Background and rationale. The control of Endothelin-1 (ET-1)-mediated intrahepatic vasoconstriction in cirrhosis is beneficial for the alleviation of relevant complications. Cirrhosis is accompanied by hypogonadism and altered sex hormone status. Besides- sex hormones have vasoactive effects- but it is unknown if they influence vascular function in cirrhosis. This study aimed to investigate the roles of sex hormones in hepatic vascular reactions to ET-1 in cirrhosis. Liver cirrhosis was induced in Spraque-Dawley male and female rats with common bile duct ligation (BDL). Sham-operated (Sham) rats were controls. On the 43rd day after operations- intrahepatic vascular concentration-response curves to ET-1 were obtained with the following preincubatioins: 1) vehicle; 2) 17β-estradiol; 3) progesterone; 4) testosterone. Livers from sham and BDL rats were dissected for real-time polymerase chain reaction analysis of estrogen- progesterone and testosterone receptors.
Results. Compared with sham males perfused with vehicle- sham females presented higher perfusion pressure changes to ET-1 which was reversed only by 17β-estradiol. In cirrhosis- compared with males- 17β-estradiol no longer attenuated vascular responsiveness to ET-1 in females. In females- BDL rats had lower hepatic estrogen receptor α(ERα) mRNA expression than that in sham rats.
Conclusions. The sham females showed a stronger intrahepatic vascular constrictive effect to ET-1 than sham males- which could be reversed by 17β-estradiol. However- the influence of 17β-estradiol was lost in cirrhotic females- which may be attributed- at least partly- to intrahepatic ERa down-regulation in females with cirrhosis.
Cirrhosis of liver increases intrahepatic resistance through sinusoidal fibrosis and compression by regenerative nodules (fixed component) and to vasoconstriction (functional component). The increased hepatic resistance accompanied by an increased portal inflow maintains the portal hypertensive status.1 In order to divert the heightened portal inflow and pressure, the portal-systemic collaterals develop gradually. After that, many severe complications supervene, including gastroesophageal hemorrhage and hepatic encephalopathy. The strategy of pharmacotherapy relies mainly on lowering portal inflow, portal pressure and/or inducing collateral vasoconstriction.2 Therefore, to reduce intrahepatic vascular resistance which subsequently ameliorate portal hypertension is a feasible way to control complications related to portal hypertension.
Endothelin-1 (ET-1), a potent endotheliumderived vasoactive peptide that plays a pivotal role in regulating vascular tone, has drawn much attention in its role in cirrhosis and portal hypertension.3 The plasma levels of endothelin are increased in cirrhosis, and correlate well with the severity of liver disease and portal pressure.4 On the other hand, administration of endothelin antagonist reduces portal pressure.5 Furthermore, ET-1 directly induced vasoconstriction on portal-systemic collateral circulation in portal hypertensive rats,6 suggesting the role of ET-1 in regulating portal pressure via its modulation of both intrahepatic and collateral resistances.
Hypogonadism and feminization as well as increased circulatory concentrations of estradiol and decreased concentrations of testosterone have been noted in cirrhotic patients.7,8 It has been reported that hypothalamic-pituitary dysfunction,9 changes in sex hormone binding proteins, hepatic sex hormone receptors, or in sex hormone metabolism may contribute to these derangements.10 In humans with cirrhosis, there is also an increased conversion of weak androgens to estrogens.11 The increased conversion of testosterone to estradiol in rats with portal vein bypass has been demonstrated as well.12
Numerous studies have established that gender and sex hormones are associated with the cardiovascular and cerebral events. For instance, coronary artery disease and hypertension occur more frequent in men and postmenopausal women than premenopausal women.13 A stepwise decrease of cerebrovascular reactivity from the 4th to 5th decades in women further suggests a possible influence of estrogen.14 Additionally, intravenous estradiol administration in postmenopausal women elicits an increase in coronary blood flow and cross-sectional area and a decrease in coronary artery resistance.15 In fact, endothelial cells from female rat aorta release more nitric oxide (NO) than those from male aorta.16 Furthermore, generation of superoxide anions, which increases NO metabolism, is also greater in aortas from male than in those from female rats,17 which may additionally explain the differences in the endothelium-dependent responses observed between male and female animals.16
Although much evidence indicates the altered vascular reactivity to vasoactive mediators associated with gender and sex hormones, the corresponding influences in the intrahepatic vascular bed of cirrhosis have not been explored. Since the hepatic vascular responsiveness to vasoactive agents and resistance play pivotal roles in portal pressure modulation, this study was thus undertaken to investigate their participation on the hepatic vascular reactions to ET-1 in sham and common bile duct ligation (BDL)-induced cirrhotic rats.
Material and MethodsAnimal modelMale Sprague-Dawley rats weighing 240-270 g at the time of surgery were used for experiments. The rats were housed in plastic cage and allowed free access to food and water. All rats were fasted for 12 h before the operation. Under ketamine anesthesia (100 mg/kg, intramuscularly), rats with secondary biliary cirrhosis were induced by BDL.18 The common bile duct was exposed through a midline abdominal incision. The common bile duct was catheterized by a PE10 catheter and doubly ligated with 3-0 silk. The first ligature was made below the junction of the hepatic ducts and the second ligature above the entrance of the pancreatic duct. Then 10% formalin (~100 μ1/100 g body weight) was slowly injected into the biliary tree to prevent the subsequent dilatation of the ligated residual bile duct. The PE-10 catheter was then removed and the ligatures tightened, followed by section of the common bile duct between the ligatures. The incision was then closed and the animal allowed recovering. A high yield of secondary biliary cirrhosis was noted five weeks after the ligation.18,19 To avoid the coagulation defects, BDL rats received weekly vitamin K injection (50 μg/kg intramuscularly).19 This study had been approved by Taipei Veterans General Hospital Animal Committee. All animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86-23 revised 1985).
Systemic and portal hemodynamic measurementsThe right femoral artery of rats was cannulated with a PE-50 catheter that was connected to a Spectramed DTX transducer (Spectramed Inc., Oxnard, CA, USA). Continuous recordings of mean arterial pressure (MAP), heart rate (HR) and portal pressure (PP) were performed on a multi-channel recorder (model RS 3400, Gould Inc., Cupertino, CA, USA). The mesenteric vein was cannulated with a PE-50 catheter connected to a Spectramed DTX transducer. The abdominal cavity was closed and the PP was recorded on a Gould model RS 3400 recorder.20
In situ perfusion of liverThe in situ liver perfusion is a modification of the technique reported by Mittal, et al.21The liver was perfused in situ via portal vein, using a non-recirculating system. Briefly, the abdomen was opened and the portal vein was then cannulated with a 16-gauge Teflon catheter. The temperature around the perfusion area was maintained at approximately 37 ± 0.5 °C. The liver was immediately perfused with Krebs solution. The perfusate was equilibrated with carbogen gas (95% O2-5% CO2) by a silastic membrane lung.22 Thereafter, the thorax was opened and the supra-diaphragmatic part of inferior vena cava (IVC) was cut to ensure an adequate outflow without any resistance. The liver was thus perfused with a constant flow rate of 40 mL/min through the portal vein to determine the cumulative concentration-response curves. To monitor and record continuously the pressure in the intrahepatic vasculature, a Spectramed DTX transducer attached to the Gould model RS 3400 recorder was connected to a side arm placed just proximal to the perfusion cannula.
The cumulative concentration-response curves of intrahepatic vessels were determined by using final concentration of 10−10, 10−9, 3 x 10−9, 10−8, 3 x 10−8 and 10−7M of ET-1 in the perfusate. After testing experimental agents, the contracting capability of the intrahepatic vessels were challenged with a 125 mM KCl solution at the end of experiments.
RNA extraction and real-time quantitative PCRLivers were isolated, cut, and stored in −80 °C until further analysis. Total RNA was extracted with the RNeasy Mini Kit (Qiagen GmbH, Hilden, Germany). One microgram of total RNA was reverse-transcribed with Superscript II reverse transcriptase and poly dT priming (Life Technologies, Rockville, MD). Quantitative RT-PCR was carried out on a LightCycler (LightCycler 480, Roche Diagnostics, Mannheim, Germany). The primers are:
- •
Hypoxanthine phosphoribosyl-transferase (HPRT): 5’-CTCATGGACTGATTATGGACAGGAC3’(sense), 5’-GCAGGTCAGCAAAGAACTTAT AGCC-3’(antisense).
- •
ERα: 5’-CTGACAATCGACGCCAGAA-3’(sense) 5’-CAGCCTTCACAGGACCAGAC-3’ (antisense). 5’-CtTGCCCACTTGGAAACATC-3’ (sense). 5’-CCAAAGGTTGATTTTATGGCC-3’ (antisense).
- •
PR-A+B: 5’-CTTTGTTTCCTCTGCAAAAATTG-3’ (sense). 5’-GTATACACGTAAGGCTTTCAGAAGG-3’ (antisense).
- •
PR-B: 5’-CAGACCAACCTGCAACCAGAA-3’ (sense). 5’-AGTCCTCACCAAAACCCTGGG-3’ (antisense).
- •
AR: ’-ACCCTCCCATGGCACATTTT-3’ (sense). 5’-TTGGTTGGCACACAGCACAG-3’ (antisense).23,24
They are based on rat mRNA sequences (Gen-Bank Accession No. Y00102, U57439, L16922, M20133 and U06637 respectively). The first segment of the amplification cycle consisted of a dena-turation program. The second consisted of denaturation, primer annealing, elongation and quantification program repeated for 40 cycles. The third consisted of a melting curve program. The final consisted of a cooling program. Abundance of mRNA was determined by real-time RT-PCR normalized to abundance of HPRT mRNA. LightCycler analysis software (Roche Diagnostics, Mannheim, Germany) allowed the quantitative analysis.
Study protocolOn the 43rd day after sham or BDL surgeries, BW, MAP, PP, and HR were measured. After that, concentration-response curves of intrahepatic vascular bed to ET-1 of male or female, sham or cirrhotic rats were obtained with the liver incubated in the following agents since 30 min prior to ET-1 administration until the end of the perfusion study:
- •
Vehicle.
- •
Estradiol (1 μM).
- •
Progesterone (1 μM).
- •
Testosterone (1 μM).
In another parallel groups, livers from sham and BDL rats were dissected for real-time PCR analysis of estrogen (ERα, ERβ), progesterone (PRA+B, PR-B) or androgen receptors (AR) mRNA expressions. Baseline perfusion pressures (baseline PP) before the start of perfusion experiments were also measured.
DrugsEndothelin-1, 17-β estradiol, progesterone, testosterone and the reagents for preparing Krebs solution were purchased from Sigma (Sigma Chemical Co, St. Louis, MO, USA). All the solutions were freshly prepared on the days of experiment.
Data analysisThe results are expressed as mean ± SEM. The changes in perfusion pressure (mmHg) over baseline were calculated for each concentration in each preparation. Statistical analyses were performed using the unpaired Student’s t-test, one-way ANOVA or two-way repeated measures ANOVA as appropriate. Results are considered statistically significant at a two-tailed P value less than 0.05.
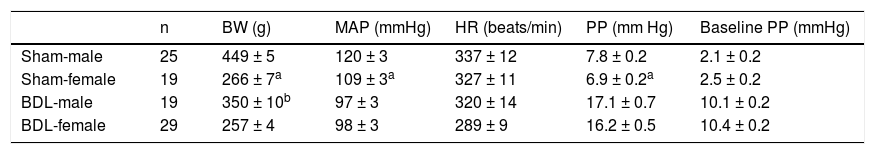
ResultsBaseline hemodynamicsTable 1 depicts the BW, MAP, PP, HR and baseline PP in females or males underwent sham or BDL surgery. In sham-operated rats, the female sham rats showed lower BW, MAP and PP than those of the male rats. The baseline PP was not significantly different between the two groups. In BDL rats, there was no significant difference in MAP, PP, HR and baseline PP except that female BDL rats had lower body weights than male BDL rats.
Body weight and baseline hemodynamics of sham-operated and bile duct ligated rats.
| n | BW (g) | MAP (mmHg) | HR (beats/min) | PP (mm Hg) | Baseline PP (mmHg) | |
|---|---|---|---|---|---|---|
| Sham-male | 25 | 449 ± 5 | 120 ± 3 | 337 ± 12 | 7.8 ± 0.2 | 2.1 ± 0.2 |
| Sham-female | 19 | 266 ± 7a | 109 ± 3a | 327 ± 11 | 6.9 ± 0.2a | 2.5 ± 0.2 |
| BDL-male | 19 | 350 ± 10b | 97 ± 3 | 320 ± 14 | 17.1 ± 0.7 | 10.1 ± 0.2 |
| BDL-female | 29 | 257 ± 4 | 98 ± 3 | 289 ± 9 | 16.2 ± 0.5 | 10.4 ± 0.2 |
BW: body weight. MAP: mean arterial pressure. HR: heart rate. PP: portal pressure. Baseline PP: baseline perfusion pressure. BDL: bile duct ligation.
Before the perfusion experiments, baseline BW and hemodynamic parameters were not significantly different among sham or BDL rats receiving vehicle, 17β-estradiol, progesterone or testosterone perfusion (P > 0.05).
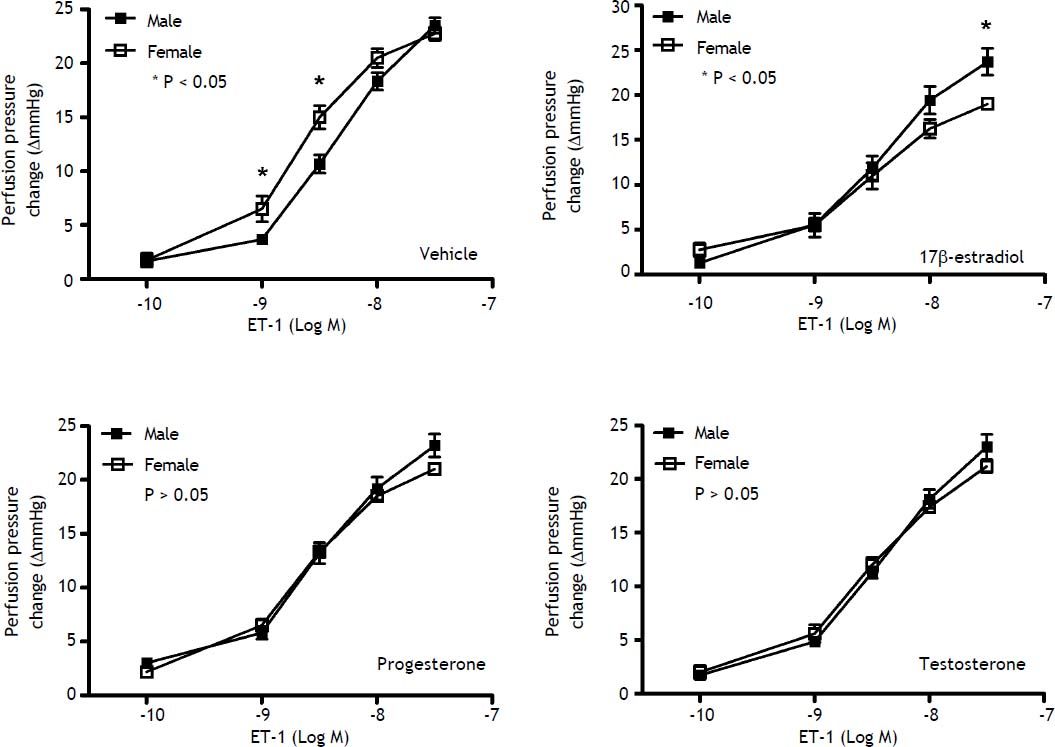
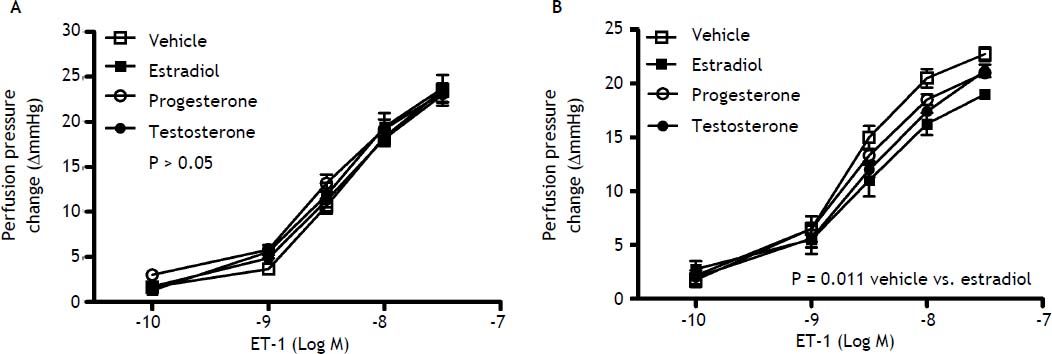
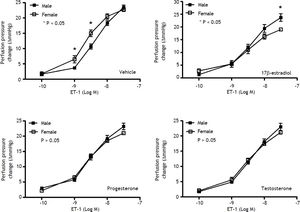
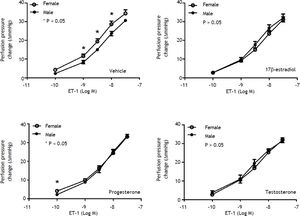
Effects of sex and sex hormones on intrahepatic vascular concentration-response relationships to ET-1 in sham ratsWhen preincubated with vehicle, the responses to ET-1 were higher in female rats at the concentrations of 10−9 M (6.5 ± 1.2 vs. 3.7 ± 0.3 mmHg, P = 0.025) and 3 x 10−9 M (15.0 ± 1.1 vs. 10.7 ± 0.8 mmHg, P = 0.013) whereas 17β-estradiol preincubation elicited lower perfusion pressure changes to ET-1 at the concentrations of 3x10−8 M (19.0 ± 0.4 vs. 23.7 ± 1.5 mmHg, P = 0.02) in female rats compared with those in male rats (Figure 1). The perfusion pressure changes caused by testosterone and progesterone were not significantly different between male and female rats (P > 0.05) (Figure 1). Two-way repeated measures ANOVA shows that compared with vehicle preincubation, 17β-estradiol elicited a lower perfusion pressure changes to ET-1 in female shamrats (P = 0.011) (Figure 2B). On the other hand, there was no significant difference of perfusion pressure changes in male sham rats perfused with vehicle, 17β-estradiol, progesterone, or testosterone (P > 0.05) (Figure 2A).
Concentration-response curves to ET-1 in intrahepatic vascular beds of male vs. female sham-operated rats pre-in-cubated with vehicle, testosterone, progesterone or 17β-estradiol, expressed as absolute increase over baseline value. Female rats pre-incubated with vehicle showed a stronger vasoconstrictive response to ET-1 but a weaker response to ET-1 when being pre-incubated with 17β-estradiol (* indicates a P < 0.05).
Con1centration-response curves to ET-1 in intrahepatic vascular beds of sham-operated male (A) and female (B) rats pre-incubated with vehicle, testosterone, progesterone or 17β-estradiol, expressed as absolute increase over baseline value. As compared with vehicle, 17β-estradiol perfusion elicited lower perfusion pressure changes to ET-1 in female rats.
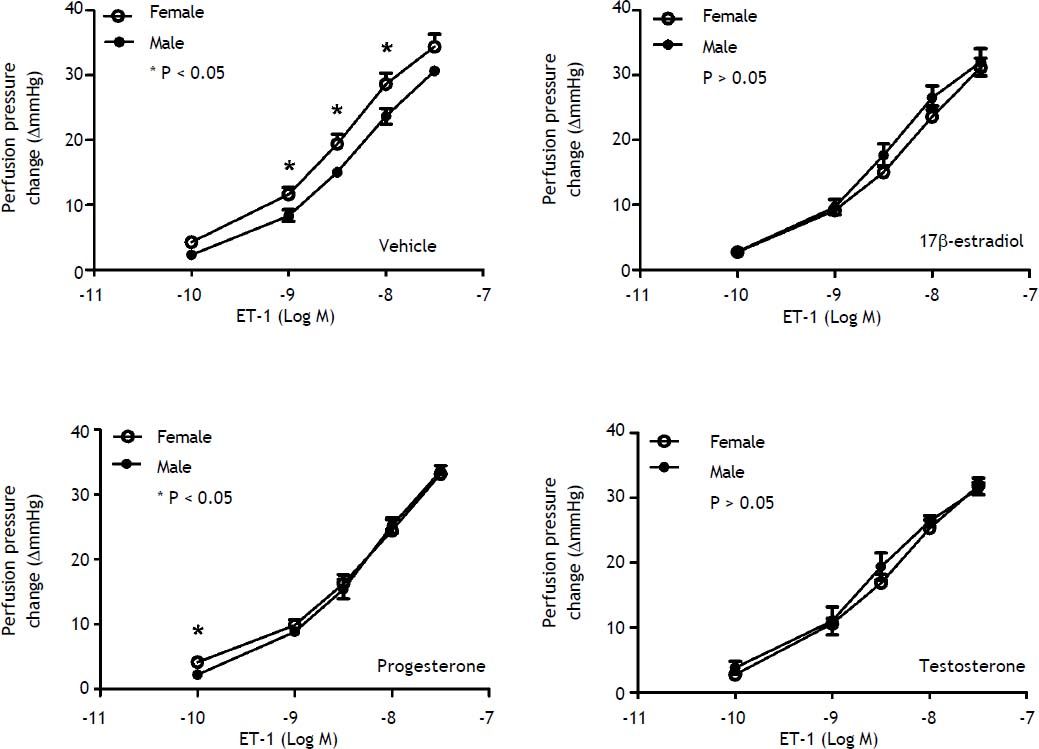
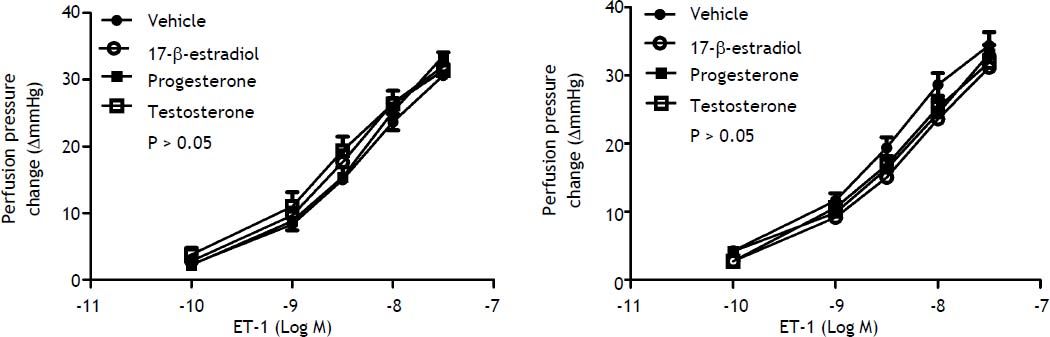
When preincubated with vehicle, the responses to ET-1 were higher in female cirrhotic rats at the concentrations of 10−9 M (11.6 ± 1.1 vs. 8.3 ± 0.9 mmHg, P = 0.046) and 3 x 10-9 M (19.4 ± 1.5 vs. 15 ± 0.6 mmHg, P = 0.025) and 10-8 M (28.6 ± 1.7 vs. 23.7 ± 1.2 mmHg, P = 0.042). 17β-estradiol preincubation abrogated the differences that the perfusion pressure changes were not significantly different between female and male cirrhotic rats (P > 0.05) (Figure 3). Vehicle, 17β-estradiol, progesterone and testosterone did not induce significantly different hepatic perfusion pressure changes to ET-1 in either male (P > 0.05) (Figure 4A) and female (P > 0.05) (Figure 4B) cirrhotic rats.
Concentration-response curves to ET-1 in intrahepatic vascular beds of male vs. female BDL rats pre-incubated with vehicle, testosterone, progesterone or 17β-estradiol, expressed as absolute increase over baseline value. Female rats pre-incubated with vehicle showed a stronger vasoconstrictive response to ET-1 but not significantly modified by 17β-estradiol (*indicates a P < 0.05).
Concentration-response curves to ET-1 in intrahepatic vascular beds of cirrhotic male (A) and female (B) rats preincubated with vehicle, testosterone, progesterone or 17β-estradiol, expressed as absolute increase over baseline value. There was no significant difference among these groups.
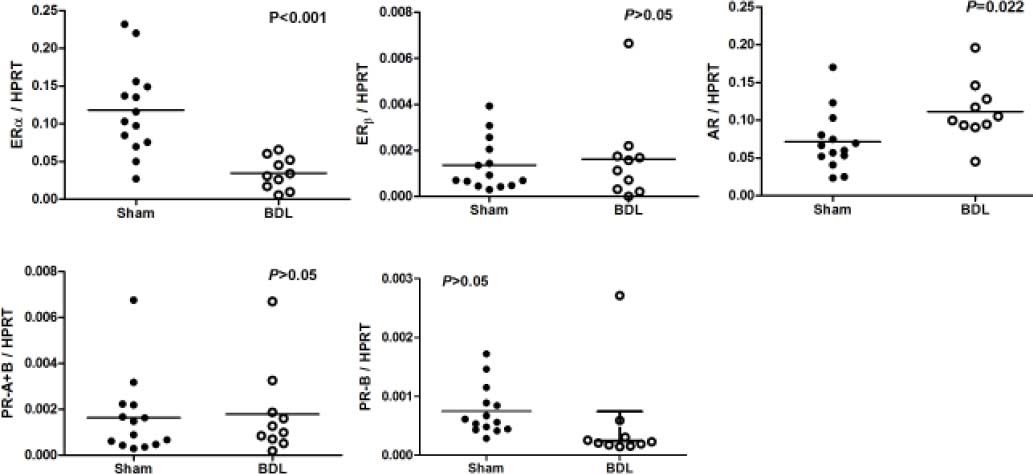
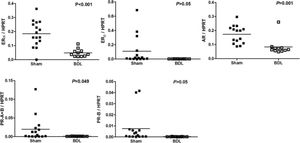
Figure 5 reveals the ERa, ERb, AR, PR-A+B and PR-B of female rats underwent sham or BDL surgeries. Female rats with BDL-induced cirrhosis had significantly lower hepatic mRNA expression of ERα (ERα/HPRT: 0.0347 ± 0.0066 vs. 0.1180 ± 0.0158, P < 0.001 ) and higher expression of AR (AR/HPRT: 0.1115 ± 0.0126 vs. 0.0712 ± 0.0105, P = 0.022).
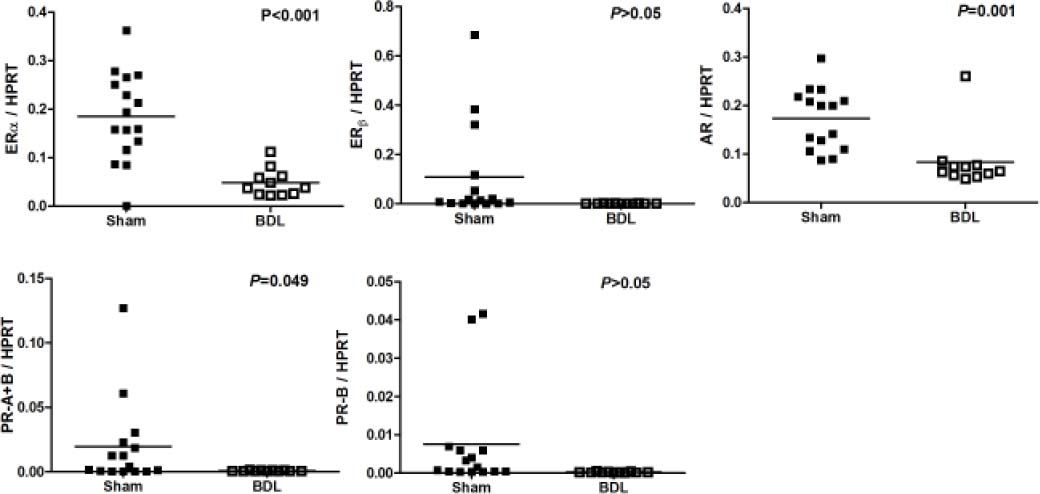
Figure 6 depicted the ERα, ERβ, AR, PR-A+B and PR-B of male rats underwent sham or BDL surgeries. Male rats with BDL-induced cirrhosis had significantly lower hepatic mRNA expression of ERα (ERα/HPRT: 0.0485 ± 0.0086 vs. 0.1971 ± 0.0204, P < 0.001), AR (AR/HPRT: 0.0834 ± 0.0180 vs. 0.1731 ± 0.0163, P = 0.001), and PR-A+B (PR-A+B/ HPRT: 0.0007 ± 0.0001 vs. 0.0197 ± 0.0088, P = 0.049).
DiscussionIn this study, female sham rats had significantly lower MAP and PP than those of the male rats. Compatible with our current finding, Khoury and colleagues found that men had higher blood pressure than did women by ambulatory blood pressure monitoring on 131 men and women.25 In an animal study, male spontaneously hypertensive rats (SHR) have higher blood pressure than do females of similar ages.26 Androgens, such as testosterone, have been implicated in the mechanism. Interestingly, the baseline hepatic perfusion pressure was similar between male and female sham rats. Since we used fixed flow rate perfusion system, this indicates that the intrahepatic resistance was not influenced by gender. Furthermore, PP is the net effect exerted mainly by portal inflow and hepatic resistance, so the lower PP in females may be related to systemic hypotension with reduced portal inflow. On the other hand, in cirrhotic rats, the hemodynamic parameters were not statistically different between females and males. Liver cirrhosis has been characterized by hyperdynamic circulation with systemic arterial hypotension. Our result implies that this overwhelming phenomenon may not be overcome by the potential influence of gender on hemodynamics.27
It has been noted that estradiol administration to isolated vascular tissue for 30 minutes attenuated large coronary arterial contractions to ET-1.28 Sudhir, et al. found that intracoronary estradiol reduced the vasoconstrictor response to intracoronary ET-1 in pigs.29 The attenuating effects of estradiol on ET-1-induced vasoconstriction in the portal venous system following trauma-hemorrhage in the rats have also been demonstrated.30 Regarding the relevant studies on liver, estrogen receptors have been identified in rat liver sinusoidal endothelial cells (SEC), supporting the ability of estradiol in regulating intrahepatic microcirculation.31
In the current study, female rats, either sham or cirrhotic, showed higher intrahepatic vascular responsiveness to ET-1 than male rats. Regarding the gender-related vascular reactions to ET-1, regional differences should be considered. It has been indicated that the hindquarter vasoconstrictor effects elicited by ET-1 were significantly higher in male than female rats.32 On the other hand, compatible with our results, femoral and brachial arteries from female pigs developed greater contractile force in response to ET-1 than those from male pigs.33 The similar finding was found in coronary arteries of pigs and the difference was not related to estrogen levels in female pigs, which is probably due to differences in the regulation of intracellular calcium.34 Barber, et al. have also reported that coronary arteries from female pigs generate more forceful contractions in response to ET-1 compared with male pigs and that this difference was mediated by increased affinity of the endothelin receptors in coronary vascular smooth muscle of female pigs.35 However, Jiang, et al. did not detect a difference of rabbit coronary arterial responses to ET-1 from male and nonpregnant female rabbits unless estradiol was added to the organ chamber.28 The vasoconstriction increment made by ET-1 on rat tail artery segments was also similar in both genders.36 Although the different conclusions might result from different experimental models in different species and vascular beds, further exploration for the mechanism in intrahepatic circulation is required.
The stronger intrahepatic vascular constrictive effect to ET-1 in sham female rats could be reversed by 17β-estradiol preincubation. However, in BDL-induced cirrhosis, 17β-estradiol only abrogated the difference in ET-1 responses between male and female rats. Furthermore, although 17β-estradiol induced lower perfusion pressure changes than vehicle, progesterone or testosterone in sham female rats, this was not found in female cirrhotic rats, suggesting a poorer modulatory role of estradiol in cirrhosis. This may be ascribed to intrahepatic ER« down-regulation in cirrhotic females as compared with sham females. Estrogens themselves regulate the ER expression. In cells with high ER content, estradiol caused ER down-regulation.37 In fact, increased circulatory concentrations of estradiol have been noted in cirrhotic patients.7,8 In humans with cirrhosis, there is also an increased conversion of weak androgens to estrogens,11 which might be responsible for, at least partly, the ERa down-regulation in cirrhotic liver. In fact, we also found a significant down-regulation of intrahepatic ERa expression in cirrhotic males, although 17β-estradiol had failed to exert changes in vascular responsiveness in sham males.
The vascular action characteristic of progesterone is relatively indefinite. In isolated vessels, progesterone reverses the beneficial effects of estrogens.38 In the study reported by Lamping, et al.,39 progesterone alleviates the vasoconstrictive effect of ET-1, but is weaker than that of estradiol. In this study, progesterone did not induce different hepatic perfusion pressure changes to ET-1 in either male or female, sham or cirrhotic rats and irrespective of intrahepatic PR expressions. The influence of progesterone in intrahepatic circulation may be negligible.
The mechanism of the vascular effects of testosterone seems rather complex. Testosterone relaxed coronary microvessels preconstricted with ET-1 similarly to estradiol and progesterone whereas it had no direct effect on the dose-response curves to ET-1.39 Furthermore, relaxation of coronary microvessels to testosterone was not mediated by release of vasodilator prostaglandins or NO.39 Studies of the testosterone effect on rabbit coronary arteries suggest that it is independent of endothelium and is not mediated by stimulation of guanylate cyclase.40 Yue, et al. had also found an acute vasodilatory effect of testosterone in the rabbit aorta and coronary artery in vitro that is endothelium independent and may involve activation of vascular smooth muscle K+ channels.40 In contrast, others demonstrated that testosterone induced acute vasorelaxation that is gender and androgen receptor independent and involves both endothelium-dependent (via NO) and -independent mechanisms of action.41 Similar endothelium-dependent (via NO) and -independent vasodilatory effects of testosterone on canine coronary conductance and resistance arteries in vivo have also been reported.42 In the current study, testosterone did not induce different hepatic perfusion pressure changes to ET-1 in either male or female, sham or cirrhotic rats and was irrespective to intrahepatic TR expressions. Likewise, testosterone may not elicit significant impacts on intrahepatic circulation.
To sum up, the sham and cirrhotic female rats exerted a stronger intrahepatic vascular constrictive effect to ET-1 than their male counterparts, which was reversed by 17β-estradiol in sham but not in cirrhotic females. The reduced intrahepatic vascular response to 17β-estradiol in sham females was also abrogated in cirrhosis. This may be attributed to the down-regulation of intrahepatic ERα expression in cirrhosis.
AcknowledgementsWe thank Chen Yi-Chou for his excellent technical assistance. This study was supported by the grant from the National Science Council, Taiwan (96-2314-B-075-034-MY3). The experiments were assisted in part by the Animal Center of Department of Medical Research and Education at Taipei Veterans General Hospital.
Abbreviations- •
AR: androgen receptor.
- •
BDL: common bile duct ligation.
- •
ER: estrogen receptor.
- •
ET-1: endothelin-1.
- •
HR: heart rate.
- •
IVC: inferior vena cava.
- •
NO: nitric oxide.
- •
MAP: mean arterial pressure.
- •
PP: portal pressure.
- •
PR: progesterone receptor.