Intestinal microflora constitutes a symbiotic ecosystem in permanent equilibrium, composed mainly of anaerobic bacteria. However, such equilibrium may be altered by daily conditions as drug use or pathologies interfering with intestinal physiology, generating an unfavorable environment for the organism. Besides, there are factors which may cause alterations in the intestinal wall, creating the conditions for translocation or permeation of substances or bacteria. In cirrhotic patients, there are many conditions that combine to alter the amount and populations of intestinal bacteria, as well as the functional capacity of the intestinal wall to prevent the permeation of substances and bacteria. Nowadays, numerous complications associated with cirrhosis have been identified, where such mechanisms could play an important role. There is evidence that some probiotic microorganisms could restore the microbiologic and immunologic equilibrium in the intestinal wall in cirrhotic patients and help in the treatment of complications due to cirrhosis. This article has the objective to review the interactions between intestinal flora, gut permeability, and the actual role of probiotics in the field of cirrhotic patients.
In the gastrointestinal human tract, there are innumerable microorganisms belonging to approximately 400 cultivable species, among which are Bacteroides, Lactobacillus, Clostridium, Fusobacterium, Bifidobacterium, Peptoestreptococcus, Peptococcus, Escherichia and Veillonella.1 Under physiologic conditions, bacterial populations in the gastrointestinal tract are confined to the intestinal lumen and constitute a symbiotic ecosystem. Under abnormal conditions, bacterial translocation (BT), defined as the migration of bacteria from the intestinal lumen to the mesenteric intestinal nodes or other extra-intestinal sites, can be observed.2 BT has been studied in animal models and appears in situations of intestinal obstruction, hemorrhagic shock, sepsis, endotoxemia, severe trauma, thermal injuries, overgrowth and change in bacterial populations, as well as cirrhosis. The results of these studies demonstrate that the intestinal bacteria overpopulation, the increase in intestinal permeability, and the altered defense mechanisms in the host are the main contributing factors, thus determining that the concept of intestinal wall is functional more than anatomic. Other factors that contribute to maintain the intestinal microbiologic ecology are gastric acidity, pancreato-biliary secretions, intestinal immunologic factors, and intestinal peristalsis; these conditions are altered in cirrhotic patients. Probiotics have been postulated as microorganisms that create an equilibrium in potentially pathogen bacterial populations and also reduce BT by increasing the immunologic capacity of the host on a gastrointestinal level.3 Probiotics are defined as microorganisms resistant to digestion, capable of adhering to the digestive tract mucosa and, when ingested in sufficient amount, beneficial to the host’s health.4
BT ocurrs rarely in normal individuals, where intestinal native bacteria such as E. coli continuously translocate when their levels exceed 108/gr of microorganisms in the cecum.5 When there is a marked increase in BT or there is immunocompromise in the host, bacterial replication is produced in the mesenteric lymph nodes with eventual systemic dissemination through lymph nodes and vascular channels, reaching the liver via the portal vein.
An increase in the BT of Gram negative enteric bacteria has been demonstrated in experimental models of cirrhosis, and this has been postulated as a pathogenic mechanism of spontaneous bacteremia and spontaneous bacterial peritonitis, situations that are frequently observed in patients with cirrhosis.6 Both the patients and the animal models with cirrhosis show abnormalities in the coordination of their intestinal motor functions, causing delayed intestinal transit and favoring bacterial overpopulation, mainly composed of enterobacteria7 Oxidative damage of the intestinal wall, increased activity of the sympathetic nervous system, and altered production of nitric oxide are factors clearly identified as causing intestinal dysmotility in cirrhotic patients.8
Cirrhosis and other hepatic diseases are also associated with functional and structural alterations in intestinal mucosa, which increases intestinal permeability to macromolecules and bacteria. It has been demonstrated than an increased activity of the xanthine oxidase enzyme is an indicator of oxidative stress, increased lipid peroxidation in membrane brush cells, and abnormal intestinal transport,9 all of which are factors triggering BT.
Studies in patients with cirrhosis have demonstrated that BT of enteric organisms occurs more frequently in patients with advanced hepatic disease. It is approximately 5 times more frequent in subjects in Child-Pugh C stage than in the rest of the cirrhotic population. Within this group of patients, subjects with ascites show a higher prevalence of BT, both parameters being independent predictive factors of BT. When compared with patients in Child-Pugh A or B stage, there was no evidence of an increased prevalence of BT with respect to control patients, and it was observed that the antimicrobial treatment to decontaminate the intestinal lumen practically prevented BT in patients in Child-Pugh C stage.10
It has been demonstrated that there is a prevalence of Gram negative aerobic bacteria, which show a linear progression as the hepatic disease advances, leading to a greater translocation capacity even in an intact intestinal epithelium.11 Under these circumstances, Lactobacilli constitute an integral part in gastrointestinal microecology and promote the growth of anaerobic and Gram positive bacteria, while they inhibit the growth of Gram negative bacteria.12 Thus, bacteriotherapy with probiotics is effective in preventing BT, an effect achieved by means of an increased formation of short-chain fat acids and a reduction in colonic pH, as well as the induction of growth factors, maintenance of the proliferation and function of intestinal epithelial flora, and inhibition of adherence and invasion of enterovirulent bacteria to intestinal cells.13
Immunologic alterations and cirrhosisImmune mechanisms in mesenteric lymph nodes are compromised, with a marked increase in BT, which carries enteric bacteria and their products, endotoxins, to the systemic circulation. Cirrhotic patients show deficiencies in their bacteriostatic and serum chemotactic capacity, in neutrophil phagocytosis, and in the effector functions of circulating immune cells.14 Splenic hyperemia following portal hypertension compromises adherence and migration of phagocytic cells to the mesenteric vessels, being at the same time another mechanism contributing to altered immune response in this type of patients.15
When these mechanisms are present, cirrhotic patients show an alteration in the contents of intestinal microflora, immunity and integrity of mucosa, which explains the high incidence of BT observed, together with circulating endotoxemia, which favors an inflammatory cytokine response as an alpha tumoral necrosis factor (TNF-***entity***) and alternate production of NO.16
The use of antibiotics such as quinolones, metronidazol, and trimetroprim-sulphametoxazole reduces the amount of enteric bacteria and BT. However, the emergence of enterobacteria resistant to quinolones is increasingly higher, which has led to seek treatment alternatives.17 Agents enhacing intestinal motility have been studied; encouraging results have been found with the administration of cisapride and propanolol, which reduce the amount of bacteria by reducing intestinal transit time.8,18
One of the therapies that have emerged as a viable alternative for treatment are probiotic microorganisms, the definition of which has already been specified. Several recent studies have shown their usefulness. Chiva et al. report that oral administration of Lactobacillus johnsonii to three groups of cirrhotic rats, together with antioxidative agents (vitamin C and glutamate), alone or in combination with the probiotic, supressed BT in mesenteric lymph nodes, and reduced enterobacteria and enterococci counts on an ileal and cecal level, as well as the levels of malondialdehyde, used as a marker for oxidative damage.19 Loguercio et al. show that the administration of the probiotic VSL#3 enhanced the condition of inflammatory markers such as IL-6 and TNF in patients with hepatic diseases of different etiologies.20 Torre et al. found similar results by administering Lactobacillus casei to a group of cirrhotic patients, showing a reduction in proinflammatory markers.21
Cirrhosis and bacterial translocationThe specific functions of the reticuloendothelial system are the phagocytosis and detoxification of bacteria and endotoxins that enter the liver via the intestine through the portal vein. Some studies have reported a reduction in the amount and function of Kupffer Cells (KC), and a reduction in bactericide activity, phagocytosis and chemotaxis to bacterial products.22,23
Cirrhotic patients frequently show complications related to bacterial infections, which have been observed in 30 to 50% of hospitalized patients.24 Patients with ascites show cases of complicated peritonitis without an apparent intraperitoneal cause of infection, this entity being known as spontaneous bacterial peritonitis (SBP), and observed in 8 to 13% of cirrhotic patients with ascites, with a mortality rate of 57 to 98%.25 This contrasts with the mortality rate of cirrhotic patients with or without an infection of other type during their hospitalization period, showing 30 and 6%, respectively.26
c.1.- Physiopathology of hepatic damageBacterial overpopulation, BT, the increase in intestinal permeability, and the altered defense mechanisms in cirrhotic patients lead to the activation of monocytes and lymphocytes, causing an elevation in the serum levels of pro-inflammatory cytokines such as TNF-***entity***, IL-6, IFN ***entity*** and IL-1 mainly.8 Likewise, constant endotoxemia caused by overpopulation and BT exacerbate cytokine response and favor the production of nitric oxide, which directly influences hyperdynamic circulation in cirrhotic patients.25 See table I.
Once in the bloodstream, endotoxins promote the hepatic synthesis of a bacterial lipopolysaccharide binding protein (LBP), which favors binding to the CD14 site of the LPS receptor.28 This binding translates the signal to the nucleus, thus determining the onset of the inflammatory cascade leading to cytokine production. Under these conditions, serum concentration of CD14 reflects the amount of endotoxins and cell activation in their charge, and it has been found that treatment with norfloxacine normalizes the LBP and CD14 levels.29
Under normal conditions, the production of nitric oxide (NO) is regulated by oxide sintetases. Three iso-forms have been identified: inducible NO sintetase, endothelial sintetase, and neuronal sintetase. The inducible isoform is synthesized de novo in macrophages, vascular smooth muscle cells after induction by lipopolysaccharides, and cytokines.30 Once this sintetase is expressed, great amounts of NO are produced for long periods of time, regardless of the mechanical or hemodynamic stimuli.31
The endothelial form produces NO for short periods in response to various endogenous and exogenous stimuli, including physical stimuli. Its activation depends on protein G under stimulation with acetylcholine, bradicinine, catecholamine and hormones. Its production derives from sinusoidal endothelial cells which are required to maintain hepatic microcirculation,32 and therefore on this level NO is in charge of maintaining the intrahepatic vascular tone.
Finally, the neuronal form is activated by nerve depolarization, and is constitutively expressed in the brain.
Under these conditions, the prevention of infectious complications is important to enhance the prognosis and the patients’ life quality.
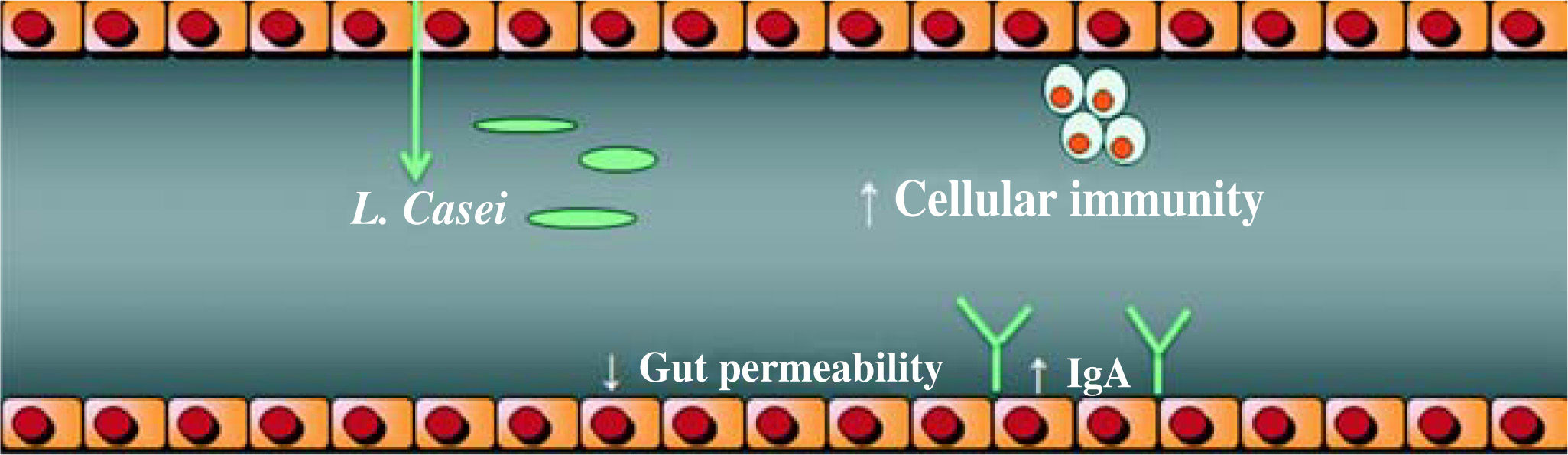
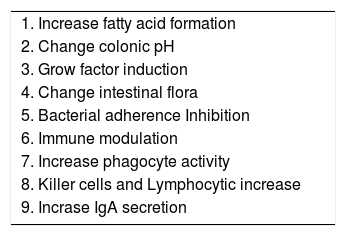
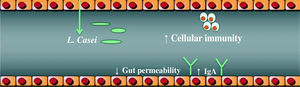
Probiotics actionIt has been demonstrated that probiotics, especially Lactobacilli strains, enhance the host’s immunity and resistance to infection by means of an increase in the phagocytic activity and an increase in IgA secretion. They enhance the induction of cell immunity by means of an increase in Killer cells and T lymphocytes, (Figure 1), among other mechanisms identified, (Table II).33–34 Given these findings, the potential benefits of Lactobacilli in chronic hepatocellular disease cannot be excluded. Nowadays, the debate focuses on the exact amount of Lactobacilli to achieve colonization and beneficial effects, the time period of administration, and the strain to be administered.
Probiotics and mechanism of action.
| 1. Increase fatty acid formation |
| 2. Change colonic pH |
| 3. Grow factor induction |
| 4. Change intestinal flora |
| 5. Bacterial adherence Inhibition |
| 6. Immune modulation |
| 7. Increase phagocyte activity |
| 8. Killer cells and Lymphocytic increase |
| 9. Incrase IgA secretion |
Bifidobacteria and lactobacilli are the main species to be considered within the category of probiotics. In general, they are species considered as non- pathogen to the human organism, which is implicit in the definition of probiotics. There have been reports on isolated cases of endocarditis and hepatic abscess mainly in immunocompromised patients in less than 2% of the cases. In such cases, the proposed action mechanism consisted in higher platelet aggregation, collagen and fibrinogen binding, and production of glycosidases and proteases.35 However, upon collecting the data of the cases, it was confirmed that the Lactobacillus causing both episodes was not the product of any experiment, but it was secondary to intestinal native flora which was a product of the patient’s diet.36
Based on the above data, it can be asserted that the use of probiotics in humans is safe and it has a very low risk of adverse reactions.
Research linesThere are numerous research lines regarding the use of probiotics in the treatment of hepatic diseases. The greatest steps forward are focused mainly on the treatment of the non-alcoholic fatty liver disease (NAFLD) and of patients with alcohol damage and subsequent elevated intestinal permeability. To a less extent, steps are taken towards modifications of cirrhotic patients’ hemodynamic profile.
NALFD is the most common cause of chronic hepatic disease. It has been suggested that 10-24% of the American population has it,37 which makes it an entity three times more common than DM, and 5 to 10 times more common than HCV infection.
There are numerous hypotheses on its pathogenesis, establishing the existence of several initial stages with a final common path, where endogenous and exogenous toxins, including alcohol and LPS, amplify the stress signal due to an increase in the production of proinflammatory cytokines (TNF-***entity***mainly), which exacerbates the resistance to insulin and leads to a greater damage due to oxidative stress and hepatocyte dysfunction.
Therefore, one of the endogenous factors contributing to the pathogenesis of NAFLD is intestinal bacterial flora. This is based on the increased production of endogenous ethanol and a direct activation of inflammatory cytokines in the epithelial lumen, non-parenchymatose hepatic cells, or both, in response to bacterial LPS.
Another identified cause is bacterial overpopulation in patients who have undergone bariatric surgery: those patients developed NAFLD in the long-term. These statements are based on the fact that treatment with metron- idazol reverts hepatic damage and damage recurs rapidly after liver transplant in previously operated patients.
Under these circumstances, the importance of intestinal flora in hepatic disease is evident, and treatment options such as antibiotics or probiotics may influence the evolution of the disease. Probiotics are more affordable than antibiotics, they are less expensive, they do not have negative effects on the long term (such as bacterial resistance in the case of antibiotics) and their role goes beyond the eradication of pathogen bacterial flora by means of competitive inhibition; they also contribute to modify the inflammatory cascade by cytokine immunomodulation, to enhance the function of the epithelial wall, and to directly reduce proinflammatory cytokines by regulating the nuclear factor kappa B (NF kB).38
In a recent study, Zhiping et al. used a combination of probiotics consisting in bifidobacteria, lactobacilli and Streptococcus thermophilus, and antibodies against TNF-***entity***in rats induced with NAFLD; they found that after 28 days of treatment there was an enhancement in hepatic histology, ALT levels, and indirect markers of insulin resistance.39 These findings are explained by a reduction in the content of total hepatic fatty acids and beta oxidation, while the enhancement in beta oxidation parameters is attributed to a reduced hepatic activity of the Jun- N-terminal kinase (JNK), a result produced by the immunomodulating action of the probiotic, together with the known effects on NF-kB, which normalizes the abnormalities in mitochondriae and in peroxisomes altered in ***entity***oxidation.
It has also been demonstrated that probiotics are effective in the treatment of hemodynamic alterations in cirrhotic patients. De Santis et al. administered a combination of probiotics consisting of (Streptococcus thermophilus, Lactobacillus acidophilus, L. plantarum, L. casei, L. bulgaricus and S. Faecium) during 1 month to a patient with postnecrotic hepatic cirrhosis secondary to HCV. After one month of treatment, there was an increase in the average rate of portal vein flux, with a reduction in splenic vein flux, parameters which are clearly related to the changes in intestinal microflora secondary to the use of the probiotics preparation, thus concluding that such preparation acts as a regulator of portal vein pressure.40