Introduction and aim. Given that early identification of non-alcoholic fatty liver disease (NAFLD) is an important issue for primary prevention of hepatic disease, the objectives of this study were to evaluate the efficacy of the product of triglyceride and glucose levels (TyG) for screening simple steatosis and non-alcoholic steatohepatitis (NASH) in asymptomatic women, and to compare its efficacy vs. other biomarkers for recognizing NAFLD.
Material and methods. Asymptomatic women aged 20 to 65 years were enrolled into a cross-sectional study. The optimal values of TyG, for screening simple steatosis and NASH were established on a Receiver Operating Characteristic scatter plot; the sensitivity, specificity, and likelihood ratios of TyG index were estimated versus liver biopsy. According sensitivity and specificity, the efficacy of TyG was compared versus the well-known clinical biomarkers for recognizing NAFLD.
Results. A total of 50 asymptomatic women were enrolled. The best cutoff point of TyG for screening simple steatosis was 4.58 (sensitivity 0.94, specificity 0.69); in addition, the best cutoff point of TyG index for screening NASH was 4.59 (sensitivity 0.87, specificity 0.69). The positive and negative likelihood ratios were 3.03 and 0.08 for simple steatosis, and 2.80 and 0.18 for NASH. As compared versus SteatoTest, NashTest, Fatty liver index, and Algorithm, the TyG showed to be the best test for screening.
Conclusions. TyG has high sensitivity and low negative likelihood ratio; as compared with other clinical biomarkers, the TyG showed to be the best test for screening simple steatosis and NASH.
Non-alcoholic fatty liver disease (NAFLD) is the most frequent liver disorder, reaching a prevalence of 15–30% in Western populations;1-4 prevalence that rises to 58% and 98% in overweight and obese individuals, respectively.5
Insulin resistance, that increases lipolysis6 and stimulates de novo lipogenesis promoting the production and storage of triglycerides in the liver,7 as well as fat stores, which are directly associated with liver inflammation,8 play an important role in the pathogenesis of NAFLD.
Given that, typically, individuals with NAFLD are asymptomatic and that among individuals with liver fat storage greater than 33% diagnosis of NAFLD usually is a casual finding,9–14 the early diagnosis of NAFLD remains as an important challenge. On this regard, several non-invasive tests such as the elevated ALT and AST levels, AST:ALT ratio greater than 1, the ALT: triglycerides ratio greater then 7.0, fatty Liver Index, the NAFLD fibrosis score, the FibroMeter, hepatic ultrasonography, computed tomography, nuclear magnetic resonance imaging, the Fibroscan, and transient elastograph, have been proposed for the early recognition of NAFLD.9,15
Recently, we proposed16 and validated versus the euglycemic insulin clamp,17 an index for screening insulin resistance, the product of fasting triglycerides and glucose levels (TyG). Since it was proposed, the TyG index has been evaluated in Mexican-American, Caucasian, Koreans, Chinese, Argentine, Italians, Brazilians, and Mexican adults,18–25 consistently showing that the TyG index properly identifies the presence and severity of insulin resistance in apparently healthy subjects.
Taking into account that insulin resistance is involved in the pathogenesis of NAFLD6,7 and that the TyG index is a well-marker for screening insulin resistance, objectives of this study were to evaluate the efficacy of the TyG index for the screening of simple steatosis and non-alcoholic steatohepatitis (NASH) in asymptomatic women, and compare its efficacy vs. other proposed clinical biomarkers.
Material and MethodsWith the approval of protocol by the Mexican Social Security Institute Research Committee, and after obtaining the subject informed written consent, a cross-sectional study was carried out in accordance with the principles of the Declaration of Helsinki as reviewed in 2000.
The sampling strategy was based on advertising to general population of Durango, city in northern México, to invite women to participate. The eligible population was of asymptomatic women aged 20 to 65 years. All women underwent anthropometric measurements, routine blood chemistry, and hepatic biopsy.
Alcohol consumption equal or greater than 20 g per day, smoking, positive markers of viral or autoimmune hepatitis, previous diagnosis of acute or chronic liver disease, renal failure, neoplasia, cardiovascular disease, pregnancy, and intake of contraceptives or hepatotoxic drugs were exclusion criteria.
Liver biopsy was performed using tru-cut needle guided by ultrasound; two expert pathologists, who were blinded to clinical and laboratory data, performed the histological interpretation.
Women of the control group were eligible among women without hepatic symptoms and signs, who required upper abdominal surgery by any other health problem (e.g. cholecystectomy, the most frequent); previous signed informed consent, liver biopsy was performed during surgical procedure.
According to the biopsy report, women were included into the groups with normal liver, simple steatosis and NASH.
DefinitionsBased on liver biopsy, normal liver was defined by no significant histological changes; simple steatosis, by the presence of hepatic steatosis with no evidence of hepatocellular injury in the form of ballooning of the hepatocytes; and NASH by the presence of hepatic steatosis, inflammation, and ballooning, with or without fibrosis.26
The TyG index was defined as the Ln[fasting triglycerides (mg/dL) x fasting glucose (mg/dL)/2].27
The efficacy of TyG index for the screening of simple steatosis and NASH was compared with the following clinical biomarkers, all of them validated versus hepatic biopsy: SteatoTest,28 NashTest,29 Fatty liver index,30 and Algorithm by Lin.31
MeasurementsUsing a fixed scale with stadimeter (Tanita TBF-215, Tokyo, Japan), with the women in the standing position, in light clothing and without shoes, the weight and height were measured. The precisions of weight and height measurements were 0.1 kg and 0.01 m. Body mass index (BMI) was calculated as weight (kilograms) divided by height (meters) squared. The waist circumference was measured to the nearest centimeter using a flexible tape measure; with the subjects in the standing position, the anatomical landmarks were the midway between the lowest portion of the rib cage and iliac crest.
AssaysA venous whole blood sample was collected after 8–10 h of fasting. Plasma glucose was assessed by glucose-oxidase method; the inter- and intra-assay coefficients of variation (CV) were 2.1, and 1.5%. Total-cholesterol (inter- and intra-assay CV of 3.0 and 2.5%) and serum triglycerides (inter- and intra-assay CV of 3.5, and 3.0%) were determined by enzymatic methods. The alanine aminotransferase levels were determined by ultra violet kinetic methods (Erlic, Tlalnepantla, Estado de México, Méx.).32
All measurements were performed using an Express 500 clinical chemistry autoanalyzer (Ciba Corning, Diagnostic Corp., Overling, Ohio).
Statistical analysisDifferences between the groups were estimated using Mann-Whitney U test for quantitative variables and χ2 test (Fisher’s exact test) for qualitative variables.
The optimal values of TyG index for screening simple steatosis and NASH were established on a Receiver Operating Characteristic scatter plot curve, for both simple steatosis and NASH; sensitivity, specificity as well as the positive and negative likelihood ratios of TyG index, were estimated versus liver biopsy.33
To compare the efficacy of TyG index, for screening simple steatosis and NASH, vs. other well-known biomarkers, we used a chart in which sensitivity and 1-specificity are plotted in the y and x axis, respectively; from the 0,0 point a line crossing the point of biomarker with the best sensitivity and specificity is drawn. In the same way, a line is drawn from the point 1,1. In this way, the chart is separated into 4 quadrants: the upper-left quadrant indicates the area of tests with best efficacy for diagnosis and screening; the upper-right quadrant is the area for tests with best screening efficacy; the lower-right quadrant is the area for tests with low efficacy for diagnosis and screening; and the lower-left quadrant is the area for the tests with the best efficacy for diagnosis.34
A p value of < 0.05 defined statistical significance. Data were analyzed using the statistical package SPSS 15.0. (SPSS Inc., Chicago IL, USA).
ResultsA total of 50 asymptomatic women with average age and BMI of 41.7 ± 12.3 years and 33.0 ± 7.1 kg/m2 were enrolled.
A total of 18 (36.0%) women had diagnosis of simple steatosis, 16 (32.0%) NASH, and 16 (32.0%) showed no changes in hepatic histology.
Table 1 shows the clinical and biochemical characteristics of target population. Women with simple steatosis and NASH exhibited the highest percentage of obesity and TyG index, whereas women with NASH had higher cholesterol and triglycerides levels than women with normal liver.
Characteristics of the target population.
| N | Normal liver 16 | Simple steatosis 18 | NASH 16 | P value |
|---|---|---|---|---|
| Age, years | 39.6 ± 14.5 | 45.7 ± 11.0 | 39,31 10,756 | 0.23 |
| Normal weight, n (%) | 4 (25.0) | 1 (5.5) | 1 (6.2) | 0.04*** |
| Overweight, n (%) | 6 (37.5) | 2 (11.1) | 4 (25.0) | 0.07 |
| Obesity, n (%) | 6 (37.5) | 15 (83.3) | 11 (68.8) | 0.04*** |
| Body mass index, kg/m2 | 28.4 ± 5.1 | 37.4 ± 7.7 | 32.6 ± 5.2 | 0.001* |
| Waist circumference, cm | 97.0 ± 13.3 | 105.8 ± 19.6 | 104.4 ± 11.3 | 0.22 |
| Systolic blood pressure, mmHg | 114.3 ± 24.4 | 127.1 ± 14.91 | 19.3 ± 20.9 | 0.19 |
| Diastolic blood pressure, mmHg | 69.3 ± 12.3 | 84.9 ± 11.3 | 71.3 ± 18.4 | 0.004*,† |
| Fasting glucose, mmol/L | 5.3 ± 2.5 | 5.7 ± 1.2 | 6.7 ± 3.7 | 0.32 |
| Total cholesterol, mmol/L | 4.4 ± 0.8 | 5.2 ± 1.3 | 5.8 ± 1.8 | 0.02** |
| Triglycerides, mmol/L | 1.1 ± 0.4 | 2.1 ± 1.3 | 2.6 ± 2.0 | 0.01** |
| AST, U/L | 41.7 ± 21.4 | 58.0 ± 29.3 | 83.8 ± 153.9 | 0.41 |
| ALT, U/L | 42.7 ± 24.2 | 67. 9 ± 30.7 | 102.5 ± 192.7 | 0.32 |
| TyG index | 4.50 ± 0.28 | 4.85 ± 0.22 | 4.95 ± 0. 37 | 0.001*,† |
Data are mean ± standard deviation; AST, aspartate aminotransferase; ALT, alanine aminotransferase; TyG index, Ln[fasting triglycerides (mg/dL) X fasting glucose (mg/dL)/2].17
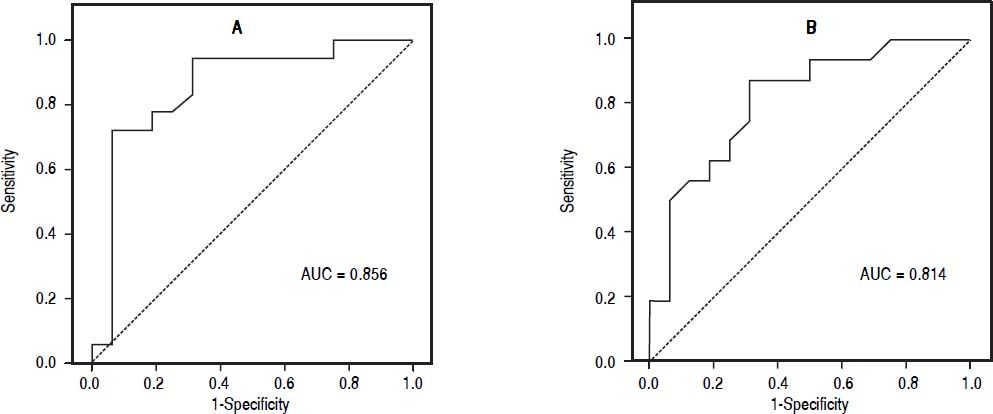
The best cutoff point of TyG index for screening simple steatosis was 4.58 (sensitivity 0.94, specificity 0.69); in addition, the best cutoff point of TyG index for screening NASH was 4.59 (sensitivity 0.87, specificity 0.69) (Figure 1). The positive and negative likelihood ratios were 3.03 and 0.08 for simple steatosis, and 2.80 and 0.18 for NASH.
Receiver operating characteristic curve. Sensitivity represents true-positive results, and 1-specificity, false-positive results. The best cutoff point of TyG index for identify simple steatosis and NASH were 4.58 and 4.59, respectively, which showed the highest sensitivity and specifcity.
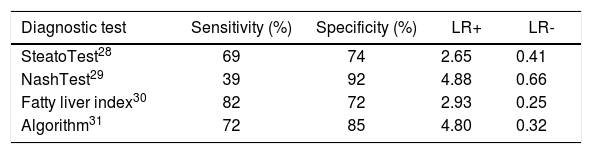
Table 2 shows the diagnostic characteristics of the clinical biomarkers that have been evaluated versus hepatic biopsy. The Algorithm, showed the best sensitivity and specificity; thus, it was the reference for drawing the chart to compare the efficacy of clinical biomarkers for recognizing NAFLD.
Characteristics of the clinical biomarkers evaluated for recognizing NAFLD.
| Diagnostic test | Sensitivity (%) | Specificity (%) | LR+ | LR- |
|---|---|---|---|---|
| SteatoTest28 | 69 | 74 | 2.65 | 0.41 |
| NashTest29 | 39 | 92 | 4.88 | 0.66 |
| Fatty liver index30 | 82 | 72 | 2.93 | 0.25 |
| Algorithm31 | 72 | 85 | 4.80 | 0.32 |
LR+: Positive likelihood ratio. LR-: Negative likelihood ratio.
The TyG index was the best test for screening simple steatosis and NASH. NashTest was the best test for diagnosing NAFLD. The SteatoTest the worst test for diagnosing and screening NAFLD (Figure 2).
Usefulness of clinical diagnostic tests to identify NAFLD. The Algorithm test,31 was the reference test for drawing the chart. TyG index to identify simple steatosis and NASH are presented as white square and black square, respectively.
Results of this study demonstrate that the TyG index has high sensitivity and low negative likelihood ratio, strongly suggesting that the TyG index may be a useful clinical biomarker for screening simple steatosis and NASH in asymptomatic women. As compared with other well-known clinical biomarkers for NAFLD, the TyG index was the best test for screening simple steatosis and NASH.
Hepatic fat accumulation is associated with insulin resistance, a common finding in patients with NAFLD;8,35–43 in addition, the NAFLD is characterized by the increase of adipose peripheral tissue,42-44 insulin resistance at adipocyte level,45–47 and inhibition of fatty acid oxidation, findings suggesting that insulin resistance plays an important role in the pathogenesis of hepatic steatosis.48 On this regard, biomarkers of insulin resistance emerge as promising tool for screening NAFLD.
In agree with the abovementioned statement, the TyG index, a biomarker to identify insulin resistance, showed its efficacy as a tool for screening simple steatosis and NASH. Keeping in mind that objective of screening is the early detection of disease, our results suggests that TyG index, with the highest sensitivity and lowest negative likelihood ratio, may be useful for screening simple steatosis and NASH.
Several index have been proposed for the early recognition of NAFLD. Among them, the SteatoTest and NashTest,28,29 which are expensive and not available in most laboratories of undeveloped countries. In addition, fatty liver index and algorithm,31,30 which although are accessible, involve numerous variables and complex calculations, which limit its use in the clinical setting. As compared vs. these biomarkers, the TyG index showed to be the best test for screening simple steatosis and NASH, with the advantage that TyG index is a simple, inexpensive, reliable, and reproducible biomarker, based on routine laboratory tests available in all clinical laboratories.
Recently, Fedchuk, et al.49 evaluate the diagnostic value of TyG index in patients with suspected NAFLD. The AUC of TyG index reported by Fedchuk, et al.49 was similar to the AUC of our study, supporting the statement that the TyG could be a useful index to identify NAFLD.
Several limitations of our study deserve to be mentioned:
- •
Because the required liver biopsy among healthy participants of control group was performed during upper abdominal surgery due to cholecystectomy, procedure indicated in women but not in men, our data cannot be applied to men. Further studies are needed to validate the efficacy of TyG index for early detection of simple steatosis and NASH in men.
- •
The small sample size could be a source of bias; however, women in the control group, showed a significantly lower BMI, waist circumference, diastolic blood pressure, total cholesterol, triglycerides, and TyG index than women with simple steatosis or NASH, data indicating that a possible type II error in analysis of data was avoided and supports the appropriate power of sample size.
Compared versus liver biopsy, the TyG index, as clinical biomarker for screening simple steatosis and NASH in asymptomatic women, has high sensitivity and low negative likelihood ratio. As compared with other clinical biomarkers for recognizing NAFLD, the TyG index showed to be the best test for screening simple steatosis and NASH.
Abbreviations- •
AUC: area under curve.
- •
BMI: body mass index.
- •
NAFLD: non-alcoholic fatty liver disease.
- •
TyG: product of triglyceride and glucose levels.
Authors have none conflict of interest declared within the manuscript.
Financial SupportThis work was supported by grants from the Mexican Social Security Institute Foundation, Civil Association.