Nowadays, hepatitis C infection has been identified as the main cause of chronic liver disease, including cirrhosis and hepatocellular carcinoma in Western Countries. However, despite its large diffusion (with more than 150 million of people infected world-wide), the lack of symptoms during the acute phase, together with the indolent course of the disease over time, hampers the difficulties to assess the natural history of the disease, which still remains an interesting clinical dilemma. This complexity can also be argued from the large heterogeneity of disease complication’s rate observed when different methodological approaches were used (retrospective cohort studies, prospective cohort studies, retrospective-prospective cohort studies).
Moreover, the progression of the disease could also be dramatically affected by many variables related to the host, the virus and the environment. Finally, in the last few years, the long–term outcome of the infected subjects, has been deeply modified by the use of efficacy antiviral therapy, as shown by the better survival observed in patients who had achieved a sustained virological response after interferon treatment.
List of abbreviations:
HCV = Hepatitis C virus
HCC = Hepatocellular carcinoma
CHC = Chronic hepatitis C
ALT = Aminotransferase
HVPG = Hepatic venous pressure gradient
MELD = Model for end stage liver disease
SVR = Sustained virologic response
Magnitude of the problemHepatitis C virus (HCV) infection is the leading cause of chronic liver disease including cirrhosis and hepatocellular carcinoma (HCC), with an estimated 170 million of people infected worldwide. It is also the most common reason for liver transplantation in the Western World.1,2
The actual estimated incidence is 1-3 per 100,000 person per year,3 which is markedly decreased in the last decades. This fact is attributable to the identification of the virus in the late ’80 of the past century, which has allowed the development of a correct transfusion’s screening policy thus permitting a dramatically decrease of the onset of new infections.
The prevalence of HCV infection varies significantly among different races: in the United States it is higher among Africans Americans (3.2%) and Hispanics (2.1%) compared with non-Hispanic whites (1.5%).3
Pitfalls in assessing the natural history of HCV infectionThe natural history of HCV infection is usually characterized by the transition from acute to chronic infection, which may progress from a long-lasting asymptomatic condition up to a decompensated hepatic disease. Recovery from acute infection is estimated to occur in 10-25% of patients.
Peak of serum HCV-RNA (usually 105 up to 107 UI/ mL) could be generally observed 15 days after the infection, two weeks earlier than the increase of aminotransferase (ALT) and the appearance of symptoms. However, the recognition of the time of infection remains largely unknown since the onset of the disease is symptom less in more than 60% of cases; as a consequence, the precise date of infection can be established only in a small proportion of patients. Moreover, in the vast majority of patients, the course of the disease shows a slow evolution (up to several decades) and its progression might have been severely affected by concurrent co-morbidities conditions. For these reasons a reliable assessment of the natural history of HCV infection remains an interesting clinical dilemma.
Methodological approaches used to study the natural history of HCV infectionA wide range of study designs (i.e. case-series, cross-sectional, case-control, cohort, clinical trial) have been developed in clinical investigation; in particular, the cohort studies have been largely employed.
Epidemiology definition of «cohort»- •
Cohort: A group of individuals sharing a common characteristic
- -
Birth cohort: all individuals in a certain geographic area born in the same period (usually a year)
- -
Inception cohort: all individuals assembled at a given point based on some factor (e.g. where they live or work)
- -
Exposure cohort: individuals assembled as a group based on some common exposure
- •
e.g. radiation exposure during desert testing
- •
e.g. asbestos exposure in the shipyards
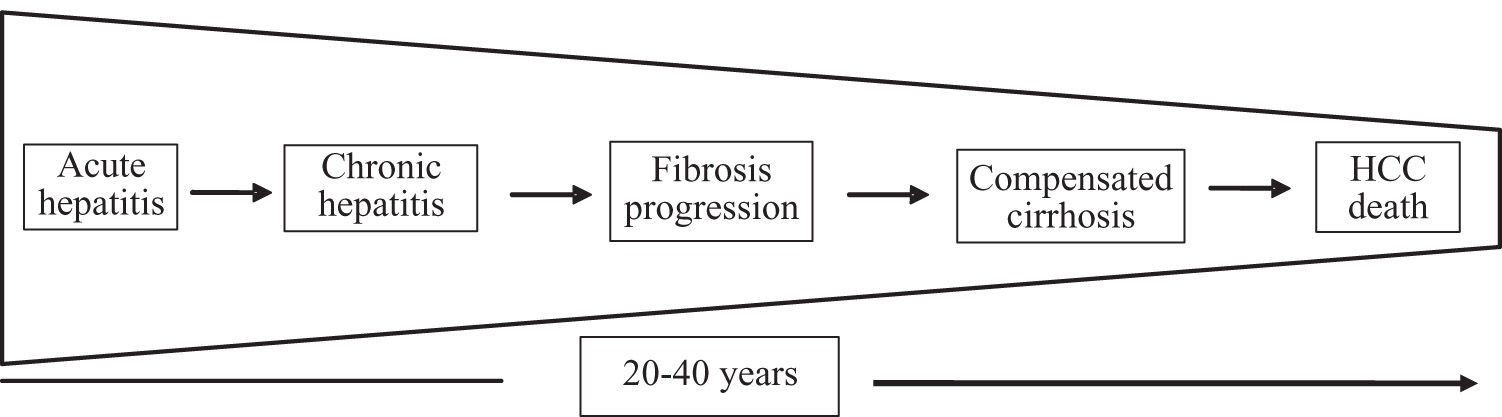
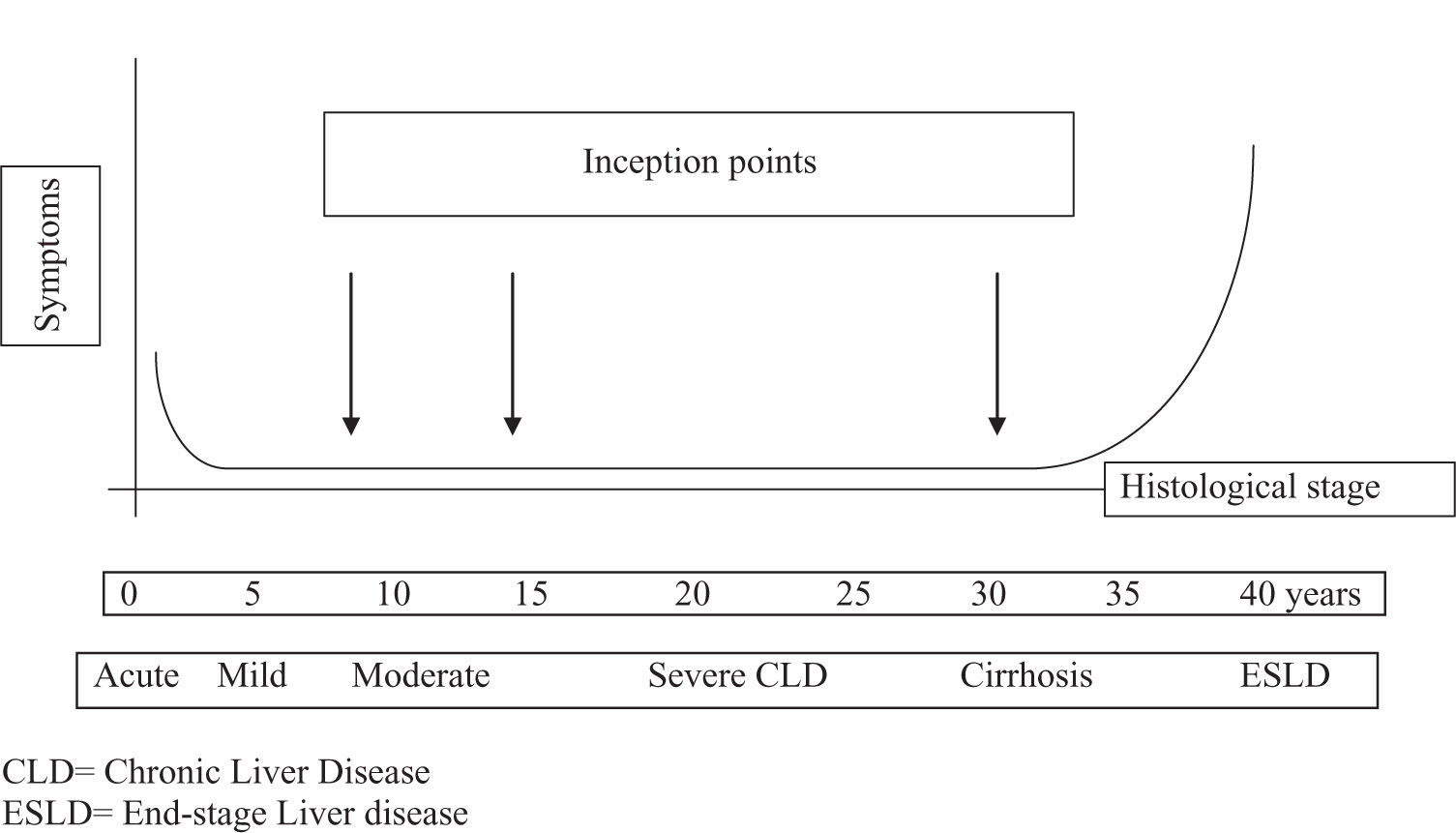
In this clinical setting, an advantageous approach used to by pass the above mentioned difficulties in the assessment of natural history of HCV infection is represented by the «analysis by segments». It is based on the concept that the progression of HCV infection can be thought as a sequence of different disease stages (Figure 1), each one with a limited and presumably known duration.
Therefore, several investigators have chosen this method in order to tentatively achieve a more correct assessment of both the disease progression and the rate of disease-related events (Figure 2).
Variety of cohort studies used in the assessment of the course of HCV infectionRetrospective cohort studies. This strategy, focusing on individuals referred to a medical division for their liver disease for a certain period of time, generally identifies the presence of cirrhosis in 17% up to 55% of chronic infected adults and hepatocellular carcinoma in 1% up to 23%.4,5 However, in this category of studies, the presence of referral bias, caused by the over-estimation of disease prevalence, which is usual in the tertiary care centres, is a major limitation.
Prospective cohort studies. This strategy is based on the prospective follow-up of patients with acute symptomatic infection. As expected, on the basis of slow progression of the disease, the rate of complications detected in these studies is significantly lower when compared to the retrospective series. They found a rate of progression to cirrhosis of 7% to 16% and HCC of 0.7% to 1.3% per year.6,7
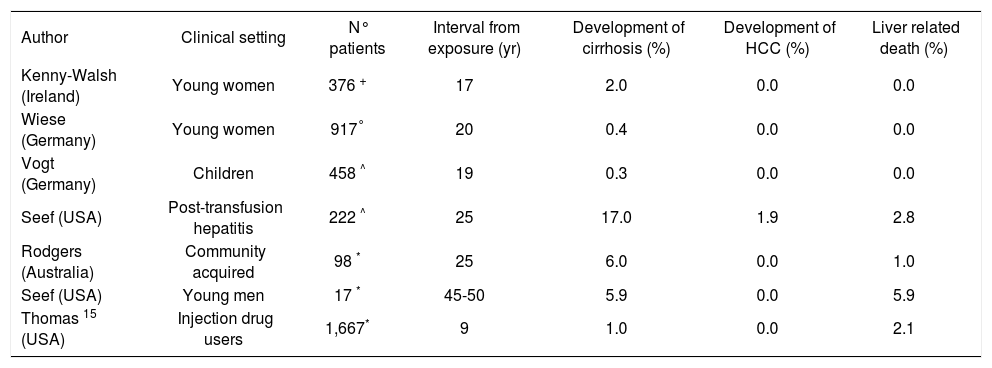
Retrospective-prospective cohort studies. Many useful information can be obtained by these studies, which are widely employed. They are characterized by the retrospective identification of a large group of individuals, exposed to the virus in the past, who were recalled at a certain time and then prospective followed-up in the long term (Table I). The two well-performed studies belonging to this class was carried out in a large series of women who had received HCV-contaminated immunoglobulin administered for prophylaxis of rhesus isoimmunization. They confirmed that the prognosis of patients with Chronic Hepatitis C (CHC) is benign and only a minority of the infected subjects ultimately developed cirrhosis.8,9
Retrospective-prospective cohort studies of the natural history of hepatitis C.
| Author | Clinical setting | N° patients | Interval from exposure (yr) | Development of cirrhosis (%) | Development of HCC (%) | Liver related death (%) |
|---|---|---|---|---|---|---|
| Kenny-Walsh (Ireland) | Young women | 376 + | 17 | 2.0 | 0.0 | 0.0 |
| Wiese (Germany) | Young women | 917° | 20 | 0.4 | 0.0 | 0.0 |
| Vogt (Germany) | Children | 458 ^ | 19 | 0.3 | 0.0 | 0.0 |
| Seef (USA) | Post-transfusion hepatitis | 222 ^ | 25 | 17.0 | 1.9 | 2.8 |
| Rodgers (Australia) | Community acquired | 98 * | 25 | 6.0 | 0.0 | 1.0 |
| Seef (USA) | Young men | 17 * | 45-50 | 5.9 | 0.0 | 5.9 |
| Thomas 15 (USA) | Injection drug users | 1,667* | 9 | 1.0 | 0.0 | 2.1 |
However, when serial liver biopsies performed twenty years after the infection were examined, these authors found histological signs of mild to moderate necro-inflammatory activity in about 40% of the HCV RNA positive women included in the study.8,10 An extremely low rate of liver disease occurrence was also reported in the study made by Vogt and coworkers, which have evaluated all children who had underwent cardiac surgery before the implementation of blood- donor screening.11
By contrast, a relatively high rate of cirrhosis and its complications was observed in the two cohorts of posttransfusion outbreak of acute hepatitis C described by others.12,13
In the same way, Seef and coworker found a 5.9% cumulative rate of cirrhosis and liver-related death during a 45 years follow-up study carried out in a small cohort of anti-HCV positive US military recruits.14 Regardless of the category of the studies, this heterogeneity could be justified by the importance of the interaction between host and virus.
Predictors of outcome and potential cofactors affecting prognosis in HCV infected peopleA long-lasting elevated necro-inflammatory activity certified either by persistent ALT elevation or histology, seems to play a crucial role in the evolution of the disease. This is also suggested by the observation that the occurrence of flares-up of ALT deeply modifies the extremely benign course of the disease in patients with persistently normal aminotransferase levels (N-ALT).16-18 However, no other indicator able to predict the speed of progression of the disease, which is characterized by the development of hepatic fibrosis up to nodular cirrhosis, in the single individual has been identified so far. In addition, it should be emphasized that the prognosis of subjects infected by HCV can also be affected by many variables and cofactors (host, virus and environment).
Viral factorsSome authors have suggested that genotype 1b could be associated with a worse than poor prognosis of the disease than other genotypes.19 However, no consensus has been reached about this point since no independent association between genotype and disease progression was observed in a large longitudinal study carried out in untreated patients.20 Contrasts also persist about the role attributable to specific quasispecies, while the amount of viral load seems not to be associated with the disease severity.21
Host factorsThe outcome of CHC could be favourably influenced by female gender and young age at infection, as suggested by Minola and coworkers.22 On the other side, the co-infection with HBV or HIV and an immune deficiency status are well-recognized conditions associated with worst prognosis as observed in young adults with hypogammaglobulineminemia infected by HCV-contaminated immune globulin.23,24
Moreover, in the last few years, a more aggressive disease has been frequently identified in individuals with a concurrent hepatic steatosis and/or an insulin-resistance condition.25 The role of HLA haplotype in determining the rate of virus clearance has also been suggested by Mangia and coworkers.26 In the same way, the significance of both iron overload and «occult» HBV infection in affecting the evolution of CHC remains still controversial.27,28
External factorsAlcohol abuse is the most important co-factor which can dramatically change the course of the disease. Despite no agreement exists about the threshold potentially dangerous, several authors have declaimed that the persistent intake of low amount of alcohol may play a role in the progression of the disease.29 Further studies are required to clarify the influence of both smoking and environmental factors, such as diet or toxic contaminants.
Natural course of HCV-induced cirrhosisDespite the natural history of HCV-induced chronic liver disease, as discussed above, remains poorly measurable, the «analysis by segments» has allowed to better characterize it in patients with compensated cirrhosis.30-33 Accordingly, in the last decades, several studies have described the course of the disease in this subset of patients. All these studies agree about the conclusion that the development of HCC, irrespective of its detection at an early stage, represents the main complication associated with mortality.
Although no data useful to predict the risk in a single individual are available, all studies have confirmed that the risk of HCC development is higher in patients with male sex, older age, co-infection with HBV and with high values of alpha-feto protein.
While it is well-established that the degree of portal hypertension, evaluated by either the presence of esophageal varices or hepatic venous pressure gradient (HVPG) assessment, is linked with the risk of decompensation, three studies have also described the association between oesophageal varices and risk of HCC32,33,36 occurrence. Interestingly, even though the presence of varices might be attributed to a longer duration of the disease, it has been suggested that its role in the development of HCC my be attributed to the impaired regional blood flow and local hypoxia induced by portal hypertension which stimulates the synthesis of angiogenic factors.34
Despite it is still debated, the role of HCV genotype 1b, the most prevalent genotype worldwide, as a risk factor associated with HCC development was recently confirmed in a prospective fashion.35,36 Accordingly, a biological plausibility of this issue was recently provided by the discover of the linkage between a mutated viral site and a different sensitive to damage- induced apoptosis, caused by an interaction with cellular onco-suppressor.37
The Model for End Stage Liver Disease (MELD) score, which is nowadays used to improve the organ allocation for transplantation, has also been identified as a potential prognostic tool in advanced liver diseases of different etiologies.38-40 However, a recent observation was made by Ripoll et al.41 who showed, in a nested cohort of cirrhotic patients without varices,42 that the MELD cut-off value of 10 was significantly associated with the probability to develop clinical decompensation. In that study, however, the follow-up was relatively short and the number of events was low. In addition, approximately 35% of the enrolled patients had non-viral, alcohol-related cirrhosis. Thus, its usefulness as a predictor of outcome in HCV-related cirrhosis remains to be confirmed.
Impact of antiviral therapy and the need for treatmentAlthough it is reasonable to believe that the clearance of the virus may lead to a reduction of complications, the lack of randomized studies hampered the difficult to achieve a reliable assessment of the impact of antiviral treatment on the long-term outcome of patients with HCV-infection.
In this clinical setting, more information have been attained in several cohort studies carried out in patients with cirrhosis. These reports, designed to assess the response to IFN therapy rather than evaluating the long- term disease outcome,30,43,44 documented a better prognosis for patients who received IFN, regardless of HCV- RNA eradication. In these survey, IFN was administered without randomization to patients who had no major contraindications. Moreover, treated (non SVR) patients had significantly better characteristics at the time of the initiation of treatment in comparison to the untreated ones. Thus, the apparent «protective» effect of IFN therapy disappeared after the adjustment for baseline patient characteristics at multivariate analysis.
However, the main reliable information emerging from these studies, which was recently confirmed in two retrospective reports,45,46 was that the achievement of SVR is significantly associated with a reduction of decompensation rate, HCC occurrence and, ultimately, liver-related mortality.
Therefore, limiting the escalating rates of hepatitis-related morbidity is dependent on successful treatment of the HCV infection and Pegylated interferon (PEG-IFN) alfa plus ribavirin (RBV) is firmly established as treatment of choice.47
Treatment of patients with cirrhosisHowever, unsatisfactory SVR rates have been attained in patients with cirrhosis (an independent risk factor associated with poor response)48 when using traditional monotherapy regimens of conventional IFN alfa or PEG-IFN alfa.49 Moreover, previous international clinical trials have almost universally listed decompensated liver disease as an exclusion criterion.50-54 Furthermore, only 15-30% of patients in these studies displayed evidence of severe liver disease and SVR rates were calculated by pooling all patients with bridging fibrosis (Knodell score F3) and complete cirrhosis (Knodell score F4), thus, including a group of patients that displayed a wide heterogeneity of liver disease, ranging from marginal bridging fibrosis to compensated cirrhosis. Because this latter group represented no more than 6% of patients, distributed among the different arms of the studies, no reliable conclusions could be reached about efficacy and safety of treatment in this subset of patients. However, with these limitations, the results emerging from these studies suggested that the treatment of patients with well-compensated HCV–induced cirrhosis using PEG-IFN alfa-2b plus RBV may be warranted, especially in «easy to treat» genotypes» (2a/c, 3a), while in the subset of the «difficult to manage» ones (1a, 1b, 4) the results are largely unsatisfactory. In this subgroup of patients the use of baseline and on-treatment predictors of response may enhance virologic outcomes and refinement of the regimens investigated is warranted. Further studies are also needed to develop treatment schedules for individual genotype populations.