Background and Aim. Statins are commonly used medications for the treatment of dyslipidemia. Although there are reported cases of hepatotoxicity related to statins, very few are associated with severe course and liver failure.
Material and methods. We used the Third National Health and Nutrition Examination Survey (NHANES III)-mortality linked files to assess the association between statin use and liver-related mortality. Patients with established causes of liver disease (HCV RNA-positive, HBs-Ag-positive, NAFLD by hepatic ultrasound, iron overload and excessive alcohol use of > 20 g of alcohol per day with elevated liver enzymes) were excluded.
Results. Of all adult NHANES III participants enrolled in 1988-1994 (n = 20,050), 9,207 individuals had sufficient demographic, clinical and medical information making them eligible for this study (age 41.26 ± 0.38, 46.76% male, 76.67% Caucasian, BMI 26.39 ± 0.38, 16.99% had diabetes or insulin resistance, 16.97% had hypertension, 65.28% had dyslipdemia). Of the entire study cohort, 90 (1.25%) participants reported using statins at the time of the interview. Median mortality follow-up for the study cohort was 175.54 months. During this period, 1,330 individuals (11.25%) died with 26 (0.17%) being liver-related deaths. For the cohort using statins, there were 37 deaths (40.15%) after a median follow-up of 143.35 months. In fact, the top cause of death for statin users was cardiac related (16 cases, 33.62%). However, after adjusting for major demographic, clinical and metabolic confounders, statin use was not associated with cardiovascular deaths in males (Hazard Ratio, 0.79, 95% Confidence Interval, 0.30-2.13), but was associated with higher risk of cardiovascular deaths in females (odds ratio, 2.32, 95% confidence interval, 1.58-3.40). Furthermore, the rate of liver-related mortality was significantly lower (p = 0.0035) among statin users compared to non-statin users.
Conclusions. After a decade of follow up, there was no association between statin use and liver-related mortality.
Coronary heart disease (CHD) is the leading cause of death for both men and women in the United States,1 potentially reducing one’s lifespan by 11.5 years.2 Statins are a class of drugs that have proven efficacy in treating dyslipidemia, an important risk factor for CHD.2 In fact, statin therapy is the treatment of choice for dyslipidemia as it significantly decreases both low-density lipoprotein cholesterol (LDL-C) and plasma total cholesterol levels.3 In long-term trials, statins have been shown to reduce cardiovascular events by 30-40%.2 More recently, additional evidence supports the efficacy of statins for the primary and secondary prevention of coronary artery disease.4 In one such study, the use of statin treatment before undergoing percutanous coronary intervention (PCI) resulted in a decline in the risk of death.5 Statin treatment also prevented future episodes of acute coronary syndrome.5 In another study of patients undergoing cardiac surgery, statin therapy before surgery was associated with a shorter in-hospital stay as well as a shorter stay in the intensive care unit.4
In other studies, statin treatment was shown to prevent atrial fibrillation6 and to reduce plaque volume.7 Furthermore, pleiotropic effects of statins have also been documented in a study documenting a reduction in pulmonary arterial hypertension.8
Despite a large amount of evidence supporting their positive impact, statins are still underused for the treatment of dyslipidemia. This is partly due to a perceived association of statins with hepatotoxicity.9 In part, recommendations in the 1970s may have been responsible for this view.2 In fact, guidelines at that time had suggested that an alanine aminotransferase (ALT) value more than three times the upper level of normal should be used to indicate liver injury caused by drug toxicity.2 Although arbitrary, this threshold became a standard for monitoring drugs in clinical trials.2 Although this standard may apply to some drugs, it cannot really be justified for patients taking statin therapy. In fact, a large proportion of patients with dyslipidemia have components of metabolic syndrome and non-alcoholic fatty liver disease (NAFLD).10 It is well known that patients with NAFLD can have fluctuating liver enzymes 2-3 times the upper level of normal.11 Applying “the standard” of the liver enzyme elevation in patients receiving the statins has contributed to a change in the management of these patients. In fact, in a recent internet-based survey of current and former statin users, it was found that the main reason patients discontinued or switched treatment was side effects and costs.12
To reduce the concerns about statin heptotoxicity, the Food and Drug Administration has recently expanded their recommendations about statin hepatotoxicity, no longer requiring routine liver enzymes monitoring.13 This recommendation was based on the conclusion that liver injury related to statin use is rare and that blood tests are not effective at predicting which patients will experience liver-related side effects.13
Additionally, assessment of statin safety by the Liver Expert Panel concluded that statins are well tolerated in patients with chronic liver disease such as NAFLD, primary biliary cirrhosis, or Hepatitis C.14 Finally, additional studies of statins in patients with chronic liver disease have supported their safety and efficacy.2,15 In summary, increasing evidence supports the fact that the association of statin use with severe liver outcomes is rare. To test this hypothesis, the aim of this study is to assess the association of statins with all-cause, cardiovascular and liver-related deaths using a population-based database.
Material and MethodsThe current study utilized data from the Third National Health and Nutrition Examination Survey (NHANES III) and the NHANES III linked mortality file. NHANES III was conducted from 1988 through 1994 by the National Center for Health Statistics (NCHS) using a complex, multi-stage, stratified, clustered sample design to obtain a representative sample of the total civilian, non-institutionalized U.S. population. The survey includes an in-home interview for demographic and basic health information, a health examination in a mobile examination center, laboratory tests and radiologic tests with ultrasonography of the gallbladder and liver. Public use data files were obtained from the NHANES Website (http://www.cdc.gov/nchs/nhanes.htm).16,17 The study was granted an exemption from full review by the Inova Institutional Review Board.
Study definitions- •
Diabetes mellitus type 2 (DM) was defined as a fasting glucose value of 126 mg/dL or greater or use of oral hypoglycemics and/or insulin.
- •
Insulin resistance (IR) was evaluated using the homeostasis of model assessment score (HOMA), which was calculated using the formula: fasting serum insulin (µU/mL) x fasting plasma glucose (mmol/L)/22.5 2.18 HOMA-IR was defined as a HOMA score of 3.0 or greater. DM and IR were combined as one variable, DM/IR, in our data analysis.
- •
Hypertension was defined as a systolic blood pressure of 140 mm Hg or greater, diastolic blood pressure of 90 mmHg or greater, or being on oral antihypertensive medications.
- •
Hypercholesterolemia was defined as increased cholesterol level of greater than 200 mg/dL, lowdensity lipoprotein level of 139 mg/dL or greater, or high density lipoprotein level less than 40 mg/ dL for men and less than 50 mg/dL for women.
- •
Elevated liver enzyme was defined as serum alanine aminotransferase level greater than 40 U/L or aspartate aminotransferase level greater than 37 U/L in men and alanine aminotransferase or aspartate aminotransferase level greater than 31 U/L in women.
- •
Obesity was defined as a body mass index greater than 30 or a waist circumference more than 102 cm in men and more than 88 cm in women.
- •
Excessive alcohol consumption was defined as more than 20 g per day for men and more than 10 g per day for women. The alcohol intake was calculated using self-reported data on amount and frequency of alcohol consumption collected as a part of the NHANES III examination questionnaire.
- •
All sera from participants were tested for core antibody to hepatitis B virus (anti-HBC). Sera testing positive for anti-HBC were tested further for the hepatitis B surface antigen (HBsAg). Chronic Hepatitis B (CH-B) was presumed in individuals with positive HBsAg.
- •
Similarly, hepatitis C antibody was tested and, if positive, hepatitis C virus (HCV) RNA was further tested. Participants with positive HCV RNA were considered to have Chronic Hepatitis C (CH-C).
- •
Alcoholic liver Disease (ALD) was presumed in participants who reported excessive alcohol use for the past year and had elevated serum amino-transferases.
- •
Elevated serum transferrin saturation is a commonly used indicator for a predisposition of iron overload. Iron overload is defined as serum transferrin saturation greater than 50%.
- •
Between 2009 and 2010, the hepatic steatosis (fatty liver) was assessed by re-reviewing the archived gall bladder ultrasound video images originally obtained in NHANES III between 1988 and 1994.16,17 Nonalcoholic fatty liver disease (NAFLD) was defined as the presence of moderate to severe hepatic steatosis from the ultrasound examination in the absence of any other evidence of chronic liver disease such as ALD, CH-B, CH-C, and iron overload.
NHANES III contains data for 33,994 persons ages 2 months and older who participated in the survey. Our analysis was limited to participants aged 20-74 at the time of the examination with complete data on demographics (age, sex, race/ethnicity), history of alcohol consumption, history of cancer but not skin cancer, history of hypertension and type II diabetes, body mass index, waist circumference, and blood pressure measured at the time of examination, as well as gradable hepatic ultrasound video images for hepatic steatosis assessment. The following tests also were required for all individuals in this study: serum glucose, triglyceride, high-density lipoprotein, aspartate aminotransferase, alanine transaminase, and transferrin saturation levels, and viral hepatitis serologies for hepatitis B virus and hepatitis C virus. Participants with history of cardiovascular diseases (congestive heart failure, stroke, heart attack) or one or more of the following liver diseases (all defined above) were excluded from the study: CH-B, CH-C, ALD, NAFLD or iron overload.
Participants’ age, sex and race/ethnicity were based on self reported data from the NHANES III household interview. Age was categorized into four groups: 20-44, 45-54, 55-64, and 65-74. Four major race/ethnic groups were reported including non-Hispanic white, non-Hispanic black, Mexican American and ‘Other’, which included all Hispanics who were not Mexican-American and also all non-Hispanics from racial groups other than white and black.
StatinsUse of statins was determined from the responses to the questions related to prescription medicines contained in the Household Adult questionnaires. During the household interview, respondents were asked a series of questions about prescription medicines used during the past month. For each medication reported, the interviewer asked to see the medication container and record the name of the product. Respondents were also asked to describe how long they had been taking the medicine.3 This information was used to ascertain participants’ current use of statins. Based on self-reported medication use, participants were categorized as statin users and non-users. To adjust for the use of other lipid-lowering medications, such as fibric acid derivatives, bile acid sequestrants, cholesterol absorption inhibitors, and miscellaneous antihyperlipidemic agents, information on the use of these medications was also obtained, and participants were grouped into users and non-users of lipid lowering agents.
MortalityThe NHANES III Linked Mortality File provides mortality follow-up data from the date of NHANES III survey participation (1988-1994) through December 31, 2006.17 Mortality ascertainment is based upon the results from a probabilistic match between NHANES III and the National Death Index (NDI) death certificate records. In addition to mortality status, the NHANES III Linked Mortality File contains months of follow-up from examination date as well as the Underlying Cause of Death 113 (UCOD_113) code to recode all deaths according to ICD-9 and ICD-10 criteria 4. In addition to overall mortality, liver-related mortality and cardiovascular mortality were also examined in this study. Liver-related mortality (UCOD_113 15, 24, 93-95) included causes of death such as viral hepatitis, hepatocellular carcinoma, alcoholic liver disease, and other chronic liver disease and cirrhosis. Cardiovascular mortality (UCOD_113 58-63, 67, 70-74) covered causes of death such as ischaemic heart diseases, heart failure, atherosclerosis, cerebrovascular diseases, aortic aneurysm and other diseases of arteries, arterioles and capillaries. Individuals without available mortality follow-up data were excluded from the study.
Statistical analysisWe compared the baseline characteristics of participants by the use of statins using χ2 test for independence. The main outcomes under study were overall mortality, liver-related mortality, and cardiovascular mortality. Periods of risk of death were defined in months for each participant between NHANES III examination date and the date of death or the end of follow-up (December 31, 2006). If an individual did not die or died from causes other than the event of interest, his survival time was censored.
Cox proportional hazards model was used to estimate hazard ratios and 95% confidence intervals for deaths from all causes, cardiovascular disease and liver disease by status of statin use. Logistic regression was used to evaluate the association of use of statins and elevated liver enzyme at the time of the NHANES III examination. Each model was adjusted for major demographic and clinical confounders including age, sex, race/ethnicity, excessive alcohol use, obesity, diabetes/insulin resistance, elevated liver enzymes, hypercholesterolemia, and hypertension. Statistical significance was set at p < 0.05.
NHANES III is based on a complex multistage probability sample design. The sampling weights incorporate the differential probabilities of selection and include adjustments for non-coverage and non-response. Sample weights together with stratification and clustering were incorporated into our analysis to estimate variances and test for statistical significance. All analyses were performed using standalone SUDAAN 10.0 (RTI International, Research Triagnle Park, NC).
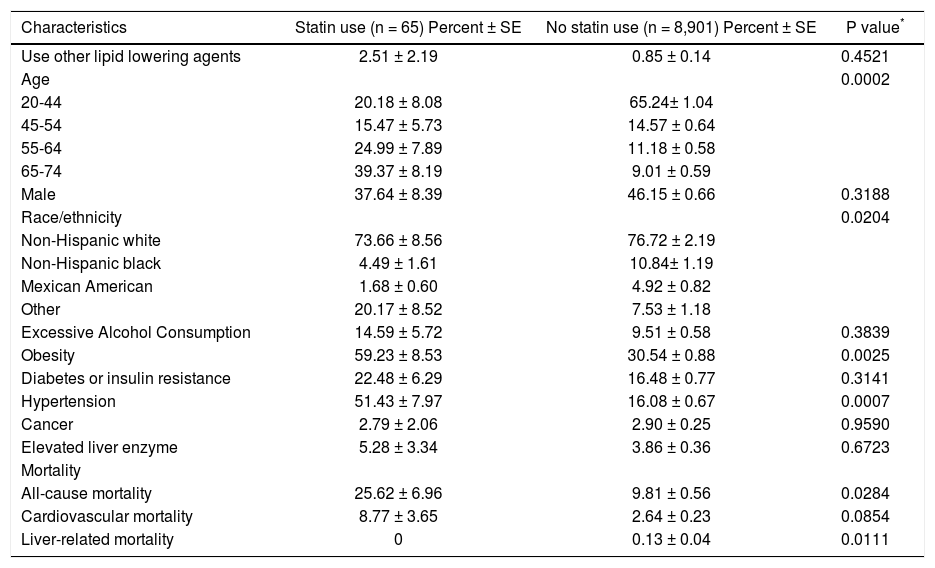
ResultsAfter applying exclusion criteria, a total of 8,966 participants remained in the analytical sample. In this cohort, the use of lipid lowering agents was documented in 1.63% (n = 146) of the cohort, with 65 individuals using statins for the treatment of dyslipidemia [lovastatin (n = 51), pravastatin (n = 9), simvastatins (n = 5)] and 81 individuals were using other lipid lowering agents [ cholestyramine (n = 16), clofibrate (n = 2), colestipol (n = 3), gemfibrozil (n = 51), niacin (n = 2), probucol (n = 7)]. The clinical and demographic information of statin users are summarized in table 1.
Characteristics of study participants by statin use from NHANES III (1988-1994).
| Characteristics | Statin use (n = 65) Percent ± SE | No statin use (n = 8,901) Percent ± SE | P value* |
|---|---|---|---|
| Use other lipid lowering agents | 2.51 ± 2.19 | 0.85 ± 0.14 | 0.4521 |
| Age | 0.0002 | ||
| 20-44 | 20.18 ± 8.08 | 65.24± 1.04 | |
| 45-54 | 15.47 ± 5.73 | 14.57 ± 0.64 | |
| 55-64 | 24.99 ± 7.89 | 11.18 ± 0.58 | |
| 65-74 | 39.37 ± 8.19 | 9.01 ± 0.59 | |
| Male | 37.64 ± 8.39 | 46.15 ± 0.66 | 0.3188 |
| Race/ethnicity | 0.0204 | ||
| Non-Hispanic white | 73.66 ± 8.56 | 76.72 ± 2.19 | |
| Non-Hispanic black | 4.49 ± 1.61 | 10.84± 1.19 | |
| Mexican American | 1.68 ± 0.60 | 4.92 ± 0.82 | |
| Other | 20.17 ± 8.52 | 7.53 ± 1.18 | |
| Excessive Alcohol Consumption | 14.59 ± 5.72 | 9.51 ± 0.58 | 0.3839 |
| Obesity | 59.23 ± 8.53 | 30.54 ± 0.88 | 0.0025 |
| Diabetes or insulin resistance | 22.48 ± 6.29 | 16.48 ± 0.77 | 0.3141 |
| Hypertension | 51.43 ± 7.97 | 16.08 ± 0.67 | 0.0007 |
| Cancer | 2.79 ± 2.06 | 2.90 ± 0.25 | 0.9590 |
| Elevated liver enzyme | 5.28 ± 3.34 | 3.86 ± 0.36 | 0.6723 |
| Mortality | |||
| All-cause mortality | 25.62 ± 6.96 | 9.81 ± 0.56 | 0.0284 |
| Cardiovascular mortality | 8.77 ± 3.65 | 2.64 ± 0.23 | 0.0854 |
| Liver-related mortality | 0 | 0.13 ± 0.04 | 0.0111 |
Participants with self-reported use of statins were compared with participants with no use of statins using χ2 test.
The median follow-up was 175.74 (maximum = 217) months for the entire cohort, 157.77 (maximum = 211) months for statin users. A total number of 1,139 (9.96% ± 0.56) participants died at the end of the follow-up, including 316 (2.70% ± 0.24) cardiovascular deaths and 19 (0.13% ± 0.04) deaths due to liver diseases. The overall mortality rate was 25.62% (19 deaths) in statin users, which was significantly higher than the rate of 9.81% (1,120 deaths) in non-users (p = 0.0284). The top causes of death for statin users were all cardiac-related. The cumulative mortality rate for cardiovascular death was 8.77% (8 deaths) for statin users, which tended to be higher than the rate of 2.64% (308 deaths) for nonusers (p = 0.0854). At the end of follow-up, none of the statin users or users of other lipid lowering drugs died from liver diseases. All of the 19 liver-related deaths occurred among participants with no use of the study drugs, resulting in a cumulative mortality rate of 0.13%, which was significantly higher than the 0 mortality rate in statin users (p = 0.0111) or lipid lowering agent users (p = 0.0101).
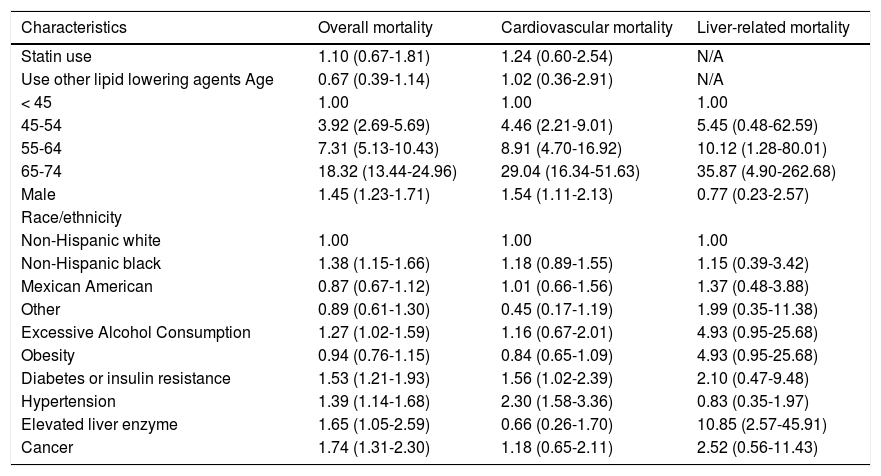
Multivariate Cox proportional hazard modelsPrevious studies have shown that participant’s demographics such as age, sex, race/ethnicity, excessive alcohol consumption, and metabolic conditions such as obesity, diabetes or insulin resistance, hypercholesterolemia and hypertension were associated with overall mortality, cardiovascular and liver-related mortality.5 Therefore, multivariate Cox proportional hazard model was used to evaluate the independent effect of statins or other lipid lowering agents on all-cause mortality and cause-specific mortality while adjusting for possible confounding effects of these variables.
All-cause mortalityFor all-cause mortality, the Cox proportional hazard model revealed that the unadjusted hazard ratio was 3.12 (95% CI: 1.69-5.75) for statin users compared to non-users (p = 0.0004). After adjusting for the use of other lipid lowering agents, demographic characteristics, and life style risk factors disease conditions, statin use was not independently associated with increased risk of overall mortality (HR, 1.10; p = 0.6313) (Table 2).
Hazard ratios (HR) and 95 confidence intervals (CI) of all-cause and cause-specific mortality, by statin use and other risk factors at baseline, NHANES III (1988-1994).
| Characteristics | Overall mortality | Cardiovascular mortality | Liver-related mortality |
|---|---|---|---|
| Statin use | 1.10 (0.67-1.81) | 1.24 (0.60-2.54) | N/A |
| Use other lipid lowering agents Age | 0.67 (0.39-1.14) | 1.02 (0.36-2.91) | N/A |
| < 45 | 1.00 | 1.00 | 1.00 |
| 45-54 | 3.92 (2.69-5.69) | 4.46 (2.21-9.01) | 5.45 (0.48-62.59) |
| 55-64 | 7.31 (5.13-10.43) | 8.91 (4.70-16.92) | 10.12 (1.28-80.01) |
| 65-74 | 18.32 (13.44-24.96) | 29.04 (16.34-51.63) | 35.87 (4.90-262.68) |
| Male | 1.45 (1.23-1.71) | 1.54 (1.11-2.13) | 0.77 (0.23-2.57) |
| Race/ethnicity | |||
| Non-Hispanic white | 1.00 | 1.00 | 1.00 |
| Non-Hispanic black | 1.38 (1.15-1.66) | 1.18 (0.89-1.55) | 1.15 (0.39-3.42) |
| Mexican American | 0.87 (0.67-1.12) | 1.01 (0.66-1.56) | 1.37 (0.48-3.88) |
| Other | 0.89 (0.61-1.30) | 0.45 (0.17-1.19) | 1.99 (0.35-11.38) |
| Excessive Alcohol Consumption | 1.27 (1.02-1.59) | 1.16 (0.67-2.01) | 4.93 (0.95-25.68) |
| Obesity | 0.94 (0.76-1.15) | 0.84 (0.65-1.09) | 4.93 (0.95-25.68) |
| Diabetes or insulin resistance | 1.53 (1.21-1.93) | 1.56 (1.02-2.39) | 2.10 (0.47-9.48) |
| Hypertension | 1.39 (1.14-1.68) | 2.30 (1.58-3.36) | 0.83 (0.35-1.97) |
| Elevated liver enzyme | 1.65 (1.05-2.59) | 0.66 (0.26-1.70) | 10.85 (2.57-45.91) |
| Cancer | 1.74 (1.31-2.30) | 1.18 (0.65-2.11) | 2.52 (0.56-11.43) |
The following factors were associated with increased all-cause mortality: older age, male gender, non-Hispanic blacks race/ethnicity, excessive alcohol consumption, presence of DM or insulin resistance, hypertension, or elevated liver enzymes (Table 2).
Cardiovascular mortalityThe unadjusted hazard of dying from cardiovascular disease was significantly higher in statin users than non-users (HR, 3.90, p = 0.0019). However, after adjusting for the other important covariates, the association was no longer significant (HR, 1.24; p = 0.5548) (Table 2). In addition to older age, male gender, having DM or insulin resistance or hypertension increased the risk of cardiovascular mortality (Table 2).
Liver-related mortalitySince no liver-related deaths occurred among users of statins or other lipid lowering agents, the independent relationship between these drugs and liver-related mortality could not be determined. The evaluation of other risk factors for liver-related death showed that having elevated liver enzymes was significantly associated with higher risk of liver-related death (p = 0.0003). Additionally, older age was also associated with higher risk of liver-related death (Table 2).
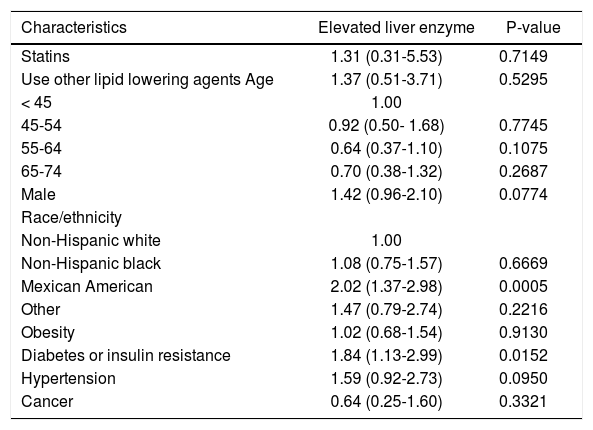
Statins and liver enzyme elevationsThere were a total of 442 participants who had elevated liver enzymes at the time of the NHANES III examination, 6 of them were from statin users. The rate of elevated liver enzymes was 5.28% in statin users and 3.86% in non-users (p = 0.6723). Multivariate analysis showed that statin use was not independently associated with elevated liver enzymes (OR, 1.31, 95% CI, 0.31-5.53) (Table 3).
Odds ratios (OR) and 95 confidence intervals (CI) of elevated liver enzyme, by statin use and other risk factors at baseline, NHANES III (1988-1994).
| Characteristics | Elevated liver enzyme | P-value |
|---|---|---|
| Statins | 1.31 (0.31-5.53) | 0.7149 |
| Use other lipid lowering agents Age | 1.37 (0.51-3.71) | 0.5295 |
| < 45 | 1.00 | |
| 45-54 | 0.92 (0.50- 1.68) | 0.7745 |
| 55-64 | 0.64 (0.37-1.10) | 0.1075 |
| 65-74 | 0.70 (0.38-1.32) | 0.2687 |
| Male | 1.42 (0.96-2.10) | 0.0774 |
| Race/ethnicity | ||
| Non-Hispanic white | 1.00 | |
| Non-Hispanic black | 1.08 (0.75-1.57) | 0.6669 |
| Mexican American | 2.02 (1.37-2.98) | 0.0005 |
| Other | 1.47 (0.79-2.74) | 0.2216 |
| Obesity | 1.02 (0.68-1.54) | 0.9130 |
| Diabetes or insulin resistance | 1.84 (1.13-2.99) | 0.0152 |
| Hypertension | 1.59 (0.92-2.73) | 0.0950 |
| Cancer | 0.64 (0.25-1.60) | 0.3321 |
The other groups of people who were at higher risk of elevated liver enzymes include Mexican Americans compared to non-Hispanic whites (OR, 2.02, 95% CI, 1.37-2.98) and people with diabetes/ insulin resistance (OR, 1.84, 95% CI, 1.13-2.99) (Table 3).
DiscussionThis study shows that statin use is not associated with liver-related mortality. Additionally, our data suggested that statin use was not independently associated with elevated liver enzymes which have been used as “urrogate markers”for hepatotoxicity. These results support the growing evidence that statin-induced severe hepatotoxicity is rare and their use should not be limited due to the fear for adverse liver-related outcomes.10 In fact, presence of elevated liver enzymes in Mexican Americans and patients with DM and IR suggests that the majority of causes of liver enzyme elevations are related to underlying NAFLD, rather than drug hepatotoxicity from statins.19 This data confirms the recommendation of the Expert Panel and studies from tertiary care centers that statins are generally well-tolerated and safe.9,12,13 Although there are cases of severe hepatotoxicity related to statins,20–22 our analysis suggests that statins are not associated with increased mortality. In fact, increase in the unadjusted risk of cardiovascular mortality in the statin users seems to be primarily due to the underlying conditions associated with dyslipidemia rather than the statin use itself.
The main limitation of this study was the relatively small number of users of statins which prevented us from assessing the independent factors associated with liver related mortality in statin users. Nevertheless, the long term mortality follow up of a population based cohort with in-depth clinical and laboratory data makes the study unique. Another limitation of this study is that there was no causality assessment in cases of statin-induced liver disease using the CIOMS scale.20–22
In Summary, our data suggests that statins are not associated with liver related mortality or elevation of liver enzymes. Nevertheless, in a clinical setting, rare cases of hepatotoxcity from statins can occur and clinicians must individualize their plans for monitoring and assessment of their patients accordingly.
Conflicts of InterestThere are no conflicts of interest for any of the authors.