Despite the public concern about the controversial use and abuse of marijuana, the scientific community has focused on the therapeutic potentials of cannabinoid compounds and had highlighted the importance of endocannabinoids and their receptors in physiology and disease. Endocannabinoids have been shown to be important mediators in neuroendocrine and psychiatric processes such as food intake, drug reward and energy metabolism. Cannabinoid receptors are expressed by several cell lines in the liver, such as hepatocytes, myofibroblastic cells, endothelial cells and probably cholangiocytes. A perpetuating role in liver damage for the endocannabinoid system has been proposed in several steps of chronic liver disease progression. Being a major cause of death worldwide, chronic liver disease is an important problem. New therapies are needed in order to stop or slow damage progression. This review summarizes the results of experimental studies involving the endocannabinoid system in liver disease and their clinical and therapeutical implications in hepatology.
Chronic liver disease (CLD) is the fourth cause of death among the productive Mexican population, and the projections based on mortality trends, seems uneasy.1 The main etiologies of liver cirrhosis in Mexico are alcohol consumption and chronic hepatitis C viral infection (HCV).2 According to population-based studies, 2% of Mexican adults carries chronic HCV infection.3 Another major cause of CLD is cryptogenic cirrhosis; however, after the recognition of non-alcoholic steatohepatitis4 as a progressive lesion leading to liver fibrosis,5,6 cryptogenic cirrhosis is now an uncommon diagnosis.
CLD carries a high economical burden for health care systems,5 due to its high hospitalization needs, lost days of work, and long-term treatments like antiviral treatments and transplantation.
Understanding the mechanisms associated with damage progression and liver fibrosis is imperative, and represents a major challenge in modern hepatology. There are recent reports regarding an endogenous cannabinoid system involved in fat deposition, apoptosis, fibrogenesis and CLD complications. High endocannabinoid levels in situ has been demonstrated in hepatic steatosis,6 acute and chronic hepatitis,7 and cirrhosis.8 The aim of this review is to summarize current literature on the experimental and clinical studies involving the endocannabinoid system in CLD and its complications.
Endocannabinoid system, the basicsEndocannabinoids are endogenous lipid compounds capable of binding and activating Δ9-tetrahidrocannabinol receptors.11,12 The first cannabinoid receptor recognized CB1, was cloned in 1990 from rat brain9 and in 1993, Munro et al,10 characterized a peripheral cannabinoid receptor, CB2, from promyelocytic leukemia cells. CB1 is distributed across the central nervous system in areas associated with motor control, cognition, emotional response and feeding behavior.15 CB1 is also expressed peripherally in endothelium,16 adipose tissue,17 gut18 and the liver;8 while CB2 is expressed by several leukocyte lines in peripheral blood,19 spleen, tonsils, Peyer’s patches, lymphatic ganglia,20 microglia21 and hepatic myofibroblastic cells.22 There is only a 44% affinity between CB1 and CB2, and their signaling pathways and functions are different,23 sometimes even antagonic.
Devane et al.24 isolated in 1992, the first endogenous cannabinoid ligand, arachydonoyl-ethanolamide, or anandamide, and Mechoulam et al.25 identified 2-arachy-donoyl-glycerol (2AG) in 1995, with much higher serum and brain concentrations than anandamide.
Anandamide is a weak CB1 and CB2 agonist23. Recently it has been established as an endogenous ligand for the ion-channel-type vanilloid receptor 1 (VR1).26 2AG is a strong agonist of both CB receptors. In the last decade, a number of other endocannabinoids has been isolated, all of them derived from the non-oxidative metabolism of arachydonate, like 2-arachydonoyl glyceryl ether (noladin),27 a selective CB1 agonist; O-arachy-donoyl ethanolamine (virodhamine),28 partial CB2 agonist and CB1 antagonist; and N-arachydonoyl dopamine (NADA),29 selective CB1 and VR1 agonist. The physiological relevance of noladin, virodhamine and NADA has not been well established.
Endocannabinnoids are not stored in vesicles or cells, like neurotransmitters, instead they are released upon demand from lipid precursors in the cytoplasmic membrane through enzyme activation.30
Anandamide is the product of arachydonate and phos-phoethanolamide. Arachydonate is released from cyto-plasmic membrane through enzymatic cleavage of N-arachydonoyl phosphatidilethanolamide by a Ca++-dependent N-acyl-transferase.31 Fatty acid-amide hydrolase (FAAH) rapidly inactivates anandamide, producing arachydonate and phosphoethanolamide. FAAH gene promoter is activated by leptin and progesterone and inhibited by estrogens and glucocorticoids. FAAH knockout mice show high serum anandamide levels with normal 2AG.32
2AG is produced by hydrolysis of a diacylglyceride containing arachydonate in glycerol position 2 released by a specific diacylglycerol lipase. Although 2AG can be inactivated by FAAH, the main catabolic pathway seems to be a specific monoacylglyceride lipase, producing arachydonate and glycerol.
Endocannabinoids also suffer an oxidative catabolism through lipooxygenases 12 and 15.33 12-OH-anandamide conserves its agonist capacity and 15-OH-anandamide is a FAAH inhibitor. 15-OH-2AG is a peroxisome proliferator activated receptor alpha (PPARα) agonist.34
Hepatic steatosisFat deposition is a common finding in several pathological conditions and is currently considered a cofactor in CLD.35 Hepatic steatosis is present in 90-100% of alcoholics,36 60-80% of obese subjects37 and 30-70% of chronic HCV cases.38-40 Fatty liver is associated with greater progression and fibrosis in HCV infection.41 The mechanisms leading to fat storage in these diverse situations have not been fully unraveled; however, the recent characterization of several adipokynes and novel members of the nuclear receptors family as mediators of lipid metabolism has lead to new insights into fatty liver development.
Metabolic disturbances are increasingly recognized in non-alcoholic fatty liver disease (NAFLD), and these alterations are also associated with worse progression of other CLD related to hepatic steatosis. Among other important factors, NAFLD results from poor food intake control and energy homeostasis.42 The endocannabinoid system plays a central role in both of these processes.15 Hypothalamic CB1 activation stimulates food intake,43 specially high energy containing and alcohol.44,45 Interestingly, CB1 knockout mice are resistant to obesity despite consuming the same amount of calories than wild-type mice.46 This suggests that endocannabinoids regulation in energy metabolism exceeds that of food intake. Based on these observations, Cota et al1 demonstrated CB1 expression in adipocytes and showed that its stimulation leads to lipolysis through upregulation of lipoprotein lipase.
Adiponectin is an adipocyte-derived insulin sensitizer47 and a tumoral necrosis factor alpha antagonist,48,49 it is currently considered a protective factor for the development of steatosis and steatohepatitis.50,51 There is indirect data suggesting that CB1 activation downregulates adiponectin production, as a CB1 antagonist SR141716 (rimonabant), increases its mRNA expression both in vitro (cultured adipocytes) and in vivo (adipose tissue from obese rats).52
CB1 knockout mice are resistant to diet-induced fatty liver.8 Wild-type mice with diet-induced fatty livers exhibit a 4-fold higher hepatic anandamide concentration than lean controls, apparently because their hepatic FAAH, the enzyme responsible for degradation of anandamide, is dramatically reduced.
Endocannabinoids activate CB1 receptor in cultured rat hepatocytes leading to overexpression of sterol regulatory element-binding protein 1c (SREBP-1c), increasing de novo fatty acid production rate.8 The increase in de novo lipogenesis in mice on high-fat diets can be inhibited by SR141716. SREBP-1c is membrane-associated protein that suffers a complex proteolytic cascade and activates sterol regulatory element sequences in acetyl-CoA carboxilase, fatty acid synthase, acyl-CoA synthase and steroyl-CoA desaturase genes, upregulating every major step in fatty acid synthesis and desaturation.53
Experimental overexpression of SREBP-1c in the liver leads to fatty liver,54 while experimental diabetes-related fatty liver exhibit SREBP-1c overexpression.55 Even after long periods, increased fatty acid synthesis can be maintained through hyperinsulinemia56 or alcohol consumption,57 since both of these factors directly activate SREBP-1c.
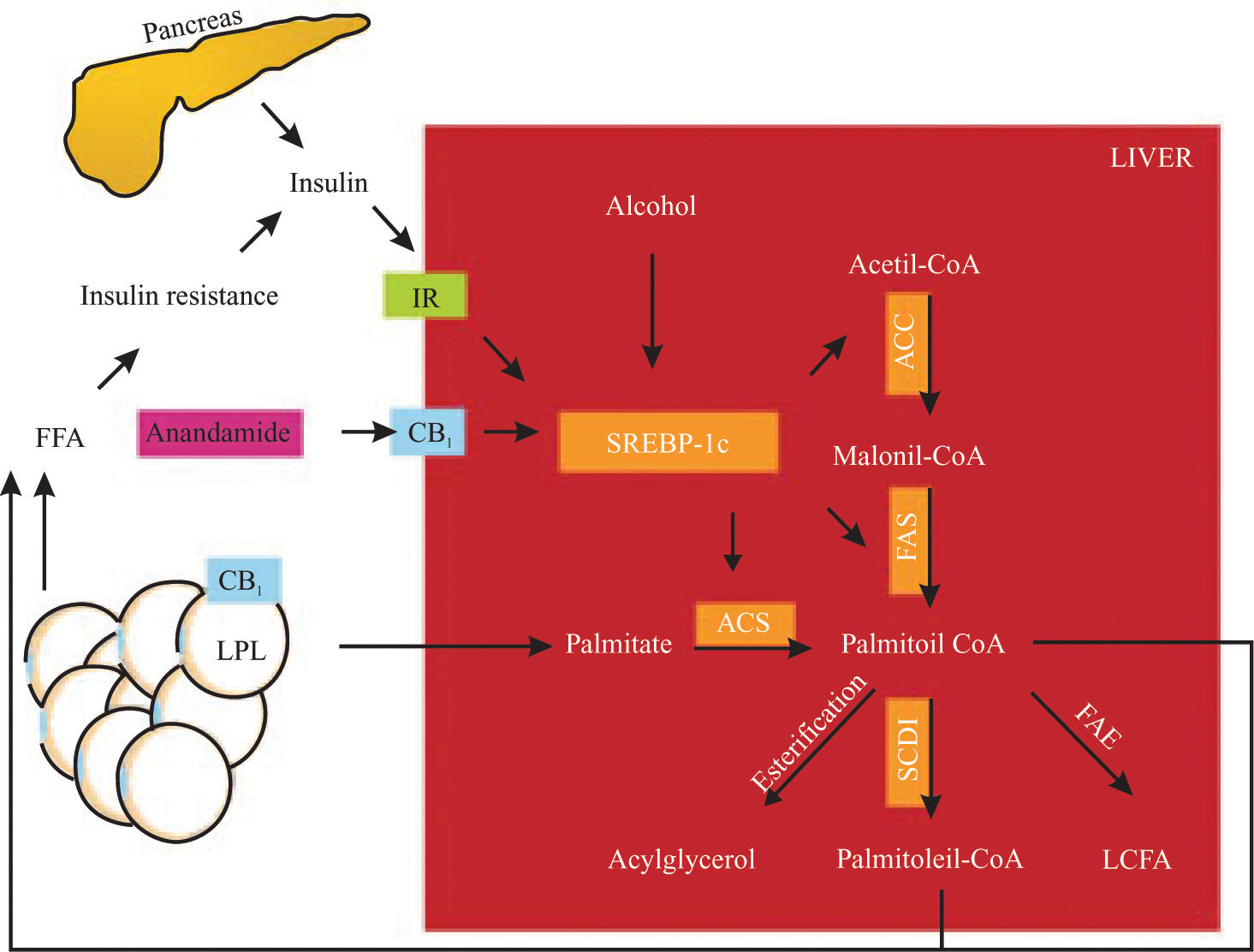
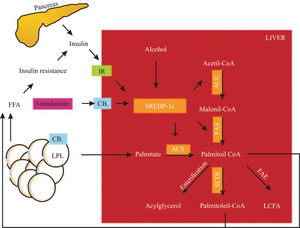
Endocannabinoids induces an elevation of free fatty acid through 1) lipolysis, 2) adiponectin downregulation in adipose tissue and 3) de novo fatty acid synthesis in the liver (Figure 1). This effect could be involved in the perpetuation of hepatic insulin resistance and fatty liver.58
SREBP-1c proteolysis is activated by insulin, alcohol and CB1 stimulation. SREBP-1c fragments upregulate the enzymes (remarked) involved in fatty acid synthesis (palmitate as an example). CB1 also activates LPL in adipose tissue, inducing lipolysis. These mechanisms raise non-esterified fatty acid in the liver and serum, increasing insulin resistance, producing hyperinsulinemia and upregulation of SREBP-1c in a vicious circle. IR, insulin receptor; CB1, cannabinoid receptor 1, LPL, lipoprotein lipase; SREBP-1c, sterol regulatory element-binding protein, ACC, acetyl-CoA carboxylase, FAS, fatty acid synthase; ACS, acyl-CoA synthase; SCD-1, steroyl-CoA desaturase 1; FAE, fatty acid elongase; LCFA, long chain fatty acid. Hepatic fatty acid synthesis and the role of CB1 activation.
Chronic damage to the liver results in fibrosis due to the accumulation of extracellular matrix proteins. This process is characteristic of most types of CLD. Advanced fibrosis leads to cirrhosis, liver failure, and portal hypertension and often requires liver transplantation. Once thought to be a passive and irreversible process, liver fibrosis is currently considered as a reversible wound-healing response to chronic damage. The study of the hepatic stellate cell (HSC) in the past decades have generated interest in potential interventions in the progression and reversion of liver fibrosis.59
HSC’s are located in perisinusoidal space and function as a major vitamin A store. HSC’s morphology and function are regulated by several stimuli, such as proinflammatory cytokines and growth factors, which activate HSC’s, inducing a phenotypical change into a collagen-producing, contractile cell.60
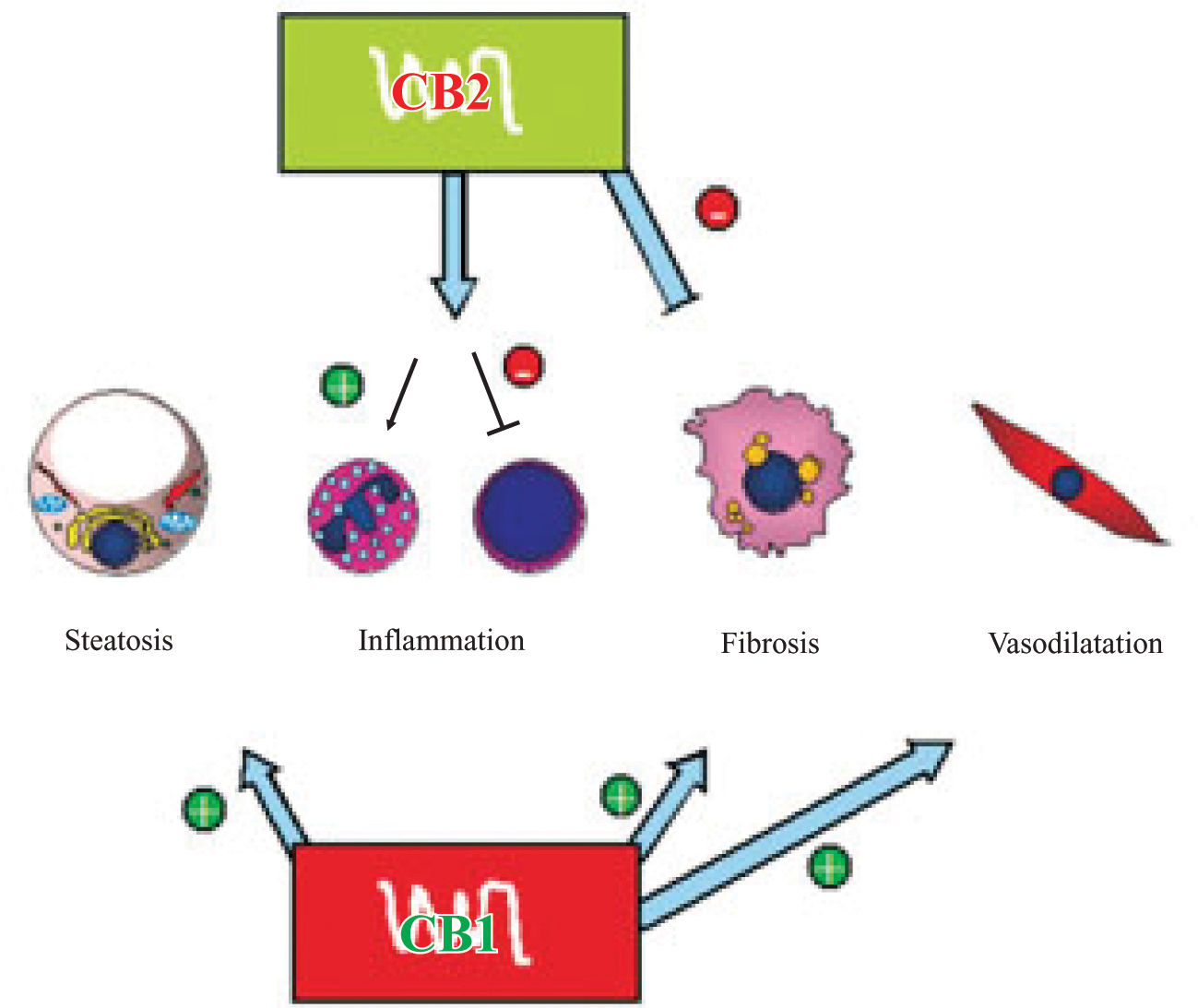
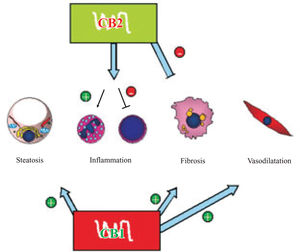
The endocannabinoid system might play an important role in liver fibrogenesis through of HSC’s activity, modulation (Figure 2). HSC’s express both CBj and CB2 receptors.61,62 CBj-knockout mice exhibit a reduced fibro-genic score after CCl4 administration with similar necro-inflammatory response than wild-type mice,61 while CB2-knockout mice shows an increased fibrogenic score and hydroxyproline content62 than wild-type mice after the same stimulation.
CB1 receptor stimulation induces fatty acid synthesis in hepatocytes and lipolysis in adipocytes, CB1 knockout mice are resistant to diet induced fatty liver. CB2 has a non-cleared role in inflammation, it upregulates IL-8 (neutrophil-activating cytokine) and downregulates TNF-α and IL-6 (T lymphocytes cytokines). CB1 and CB2 in activated hepatic stellate cells have antagonist roles in liver fibrogenesis, the former inducing and the latter inhibiting. Lastly, CB1 seems to have a role in the hyperdynamic state in portal hypertension through vascular receptors. Pathophysiological implications of endocannabinoids in chronic liver disease.
CB2 activation reduces collagen synthesis and DNA synthesis in HSC’s. Julien et al.62 showed that CB2 stimulation increased caspase 3-like activity and Siegmund et al.63 demonstrated that anandamide at high concentrations induces apoptosis in HSC’s in a non-CB, cholesterol membrane dependent manner. The same effect was previously shown in HepG2 cells by Biswas et al.,9 however, they did not analyze this in normal hepatocytes.
A recent human case-control study, among 270 consecutive untreated patients with chronic HCV infection of known duration undergoing liver biopsy, Hezode et al.64 studied the relationship between Cannabis smoking and histological findings. Half of the patients studied were non-smokers, a third were daily smokers and the rest occasional smokers. These investigators observed that older age at infection, hepatic steatosis and alcohol consumption were related with higher METAVIR fibrosis scores, and also that daily Cannabis smoking was an independent risk factor for higher fibrosis progression rate in multivariate analysis (OR = 3.4, 95% CI 1.5-7.4). These results highlight the role of cannabinoids in liver fibrosis, but raise an interesting question concerning the relevance of CB1 over CB2 activation under the same ligand availability; and the actual ability of endocannabinoids to induce fibrosis on activated HSC’s under concentrations reported in CLD.
End-stage liver disease complicationsPortal hypertensionPortal hypertension (PH) is one of the most common complications of end-stage liver disease (ESLD) due to mechanical and humoral factors.65 ESLD is also associated with arteriolar splanchnic vasodilatation and a hyper-dynamic circulatory state.66 Portal hypertension-related morbidity and mortality has capital importance in ESLD patients.
Anandamide worsens and SR141716 prevents lipopolysaccharide-induced hypotension during septic shock67 and hypotension in cirrhotic rats.68,69 Macrophages and platelets from cirrhotic patients has been shown to produce endocannabinoids and when administered to normal rats induce marked hypotension.67 These results had lead to the recognition of anandamide as a vasoactive component, and the endocannabinoids system has been postulated as mediators of circulatory hyperdynamic state in cirrhosis.
It should be noted that circulating anandamide levels are almost 2-fold increased during liver cirrhosis compared to normal controls.10 Being volatile compounds, this fact ensure closer examination. Endotoxemia is a well characterized feature of liver cirrhosis.70 Portosystemic shunts and decreased Kupffer’s cells clearance are thought to cause high lipopolysaccharide serum levels.71 Platelets and macrophages had been shown to release anandamide after stimulation with lipopolysaccharide through a CD14/MAPK/PI3K pathway.72 Platelet activating factor (PAF) is elevated in serum from cirrhotic patients,73 and PAF receptor is upregulated and overesponsive in their livers.74 PAF induces 2AG production in platelets and macrophages, but not of anandamide.75 Unlike anandamide, 2AG concentration is normal in situ and in serum during liver cirrhosis.
There is evidence for a role of endocannabinoids in the vasodilated state in cirrhosis. Although not the main feature of PH, mesenteric vasodilatation is a relevant issue in ESLD. Anandamide induces mesenteric vasodilatation in normal and cirrhotic rat isolated mesenteric arteries76-78 through CBj and/or VR1 activation,79 independent of sympathetic and nitric oxide activity, and probably in an endothelium-independent fashion. CBj is expressed by endothelial and adventitial cells in second and third degree mesenteric artery branches and perivascular sensory nerves innervating these arteries which also express VR1. The expression of CBj in adventitial cells is upregulated during liver cirrhosis and the response of isolated mesenteric arteries from cirrhotic rats to anandamide is increased.77
Only one clinical study had assessed the direct relationship between anandamide concentrations and portal hemodynamics. Fernandez-Rodriguez et al.10 found no differences in anandamide levels between cirrhotic patients with and without ascites. These researchers showed that circulating anandamide levels were elevated, but were not correlated with the severity of portal hypertension, the stage of hepatic or renal dysfunction, and the activity of vasopressor and antidiuretic mediators.
Cirrhotic cardiomyopathyCirrhotic cardiomyopathy is a ESLD complication associated with the pathogenesis of hepatorenal syndrome80 and heart failure after orthotopic liver transplantation, which causes 7-15% of postoperative deaths.81 Cirrhotic cardiomyopathy is characterized by high cardiac output with normal ventricular contractility at baseline but abnormal response to inotropic stimuli, like hemorrhage, exercise and drugs.82 Several mechanisms have been involved in the reduced cardiomyocyte response in liver cirrhosis, ranging from endotoxemia to altered b-adrenergic receptor signal transduction.83,84 There is only one experimental study that evaluates the role of the endocannabinoids system in cirrhotic cardiomyopathy in a rat bile duct-ligated model.
Gaskan et al.85 isolated normal and cirrhotic left ventricular papillary muscle and assessed its contractility at baseline and after isoproterenol. Both groups had similar contractile force induced by electric stimulation, but after isoproterenol administration, cirrhotic heart muscle exhibited an impaired response that was restored by a CBj antagonist (AM251). Preincubation with anandamide blunted the contractile response to isoproterenol. Interestingly, CBj density was not significantly different between the groups and response to anandamide was similar. Therefore, the authors argue for a role of high endocannabinoids serum levels in cirrhotic cardiomyopathy.
Hepatic encephalopathyHepatic encephalopathy (HE) is a metabolic neuropsychiatric syndrome with a wide clinical spectrum, ranging from altered attention to stupor and coma.86 HE is a feature of acute liver failure (ALF) and ESLD, although the pathogenesis seems to be different.87 Currently HE is considered a prognosis-defining CLD complication.
CB1 receptors are densely expressed in a vast part of the central nervous system.22 The endocannabinoids system is thought to mediate synapses in a feedback manner based on the following observations; first, anandamide releasing enzyme is Ca++-dependent; second, 2AG synthesizing enzymes are present in the postsynaptic membrane and degradating enzymes are located in the presynaptic membrane;30 and third, CBj stimulation has been shown to modulate neurotransmitter releasing, such as dopamine, GABA, serotonine and glutamate.88 CB2 is not expressed by neurons, but it is found in microglial brain cells,21 and it is upregulated after microglial cell activation,89in vivo and in postmortem examinations of Alzheimer’s disease brains.90 2-AG has been shown to protect rat neurons from ischemia and traumatic injury and provides protection against excitotoxicity.91-93
Avraham et al.94 induced ALF in rats by thioacetamide administration. They showed that 2AG brain levels were 2.5-fold elevated and treatment with SR141716 improved neurological score, activity and cognitive function. 2AG administration lead to the same outcome and the effect on neurological score was blocked by a CB2 antagonist (SR144528). CB2 specific agonists improved neurological score. However, CBj specific agonists had no effect on this outcome.
CB2 activation leads to cytokine release control,95 this is an important issue in HE during ALF.96 CB2 receptors might alleviate brain structural and functioning damage through regulation of microglial cells, perhaps reducing brain edema and neurotoxicity. More studies are needed to understand the participation of the endocannabinoids system in HE.
ConclusionThe endocannabinoid system is a new pathway in the pathophysiology of several steps in CLD natural history and complications. Endocannabinoids provide an exciting interventional landscape in modern hepatology. CB1 antagonism opens a new therapeutic possibility in CLD, which could be expanded with the use of CB2 synthetic agonists. However, more studies are needed to understand and clarify the exact mechanisms by which endocannabinoids participate in acute and chronic liver disease.