Chronic hepatitis C virus (HCV) infection can be cured with treatment using direct-acting antivirals (DAAs). Although these drugs have been widely studied, information about certain special populations is missing. In this case report we describe a treatment-experienced patient with chronic HCV infection genotype 1b, treated with 150 mg/day simeprevir, 400 mg/day sofosbuvir, and 1,000 mg/ day ribavirin for 24 weeks, after a Roux-and-Y gastric bypass. At steady-state a pharmacokinetic curve was recorded of sofosbuvir, GS-331007, and simeprevir. Ribavirin trough plasma concentration (Ctrough) was determined. The simeprevir area under the-concentration time curve (AUClast) and Ctrough were 9.42 h.mg/L and 0.046 mg/L, respectively. Compared to what was described in the literature, simeprevir exposure was low and therefore the simeprevir dose was increased to 300 mg/day. The increased dose of simeprevir was well tolerated and Ctrough was 0.532 mg/L. Sofosbuvir AUClast and Ctrough were 0.63 h.mg/L and 0.0013 mg/L. GS-331007 AUClast and Ctrough were 21.02 h.mg/L and 0.35 mg/L. Ribavirin Ctrough was 2.5 mg/L. Sofosbuvir, GS-331007, and ribavirin exposure were comparable with levels described in literature. The patient achieved a sustained virological response twelve weeks after the completion of treatment.
Bariatric surgery is the most effective option to treat morbid obese patients and the number of patients undergoing these kind of surgeries is increased with 11% in 2013 when compared with 2008. In the Netherlands the number of bariatric surgeries almost doubled in these years; from 3,500 in 2008 to 6,807 in 2013.1,2 This is among others explained by the fact that obesity has worldwide doubled since 1980 and it is estimated that 39% of the world’s adult population is overweight [Body Mass Index (BM) ≥ 25] and 13% is obese (BMI ≥ 30).3
The goal of bariatric surgery is to decrease the intake of food and absorption of nutrients for severely obese patients, resulting in weight loss.4 As the gastrointestinal (GI) tract is substantially altered by these surgeries, drug absorption of orally administered drugs can also be altered. Little is known about the effects of a bariatric sur- gery on drug exposure and the effect of the surgery on the exposure of drugs is hard to predict. For example, gastric pH rises which could cause increased absorption of drugs soluble in higher pH ranges or decreased absorption of drugs soluble in low pH ranges. In addition, absorption could decrease, as the transit time of a drug through the GI tract is reduced. Also, the limited food intake of patients that underwent bariatric surgery alters absorption.
Direct-acting antivirals (DAAs) are highly effective drugs licensed for the treatment of chronic hepatitis C virus (HCV) infection. Combinations of at least two DAAs are used and over 90% of the patients is cured after 8 to 12 weeks of treatment. Treatment duration can be prolonged to 24 weeks, or ribavirin can be added to enhance the potency of the regimen when a patient is difficult to treat. This includes patient that are treatment-experienced, have genotype 1a or 3, or have (decompensated) cirrhosis.5
The influence of bariatric surgery on direct-acting antiviral (DAAs) is unknown, but it is previously described that the absorption of DAAs daclatasvir, simeprevir, paritaprevir, ombitasvir, and dasabuvir are, just as ribavirin, altered when taken together with food.6–9 In addition, the solubility of daclatasvir, ledipasvir and velpatasvir is dependent on gastric pH.6,10,11 Therefore, it is likely that bariatric surgery alters exposure of DAAs; possibly causing increased (toxicity) or decreased (sub therapy) plasma concentrations.
In this case report we describe efficacy, safety, and pharmacokinetics of a patient who underwent bariatric surgery and who, after relapsing to previous DAA therapy, was successfully treated for HCV-infection genotype 1b with sofosbuvir, simeprevir, and ribavirin.
Case ReportWe describe a 61-year old Brazilian female patient who presented to our outpatient clinic in 2011 with chronic HCV genotype 1b infection. She was diagnosed with HCV-infection in 2008, but the transmission route was unknown. Possible sources of infection included dental treatment or a caesarean section in Brazil.
The patient was severely obese with a BMI of 35.4 kg/ m2 (weight 84 kg, height 1.54 m). Ultrasound demonstrated hepatic steatosis without any ultrasonographic signs of cirrhosis. Evaluation of liver stiffness using Fibroscan®14,15 showed a value of 13.6 kPa, consistent with METAVIR fibrosis score F3 (severe fibrosis).16,17 A liver biopsy showed moderately active periportal inflammation and moderate periportal fibrosis with formation of septae in less than 50% of portal fields (METAVIR score A2/F2-3) and macrovesicular steatosis in 40-50% of hepatocytes with minimal pericellular fibrosis (Brunt score steatosis grade 2, fibrosis stage F1).18 Laboratory testing showed mildly elevated liver enzymes with an ALT of 77 U/L, AST of 51 U/L and gamma-GT of 57 U/L. Serum bilirubin, prothrombin time, albumin, creatinine, thrombocytes, and fasting blood glucose values were all normal. HCV RNA was 9.56 x 105 IU/mL and HBsAg and anti-HIV 1 & 2 antibodies were negative.
The patient was a non-responder to treatment with peg-interferon alfa and ribavirin in 2009. In 2013, she was included in a clinical trial and was treated with DAAs daclatasvir and asunaprevir for 24 weeks. A relapse occurred after this treatment.
Whilst waiting for registration and reimbursement of the first DAAs in the Netherlands the patient decided to undergo gastric bypass surgery in 2014 (Roux-and-Y gastric bypass). She came back to our outpatient clinic in 2015 for (re-) treatment of the chronic HCV-infection. Her weight had reduced to 59 kg (BMI 24.9 kg/m2), and transaminases had improved (ALT 48 U/L; AST 39 U/L). All other liver enzymes and liver function tests were not altered. HCV RNA load was 5.64 × 106 IU/mL and Fibroscan® showed a value of 7.8 kPa. Sequencing of the viral genome was performed on the regions NS5A and NS3 (as she had received a NS5A inhibitor and a Protease Inhibitor), which showed a high level of resistance associated substitutions (RAS) to NS5A inhibitors on the loci L31M/ I and Y93H. There were no RAS present in the NS3 gene of the viral genome. For these reasons, we decided to treat the patient with 400mg sofosbuvir once daily (Sovaldi®, Gilead Sciences, Cambridge, United Kingdom), 150mg simeprevir once daily (Olysio®, Janssen-Cilag International, Beerse, Belgium), and 1000 ribavirin per day, for a total of 24 weeks.19–21
Simeprevir and ribavirin in particular must be taken with food for adequate plasma concentrations.7 However, due to the bariatric surgery, the patient was not able to eat large meals. To study the exposure of the DAAs and ribavirin in this patient, a pharmacokinetic curve was obtained at Week 3 of DAA treatment. Blood was sampled at t = 0 (pre-dose), 2, 3, 5, 6, 8, and 24 h after intake of the DAAs. DAA plasma concentrations were determined using an inhouse made, validated high performance liquid chromatography (HPLC)-tandem mass spectometry (MS/MS) assay. Pharmacokinetic parameters were calculated using WinNonlin/Phoenix version 6.3, Pharsight Corporation, St. Louis, MO, USA. The assay lower limits of quantification for sofosbuvir, GS-331007 and simeprevir were 2.5 ng/ mL, 10 ng/mL, and 10 ng/L respectively. The precision for low, medium and high quality control (QCs) samples was < 10% for all analytes. Ribavirin plasma concentrations were determined using validated HPLC assay with Ultraviolet detection.22,23
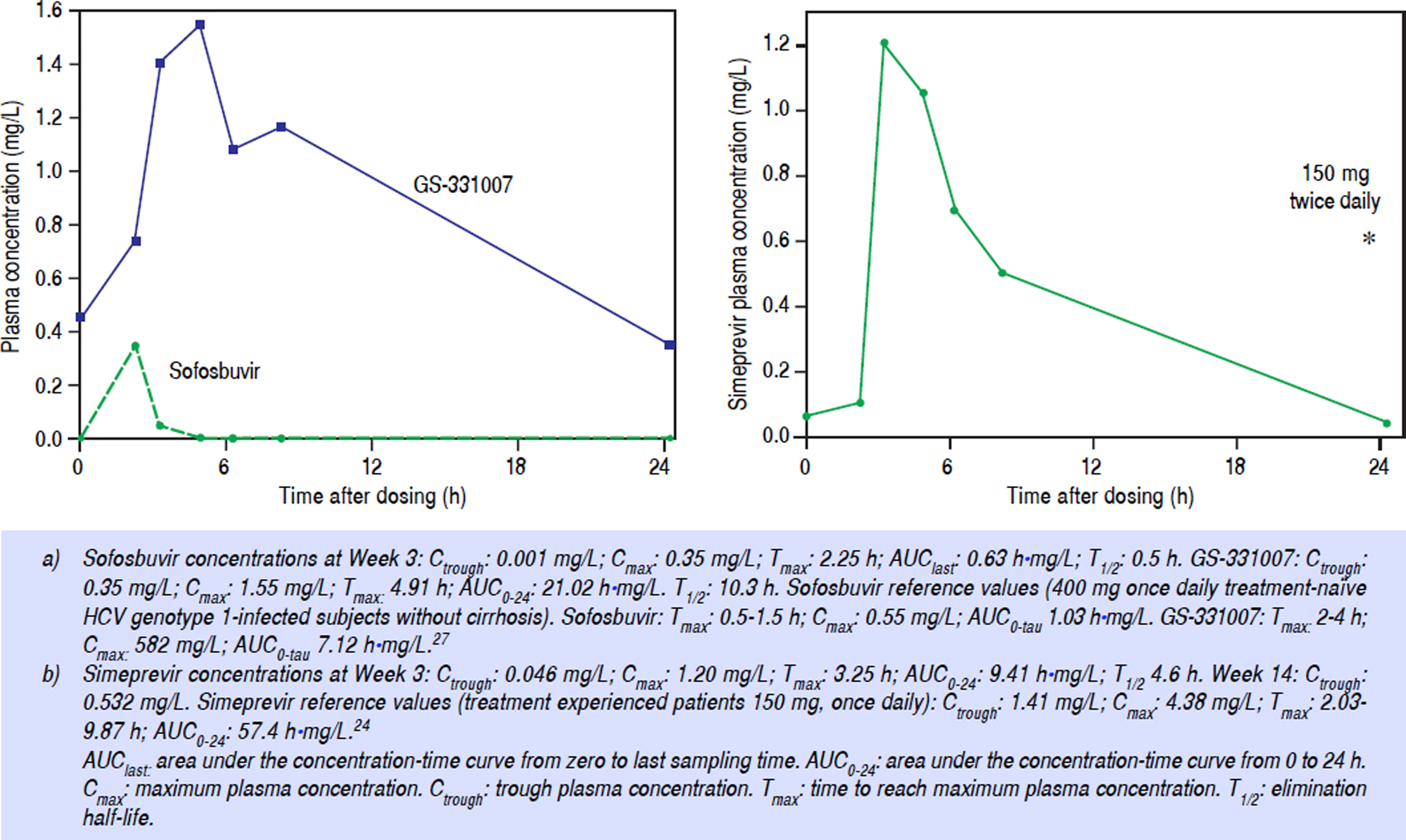
At Week 3, the area under the concentration-time curve (AUClast) for sofosbuvir was 0.63 h•mg/L, the maximum plasma concentration (Cmax) was 0.35 mg/L, and the minimum plasma concentration (Ctrough) was 0.0013 mg/L. For the main inactive metabolite of sofosbuvir, GS-331007, the AUC0–24 was 21.02 h•mg/L, Cmax was 1.55 mg/L, and the Ctrough was 0.35 mg/L (Figure 1A).
For simeprevir, at Week 3 of treatment, the AUC0–24 was 9.42 h•mg/L, the Cmax was 1.21 mg/L, and the Ctrough was 0.046 mg/L (Figure 1B). Ribavirin concentration was 2.5 mg/L. Sofosbuvir and ribavirin concentrations were considered adequate but simeprevir concentrations were sub therapeutic compared with those described in literature.24 As a result, at Week 10 of treatment, the simeprevir dose was doubled to 150 mg twice daily (taken together with food). At Week 14 trough concentrations of ribavirin and simeprevir were determined again and were 0.532 mg/ L and 3.5 mg/L, respectively. The hemoglobin concentraton had dropped from 12.3 g/dL to 9.8 g/dL.
HCV RNA was undetectable during treatment at Week 3, 4, 12, 24 (end of treatment) and 12 weeks after end of treatment (sustained virological response, SVR12).
During treatment, the main side effect was extreme fatigue. Liver enzymes, liver function tests and renal function were all normal during treatment. Twelve weeks after completion of treatment Fibroscan® showed a value of 4.6 kPa.
For this case report no formal ethical approval was obtained as all procedures were performed for regular health care purposes. The patient did not have to comply to certain extra examinations of life style rules. However, the patient gave consent for performing the pharmacokinetic curve and publication of this paper. This was recorded in the patient chart.
DiscussionWe are the first to describe a patient who was successfully treated with DAAs including an adjusted dose of simeprevir after undergoing gastric bypass surgery. Although simeprevir was not deemed to be ideal in this patient, given the food-dependent uptake, there was no alternative choice due to existing resistance to NS5A inhibitors.
We treated the patient for 24 weeks, according to national and international guidelines, as she relapsed to earlier dual NS3/NS5A DAA therapy.19–21 We also tried to enhance the potency of the treatment by adding ribavirin (at a weight-based dose).
According to the simeprevir label, the AUC increases by 60% when administered with a fatty meal or normal breakfast.7 We measured simeprevir Ctrough levels that were 97% lower than comparable reference values, and the AUC0–24 was 84% lower. Our patient was not able to have large or “normal sized” meals (i.e. a high intake of calories) anymore and we postulate that this resulted in the extremely low exposure to simeprevir. Despite the fact that HCV RNA was undetectable at that time, we doubled the dose of simeprevir to increase the plasma exposure and possibly efficacy. This dose was well-tolerated and the Ctrough plasma concentration at Week 14 (4 Weeks after doubling the dose), was approximately 11-fold higher than the Week 3 Ctrough level (62% lower than the reference value). This extreme increase is the result of the non-linear pharmacokinetics of simeprevir.
For ribavirin, we strived to attain a plasma concentration of 2.0-3.0 mg/L at steady-state.25 At Week 3 of treatment the plasma concentration was already 2.5 mg/L, which is remarkable as the patient had a low intake of food.9 These high ribavirin levels caused anemia and the patient suffered from extreme fatigue. It was considered to lower the dose of ribavirin, but because the hemoglobin levels remained stable throughout the whole course of treatment and the patient did not want a dose reduction, so the starting dose of 1,000 mg/day was continued. The high plasma concentrations of ribavirin (compared to the low plasma concentrations of simeprevir) could also be related to the low body weight of < 60 kg of the patient after gastric bypass surgery. The fact that a large or “normal” meal could not be consumed seems less important for an adequate ribavirin level as the initial dose was already relatively high.
Sofosbuvir pharmacokinetics were not affected by the gastric bypass or the low intake of food as the exposure to both sofosbuvir and GS-331007 (the main inactive metabolite of sofosbuvir) were sufficient. This was as expected because it was earlier described that a high-fat meal does not influence the plasma concentration of sofosbuvir or GS-331007.26
This case report describes a patient with chronic HCV-infection genotype 1b without liver cirrhosis, but with a relapse after earlier dual DAA-treatment, who was successfully treated with simeprevir, sofosbuvir, and ribavirin for 24 weeks after undergoing gastric bypass surgery. Adequate sofosbuvir and ribavirin plasma concentrations were achieved, however, simeprevir plasma concentrations were low when simeprevir was dosed according to the drug label (150 mg once daily).7 Both bariatric surgery and low intake of food can influence drug absorption and drug exposure. Awareness is needed when patients who underwent bariatric surgery are treated with certain drugs without any experience in this specific condition. This is especially the case for simeprevir, as absorption is dependent of food intake, it has non-linear pharmacokinetics and possibly more severe side effects when given in high dosages. Patients with a history of bariatric surgery who are treated with simeprevir should be closely monitored using, for example, therapeutic drug monitoring.
Abbreviations- •
AUCļast: area under the concentration-time curve from zero to last sampling time.
- •
AUC0–24: area under the concentration-time curve from 0 to 24 hours.
- •
BMI: body mass index.
- •
Cmax: maximum plasma concentration.
- •
Ctrough: trough plasma concentration.
- •
DAAs: direct-acting antivirals.
- •
GI: gastrointestinal.
- •
HCV: hepatitis C virus.
- •
PI: protease inhibitor.
- •
RAS: resistance associated substitutions.
- •
SVR12: sustained virological response.
EJ Smolders and SB Willemse declare that they have no conflicts of interest that are directly relevant for the content of this manuscript.
O El-Sherif has no conflicts in relation to this article, but has received conference travel support from Abbvie, Gilead, Janssen and MSD.
S Khoo has no conflicts in relation to this article, but has received research grant funding from Merck, Janssen, Novartis, Gilead and BMS.
DM Burger joins advisory boards of Abbvie, BMS, Gilead, Janssen, ViiV Healthcare and Merck and received sponsorship/research grants from BMS, Janssen, ViiV Healthcare and Merck.
Financial SupportThis study was not funded.
Author Contributions- •
EJ Smolders: study design and execution of the study, interpreting results, and drafting of the paper.
- •
SB Willemse: treating physician, study execution, interpreting results, and drafting of the paper.
- •
O El-Sherif: measurement DAA concentration, interpreting results, editing paper.
- •
S Khoo: measurement DAA concentration, editing paper.
- •
DM Burger: study design, interpreting results, editing paper.
We thank the patient for participating. Secondly we would like to thank the laboratory personnel at the Institute of Translational Medicine, University of Liverpool, Liverpool, United Kingdom and the laboratory personnel at the Clinical Pharmacy of Radboud University Medical Center, Nijmegen. We also thank the Marc van der Valk, Yuma Bijleveld, nurses, and pharmacy personnel Academic Medical Center, Amsterdam, the Netherlands, for their support during the hospital admission of the patient.