22.5 (5.3) | 12.6 (4.4) | |
| Median (range) | 25.0 (11-28) | 12.0 (6-23) | |
| MELD score, Native | 0.76 | ||
| Mean (SD) | 13.3 (4.5) | 12.9 (4.6) | |
| Median (range) | 13.0 (7-21) | 12.5 (6-23) | |
| Explanted tumor numbera | |||
| Mean (SD) | 4.6 (4.0) | 1.7 (0.9) | <0.001 |
| Median (range) | 3.0 (1-16) | 1.0 (1-5) | |
| Vascular invasion-Pathology | 0.02 | ||
| No | 13 (68.4%) | 35 (92.1%) | |
| Yes | 6 (31.6%) | 3 (7.9%) | |
| Pre-LRT Serum AFP level | 0.43 | ||
| Mean (SD) | 185.0 ( 499.4) | 53.8 (130.6) | |
| Median (range) | 16.3 (3.7-1601.4) | 7.7 (2.2-636.3) | |
| Post-LRT Serum AFP level | 0.58 | ||
| Mean (SD) | 425.1 (1689.3) | 212.1 (1118.9) | |
| Median (Range) | 12.9 (1.9-7392.0) | 7.2 (1.2-6536.8) | |
| Type of LRT | |||
| TACE | 23 (85.2%) | 35 (68.6%) | |
| TARE | 3 (11.1%) | 2 (3.9%) | |
| RFA | 1 (3.7%) | 10 (19.7%) | |
| PEI | 0 (0%) | 2 (3.9%) | |
| MWA | 0 (0%) | 2 (3.9%) | |
| Combination LRT | |||
| Yes | 3 (15.8%) | 4 (10.5%) | |
| No | 16 (84.2%) | 34 (89.5%) | |
| LRT responseb | 0.11 | ||
| Complete response | 4 (21.1%) | 15 (39.5%) | |
| Partial response | 8 (42.1%) | 17 (44.7%) | |
| Stable disease | 6 (31.6%) | 3 (7.9%) | |
| Progressive disease | 1 (5.3%) | 3 (7.9%) | |
| Responders | 12 (63.2%) | 32 (84.2%) | 0.10 |
| Non-responders | 7 (36.8%) | 6 (15.8%) |
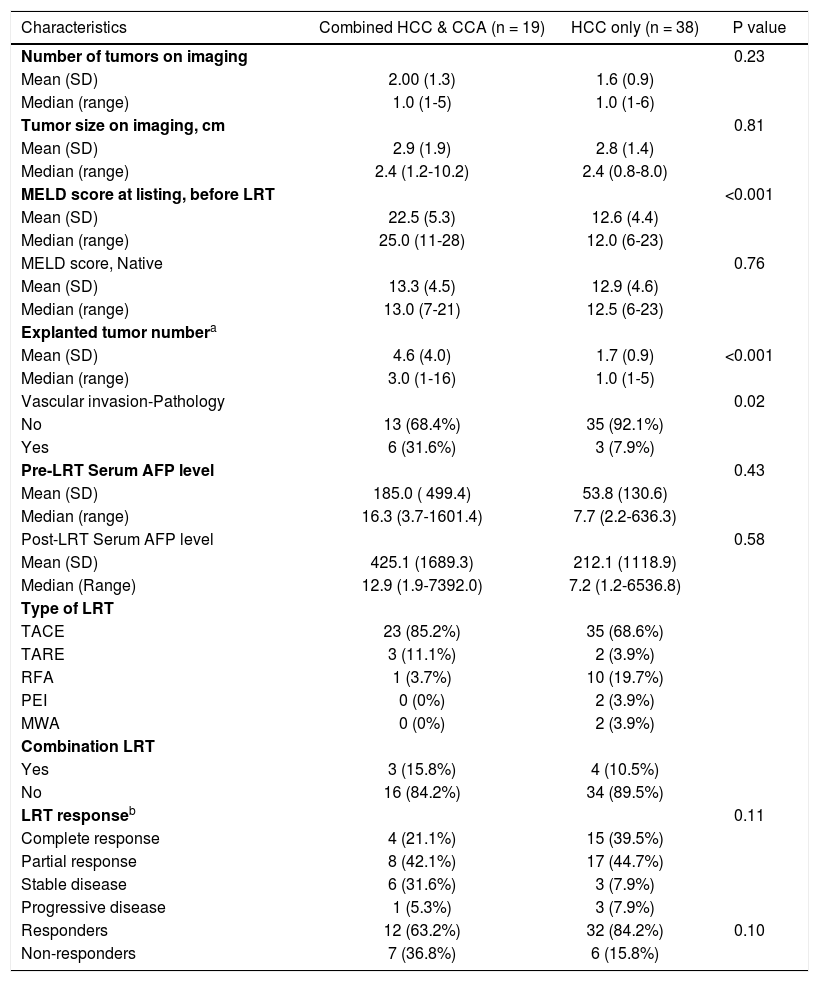
Two patients had greater than 10 tumors on expiant pathology, As a resuit, there was a higher number of tumors among the HCC-CCA patients. If these two cases were excluded, the number of tumors on expiant pathology wouid be similar for HCC-CCA (2.2) and HCC (1.7) patients.
Although some patients had complete radiologic response to pre-transpiant LRT, none had evidence of complete pathologic response. Individuais with complete or partial radiologic response were combined into one group classified as responders, whiie those with stable and progressive disease were combined and classified as non-responders. AFP: aipha-fetoprotein. CCA: cholangiocarcinoma. HCC: hepatocellular carcinoma. LRT: ioco-regionai therapy. TACE: transcatheter arterial chemoemboiization. TARE: transarteriai radioemboiization. RFA: radiofrequency abiation. PEI: percutaneous ethanoi abiation. MWA: microwave abiation.
On average, patients with HCC-CCA had 2 lesions with a mean diameter of 2.9 cm, while those with HCC had 2 lesions with a mean diameter of 2.8 cm. Compared with the HCC patients, the HCC-CCA patients had higher MELD score at listing, before LRT (22 vs. 13), a higher number of tumors on explanted liver (5 vs. 2), and were more likely to have a vascular invasion on histopathological report (32 vs. 8%) (Table 2). However, they did not differ on native MELD score (without exception points) or serum alpha-fetoprotein level post-LRT. There was a higher proportion of pre-transplant LRT responders among HCC patients (84%) than among HCC-CCA patients (63%), but the two groups were similar in terms of the time between pre- and post-LRT imaging, the number of LRT performed, or the time between post-LRT imaging and LT. The average interval between post-LRT imaging and transplantation was 3.2 months for HCC-CCA patients and 2.4 months for HCC, with a combined average of 3 months. The HCC-CCA patients underwent an average of 1.4 LRT treatments, whereas those with HCC underwent an average of 1.3 LRT treatments prior to LT.
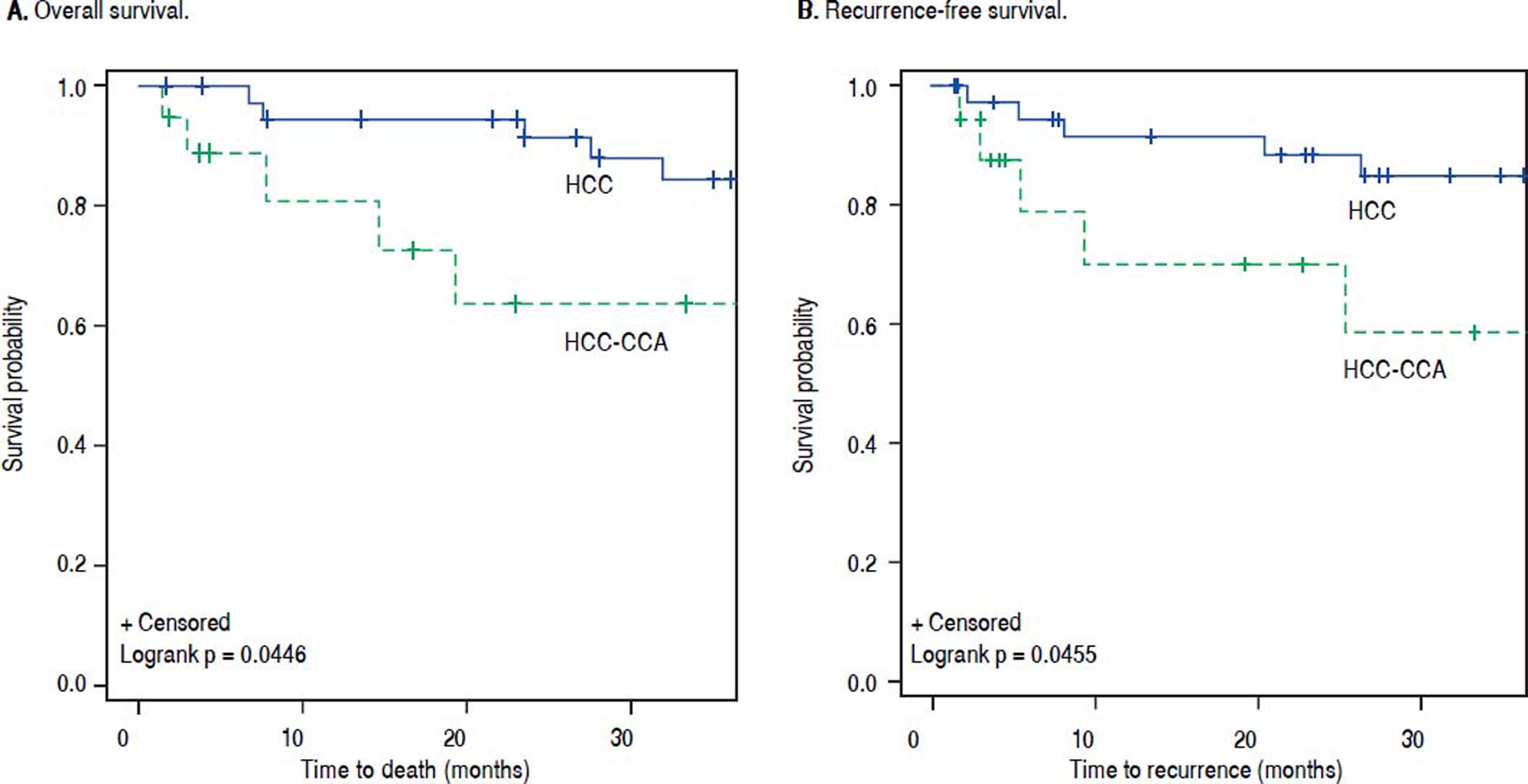
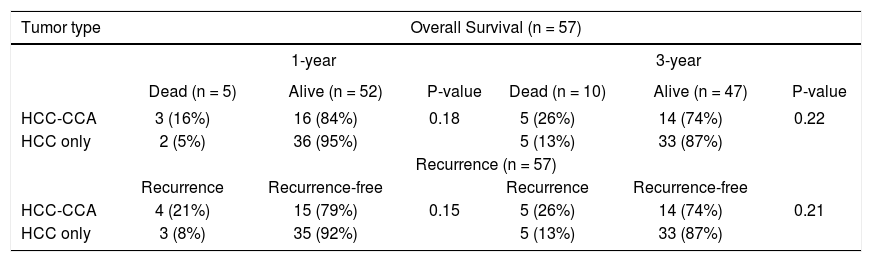
The OS and RFS rates among the HCC-CCA and the HCC patients were examined after 1 and 3 years of follow-up (Table 3). At 1 year of follow-up, OS was 84% among patients with HCC-CCA and 95% among patients with HCC (p-value = 0.18). At 3 years, the OS rates were 74% for HCC-CCA and 87% for HCC (p-value = 0.22). RFS rates at 1 and 3 years were 79% and 74%, respectively, for HCC-CCA and 92% and 87%, respectively, for the HCC patients (p-values ≥ 0.15). As shown in figure 2, OS and RFS were better across follow-up for HCC than for HCC-CCA patients (log-rank p-values for OS and RFS were each = 0.04).
Overall survival and recurrence rates among patients with combined hepatocellular-cholangiocarcinoma (HCC-CCA), and patients with hepatocellular carcinoma (HCC) only.
| Tumor type | Overall Survival (n = 57) | |||||
|---|---|---|---|---|---|---|
| 1-year | 3-year | |||||
| Dead (n = 5) | Alive (n = 52) | P-value | Dead (n = 10) | Alive (n = 47) | P-value | |
| HCC-CCA | 3 (16%) | 16 (84%) | 0.18 | 5 (26%) | 14 (74%) | 0.22 |
| HCC only | 2 (5%) | 36 (95%) | 5 (13%) | 33 (87%) | ||
| Recurrence (n = 57) | ||||||
| Recurrence | Recurrence-free | Recurrence | Recurrence-free | |||
| HCC-CCA | 4 (21%) | 15 (79%) | 0.15 | 5 (26%) | 14 (74%) | 0.21 |
| HCC only | 3 (8%) | 35 (92%) | 5 (13%) | 33 (87%) | ||
Percentages are presented as row percent because of the 1:2 matching of HCC-CCA and HCC patients, respectively.
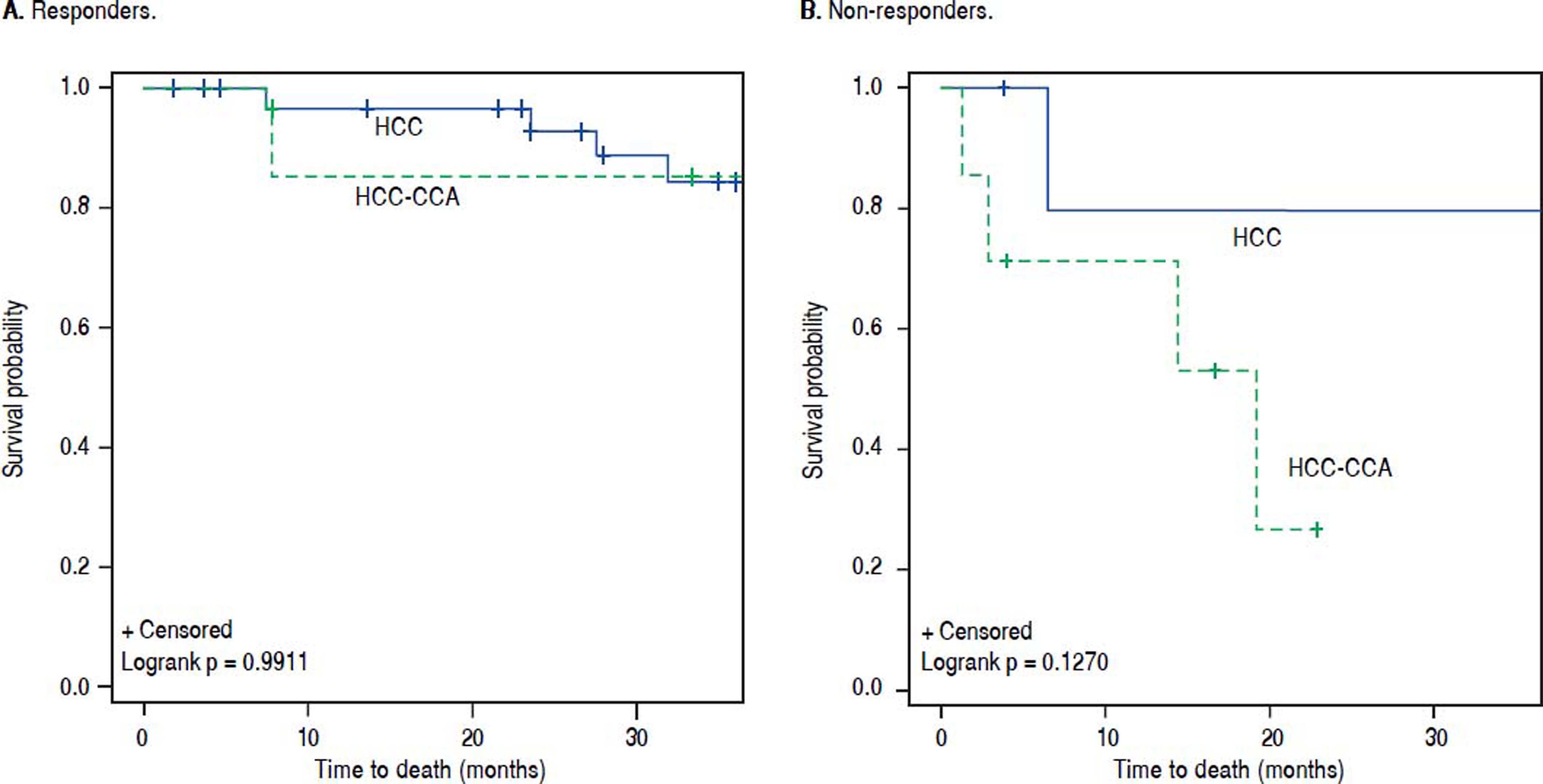
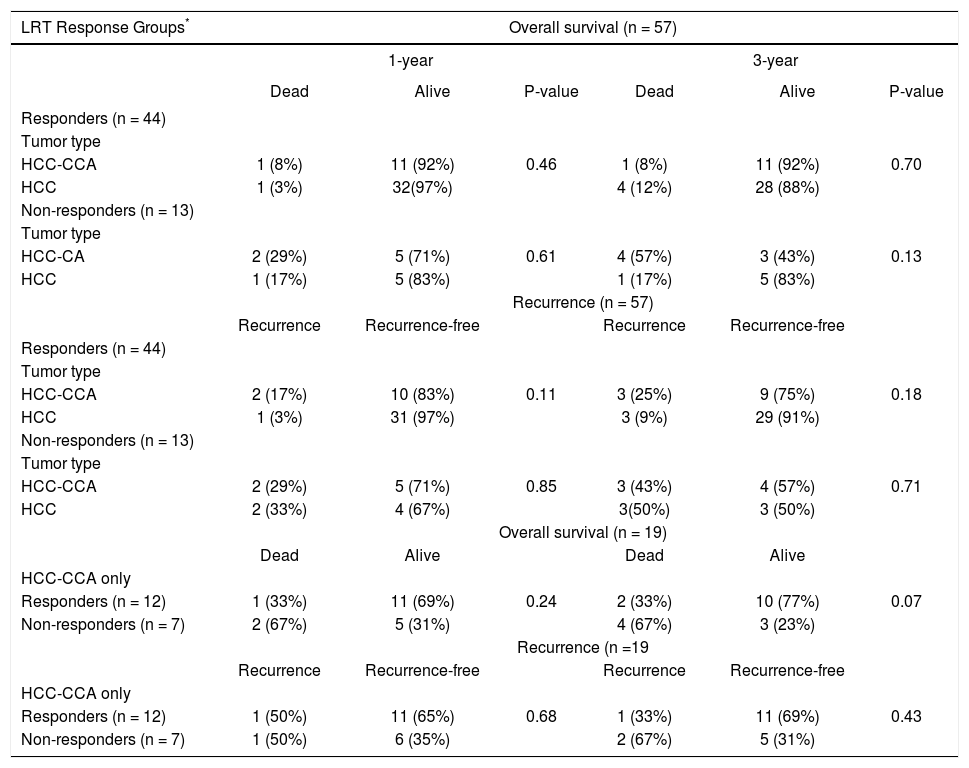
The results for overall and RFS rates were stratified by response to pre-transplant LRT (Table 4). Among responders, the OS rate at 1-year was 92% for HCC-CCA and 97% for HCC (p-value = 0.46). In comparison, 1-year OS rates were poorer among non-responders regardless of tumor type, with a 71% OS rate for HCC-CCA and 83% for HCC. The difference between HCC and HCC-CCA survival was not statistically significant (p-value = 0.61). Three-year OS was also inferior among non-responders compared to responders, and was much worse for nonresponders with HCC-CCA, such that while the 3-year OS among responders was 92% for HCC-CCA and 88% for HCC (p-value = 0.70), among non-responders, the 3-year OS rates were 43% for HCC-CCA and 83% for HCC (p-value = 0.13). Figure 3 demonstrates comparable OS pattern between HCC-CCA and HCC with no statistically significant difference between patients who responded to LRT with either HCC or HCC-CCA (log-rank p-value = 0.99). Among non-responders; however, the curves show a trend towards worse survival in the HCC-CCA group as compared with the HCC group (log-rank p-value = 0.13).
Rates of survival and recurrence among patients with combined hepatocellular-cholangiocarcinoma (HCC-CCA), and patients with hepatocellular carcinoma (HCC) only, stratified by response to loco-regional therapy (LRT).
| LRT Response Groups* | Overall survival (n = 57) | |||||
|---|---|---|---|---|---|---|
| 1-year | 3-year | |||||
| Dead | Alive | P-value | Dead | Alive | P-value | |
| Responders (n = 44) | ||||||
| Tumor type | ||||||
| HCC-CCA | 1 (8%) | 11 (92%) | 0.46 | 1 (8%) | 11 (92%) | 0.70 |
| HCC | 1 (3%) | 32(97%) | 4 (12%) | 28 (88%) | ||
| Non-responders (n = 13) | ||||||
| Tumor type | ||||||
| HCC-CA | 2 (29%) | 5 (71%) | 0.61 | 4 (57%) | 3 (43%) | 0.13 |
| HCC | 1 (17%) | 5 (83%) | 1 (17%) | 5 (83%) | ||
| Recurrence (n = 57) | ||||||
| Recurrence | Recurrence-free | Recurrence | Recurrence-free | |||
| Responders (n = 44) | ||||||
| Tumor type | ||||||
| HCC-CCA | 2 (17%) | 10 (83%) | 0.11 | 3 (25%) | 9 (75%) | 0.18 |
| HCC | 1 (3%) | 31 (97%) | 3 (9%) | 29 (91%) | ||
| Non-responders (n = 13) | ||||||
| Tumor type | ||||||
| HCC-CCA | 2 (29%) | 5 (71%) | 0.85 | 3 (43%) | 4 (57%) | 0.71 |
| HCC | 2 (33%) | 4 (67%) | 3(50%) | 3 (50%) | ||
| Overall survival (n = 19) | ||||||
| Dead | Alive | Dead | Alive | |||
| HCC-CCA only | ||||||
| Responders (n = 12) | 1 (33%) | 11 (69%) | 0.24 | 2 (33%) | 10 (77%) | 0.07 |
| Non-responders (n = 7) | 2 (67%) | 5 (31%) | 4 (67%) | 3 (23%) | ||
| Recurrence (n =19 | ||||||
| Recurrence | Recurrence-free | Recurrence | Recurrence-free | |||
| HCC-CCA only | ||||||
| Responders (n = 12) | 1 (50%) | 11 (65%) | 0.68 | 1 (33%) | 11 (69%) | 0.43 |
| Non-responders (n = 7) | 1 (50%) | 6 (35%) | 2 (67%) | 5 (31%) | ||
Individuais with complete or partial response to ioco-regionai therapy were classified as responders, whereas those with stable or progressive disease after ioco-regionai therapy were classified as non-responders. Percentages are presented as row percent because of 1:2 matching of HCC-CCA and HCC patients, respectively.
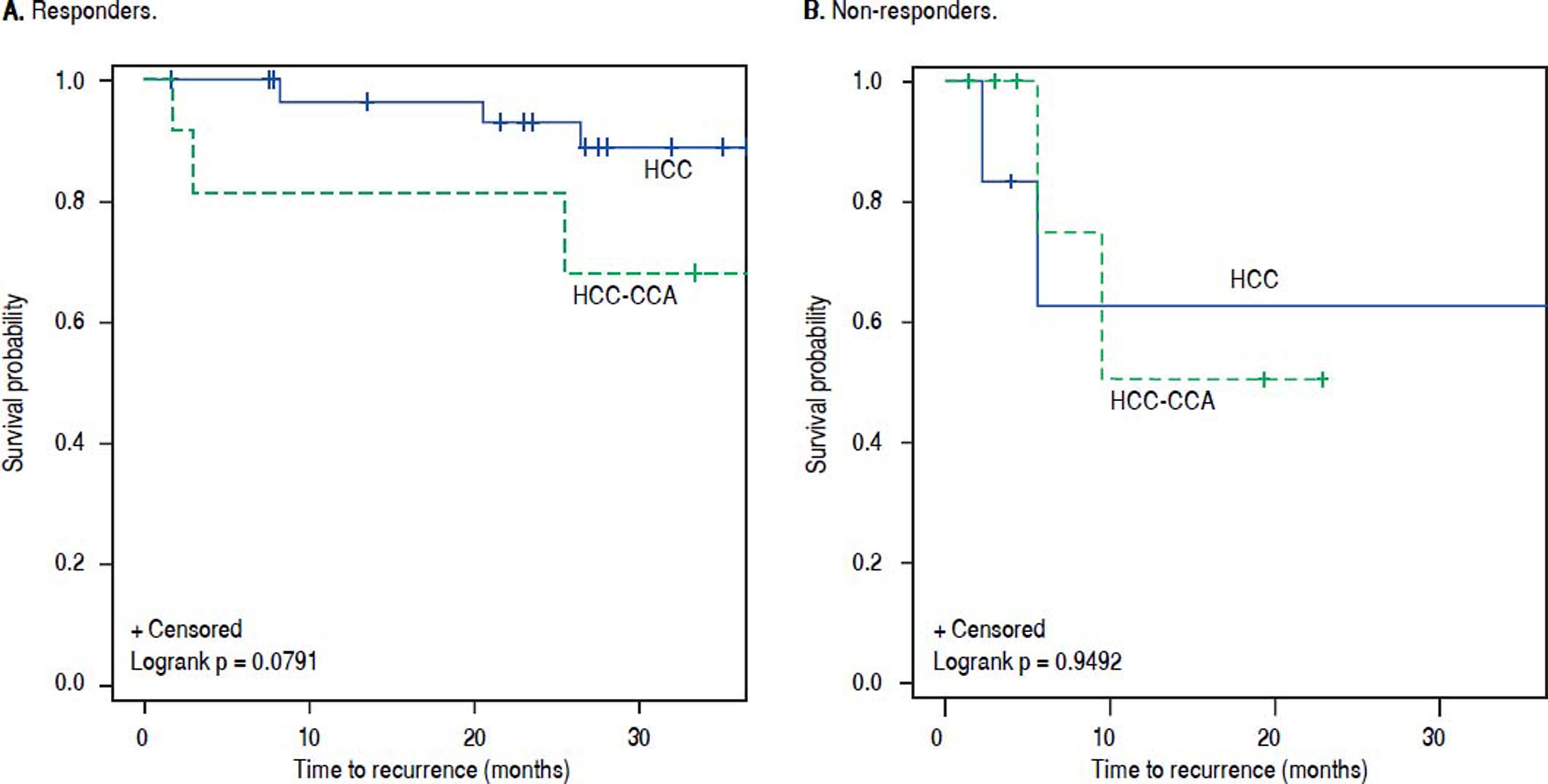
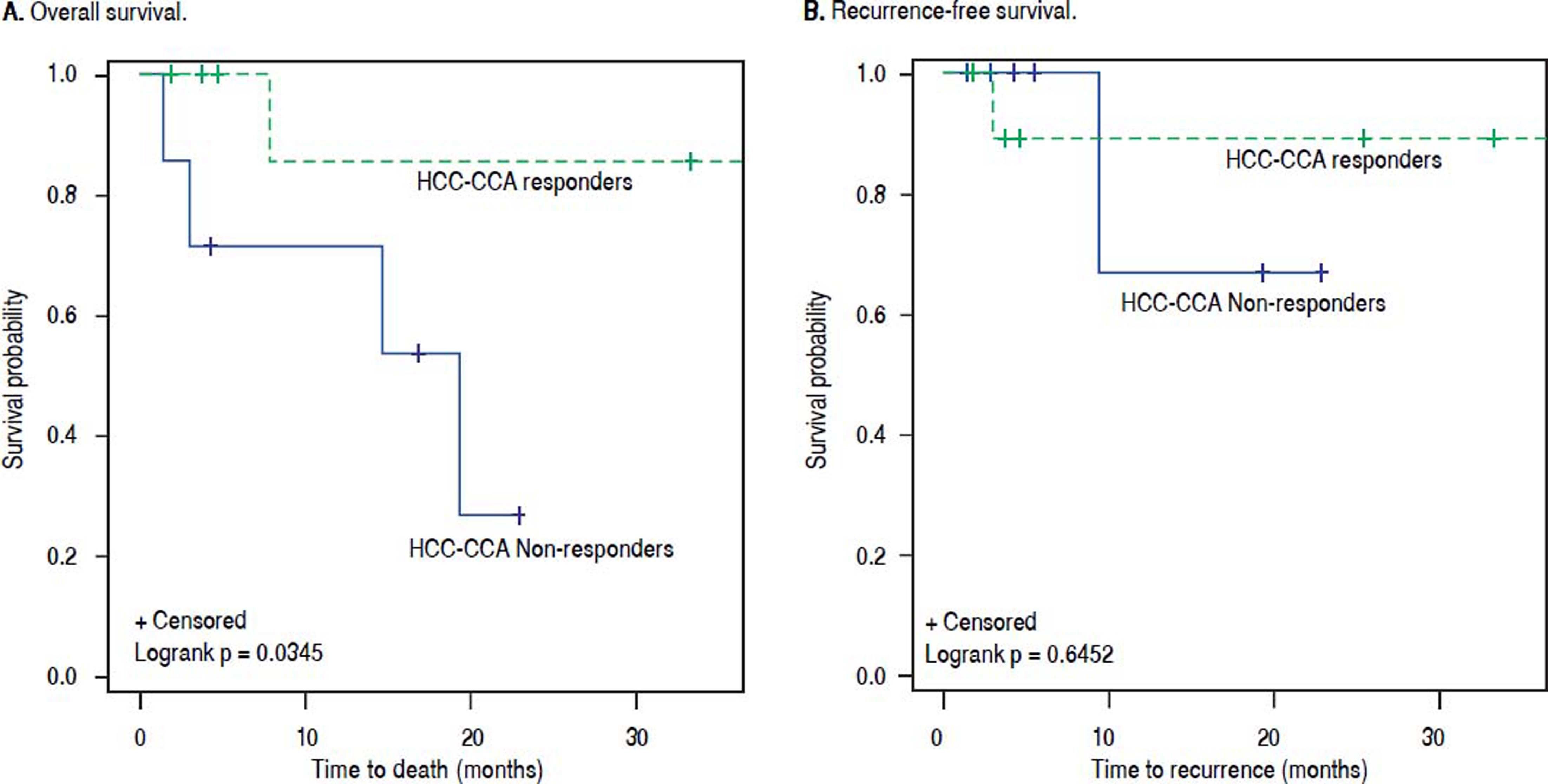
Among responders to pre-transplant LRT, the 1- and 3-year RFS rates were respectively 83% and 75% for patients with HCC-CCA, and 97% and 91% for HCC patients. Non-responders with HCC-CCA and HCC had equally poor RFS, with 1- and 3-year recurrence-free rates of 71% and 57%, respectively, for HCC-CCA and corresponding rates of 67% and 50% for HCC patients. In line with these results, the RFS curves (Figure 4) show a slightly better RFS across follow-up for responders with HCC than for responders with HCC-CCA (log-rank p-value = 0.08). In contrast, the RFS curves show similarly dismal survival across follow-up among non-responders with HCC-CCA and HCC (log-rank p-value = 0.94). Further analyses showed a generally higher survival rates among HCCCCA responders compared to HCC-CCA non-responders. Three-year overall survival was particularly higher among HCC-CCA responders (77%) compared with the HCC-CCA non-responders (23%) (p-value = 0.07). We also found a significantly better survival across follow-up among HCC-CCA responders than among HCC-CCA non-responders, with log-rank p-value = 0.03 (Figure 5). However, RFS did not differ significantly between the HCC-CCA responders and HCC-CCA non-responders (Table 4 and Figure 5).
Kaplan-Meier survival curves for (A) overall survival and (B) recurrence-free survival among combined hepatocellular-cholangiocarcinoma (HCCCCA) patients who responded to loco-regional therapy (LRT) vs. HCC-CCA patients who did not respond to LRT. Solid blue line: HCC-CCA responders to LRT. Dashed green line: HCC-CCA non-responders to LRT.
Patients with HCC-CCA are usually excluded from listing for LT due to the generally poor outcomes. To our knowledge, this is the largest study of treatment response on survival outcomes among HCC-CCA patients conducted to date. Our findings are consistent with the reported poor outcomes of HCC-CCA, in that, patients who were incidentally found to have HCC-CCA post-LT had worse OS and RFS as compared with individually matched HCC patients. These may be due, in part, to greater vascular invasion among the HCC-CCA patients. We found also that among patients who had a complete or partial response to neoadjuvant LRT, OS rates were equally favorable for HCC and HCC-CCA patients. These findings suggest that response to LRT could predict OS and RFS following LT, pending verification in independent samples, leading us to propose that a favorable response to LRT could potentially be used to identify those HCC-CCA patients who may benefit from LT.
LT provides the most favorable survival outcome for patients with primary liver cancer and even for patients with HCC-CCA, survival after LT is better than with any other therapeutic modality. Unfortunately, the scarcity of donor liver allografts and the high mortality in patients with primary liver failure have put restrictions on which patients can be considered for expedited access to donor organs in the setting of cancer.31 As a benchmark, LT offers a 4-year survival of ~85% for HCC within the Milan criteria. Many centers have modified these criteria by expanding the tumor number and size criteria for HCC or by including patients with other types of primary liver cancers (e.g., CCA) and have demonstrated similarly favorable outcomes in carefully selected patients.32,33 Response to LRT has been proposed by a number of groups to be used as a selective tool for patients with HCC because it appears to act as a surrogate for “favorable tumor biology”.27 A recent meta-analysis of incidental HCC-CCA upon explant, showed a wide 3- year RFS of 33-86% and OS of 22-70%, in comparison, the current study shows 3-year OS and RFS of 74% each.34 The heterogeneity of tumor behavior supports the use of criteria based on tumor biology rather than arbitrary size and number to identify candidates who will equally benefit from the life-saving intervention of LT and have excellent outcomes to justify expedited access to the scarce resource of a liver transplant. Our findings show that the response to LRT could identify suitable candidates with HCC-CCA who may have favorable OS after LT. To our knowledge, this is the first such report to suggest these findings.
The greatest challenge to any potential prospective trial investigating the benefits of LT for patients with HCCCCA is the limited ability to accurately diagnose these cancers in the pre-operative setting. The consistency between radiological and histopathological reports for HCC-CCA tumors is not high.15 Almost every reported experience with HCC-CCA and LT has been based upon incidental, post-LT diagnosis of HCC-CCA in the explant. Reports from some centers have suggested that incidental HCCCCA may be identified in 1-3% of all LTs performed. The accuracy of imaging-based diagnosis for HCC-CCA is low, even with large tumors, and the potential for misdiagnosis as HCC is well recognized.12,35 The imaging features of HCC-CCA are heterogeneous and overlap with those of HCC and CCA with the more dominant histopathological component determining the predominant radiographic features.36 The use of ancillary features within the LI-RADS algorithm such as rim enhancement and liver surface retraction can improve the ability to detect non-HCC tumors even when all major imaging features of HCC such as arterial phase enhancement, washout, and capsule appearance are present.35 Developing refined and specific radiological criteria of HCC-CCA with pathological validation, as well as a determination of their specificity in lesions within Milan criteria will be essential in order to enable diagnosis of HCC-CCA in the absence of pathological data. However, this goal has been stymied by the rarity of these tumors.2,12,35
The limitations of the present study include its retrospective and non-randomized nature, which precludes definitive causal inferences. The small sample size of the HCC-CCA group impeded reliable estimation of hazard ratios since such small sample sizes generally result in over-inflated risk estimates and wide confidence intervals. Major strengths of the study include the use of a standardized assessment of radiographic response to LRT, and consistent protocol based approach to patient selection for transplantation, which makes the results generalizable to HCC-CCA patients across centers and can be used to guide the management of HCC-CCA patients in different centers. However, our study is limited in generalizability because of the small sample size and the single center approach. The possibility exists that HCC-CCA patients with diagnosis established prior to transplant may have a different prognosis. This should be considered in the interpretation of results. The ability to individually match the HCC-CCA cases with HCC cases on relevant demographic and clinical factors add to the study strengths by reducing the potential impact of confounding factors. Moreover, HCC-CCA is an extremely rare cancer and this is the largest study conducted to date on treatment response among HCC-CCA patients.
In summary, this study shows that HCC-CCA has poorer post-transplant survival than HCC. However, among responders to pre-transplant LRT, HCC-CCA and HCC both had a similarly favorable OS after transplant. In contrast, OS is dismal for non-responders, particular nonresponders with HCC-CCA. The findings, therefore, indicate that a radiological response to LRT predicts favorable OS and RFS in both HCC-CCA and HCC. Response to pre-transplant LRT could potentially be useful for identifying HCC-CCA patients who will receive the greatest benefit from LT; however, the findings need verification in independent cohorts. If the findings are confirmed by other studies, response to LRT may be useful also for guiding post-transplant management of immunosuppression, surveillance protocols for recurrence of the tumor, and the use of post-transplant cancer preventive strategies. Although, this is the largest study of its kind conducted to date, prospective validation in larger cohorts is warranted.
Conflicts of InterestThe authors declares that there is no conflict of interest regarding the publication of this article.
FundingMayo Clinic.
Abbreviations- •
CI: confidence interval.
- •
CT: computed tomography scan.
- •
HCC: hepatocellular carcinoma.
- •
HCC-CCA: combined hepatocellular and cholangiocarcinoma.
- •
HR: hazard ratio.
- •
iCCA: intrahepatic cholangiocarcinoma.
- •
LRT: loco-regional therapy.
- •
MELD: Model for end-stage liver disease.
- •
mRECIST: modified response evaluation criteria in solid tumors.
- •
MRI: magnetic resonance imaging.
- •
OS: overall survival.
- •
RFS: recurrence-free survival.