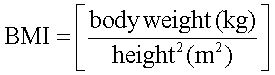Background and rational. Data on newer adipokines and interleukins in patients with nonalcoholic fatty liver disease (NAFLD) are inconclusive. The primary aim of this study was the evaluation of serum vaspin, resistin, retinol-binding protein (RBP)-4, inter-leukin (IL)-1α and IL-6 levels in NAFLD patients compared to matched controls, and their association with disease severity.
Material and methods. Twenty-nine consecutively enrolled NAFLD patients with histologically confirmed nonalcoholic simple steatosis (SS; n = 15) or steatohepatitis (NASH; n = 14) and 25 matched controls without NAFLD were recruited. Serum vaspin, resistin, RBP-4, IL-1α and IL-6 and biochemical tests were measured.
Results. Serum vaspin levels were lower and IL-6 levels higher in NASH patients than controls, but similar between controls and SS patients, or NASH and SS patients (vaspin, controls: 728.5 ± 39.3; SS: 634.6 ± 63.7; NASH: 531.5 ± 52.0 pg/mL; p for trend 0.028; IL-6, controls: 1.5 ± 0.2; SS: 2.5 ± 0.6; NASH: 3.0 ± 0.6 pg/mL; p for trend 0.032). However, after adjustment for body mass index or waist circumference, both vaspin and IL-6 did not remain significantly different between groups. Resistin, RBP-4 and IL-1α were not statistically different between groups. None of the selected adipokines or interleukins could independently differentiate NAFLD from SS, or patients with more severe from less severe histological lesions.
Conclusion. Lower circulating vaspin, but higher IL-6 levels were observed in NASH patients than controls, whereas resistin, RBP-4 and IL-1α levels were similar between groups. However, these differences did not remain robust after adjustment for body mass index or waist circumference.
Nonalcoholic fatty liver disease (NAFLD), ranges from nonalcoholic simple steatosis (SS) to nonalcoholic steatohepatitis (NASH), NASH-related cirrhosis and hepatocellular carcinoma.1 Its distribution is global, since NAFLD affects both developed and developing world populations, possibly due to the adoption of western lifestyle in the latter. Its worldwide prevalence in the general population is estimated to be 20–30% in western countries and 5–18% in Asia, and it is increasing over time.2
Adipokines, polypeptides produced by adipose tissue, affect hepatic and systematic metabolism.3 It seems that adipokine alterations, occurring during the expansion of adipose tissue, may contribute to the development of SS and to the progression to NASH.3 Examples of adipokines related to NAFLD are adiponectin and leptin. In two metaanalyses, adiponectin levels were shown to progressively decrease,4 whereas leptin levels to progressively increase5 in the sequence non-NAFLD controls - SS patients NASH patients. There is limited and inconclusive evidence for other adipokines, including resistin, retinol-binding protein (RBP)-4 and vaspin, as well as conventional cytokines, including interleukin (IL)-1α and IL-6, partly produced by immune cells infiltrating adipose tissue when it expands.3 A limitation of the most previous relevant studies was that controls were of lower body mass index (BMI) and/or waist circumference (WC), and an adjustment for BMI or WC had been performed only in a minority of them. However, adiposity is an important confounding, when considering the interplay between adipokines/cytokines and NAFLD or any obesity-related disease.3
The primary aim of this study was the evaluation of serum vaspin, resistin, RBP-4, IL-1α and IL-6 levels in patients with histologically confirmed SS and NASH compared to controls of similar gender, age, BMI and WC. Secondary aims were:
- •
The evaluation of studied adipokines and interleukins within specific hepatic histological lesions.
- •
The association of the studied adipokines and interleukins with other metabolic and inflammatory parameters, and liver function tests.
This was a single-center, cross-sectional study. NAFLD patients and controls were consecutively recruited on an outpatient basis between June 2008 and November 2010 at the Second Medical Clinic (Aristotle University of Thessaloniki, Ippokration Hospital, Thessaloniki, Greece). Determination of eligibility was based on medical history, physical examination, and liver function tests (serum aspartate transaminase [AST], alanine transaminase [ALT], gamma-glutamyl transferase [GGT], alkaline phosphatase [ALP], total and direct bilirubin) and liver ultrasound imaging performed during the screening visit. The study protocol conformed to the ethical guidelines of the Declaration of Helsinki and was approved by the local ethics committee. All participants provided a written informed consent.
Inclusion criteria for the NAFLD patients were:
- •
Age > 18 years.
- •
Bright liver on ultrasound imaging and increased liver function tests for at least 6 months before liver biopsy.
- •
Patient’s consent for liver biopsy.
NAFLD patients were subdivided into those with SS or NASH according to the criteria of NASH Clinical Research Network [NAFLD Activity Score (NAS)].6 Steatosis grade, fibrosis stage, lobular and portal inflammation, and ballooning were categorized based on the classification of NASH Clinical Research Network.6 Regarding fibrosis stage, cirrhosis (grade 4) was not included (exclusion criterion).
Individuals without NAFLD, undergoing regular checkup for professional needs at the same hospital, were recruited as controls. The controls were living in the same region, and were selected to be of similar gender, age, BMI and WC with patients. Inclusion criteria for the controls were:
- •
Age >18 years.
- •
No history of abnormal liver ultrasound imaging or abnormal liver function tests.
- •
Currently normal liver ultrasound imaging.
- •
Currently normal liver function tests.
The controls were not subjected to liver biopsy, due to obvious ethical considerations.
Exclusion criteria for both NAFLD patients and controls, as described in details elsewhere,7 were:
- •
Ethanol consumption > 20 g/day.
- •
Liver cirrhosis.
- •
Other liver disease (viral hepatitis, autoimmune hepatitis, primary sclerosing cholangitis, primary biliary cirrhosis [currently termed as primary biliary cholangitis] and overlap syndromes, drug-induced liver disease, hemochromatosis, Wilson’s disease, α1-antit-rypsin deficiency).
- •
Type I diabetes mellitus.
- •
Pancreatitis.
- •
Uncontrolled hypothyroidism or hyperthyroidism.
- •
Adrenal insufficiency.
- •
Renal failure.
- •
Thrombotic disorders.
- •
Cancer.
- •
Pregnancy.
- •
Addiction to any drug.
- •
Use of the following medications within a 12-month period before screening: estrogens, progestins, gluco-corticosteroids, thiazolidinediones, insulin, sibutramine, orlistat, rimonabant, vitamin E, vitamin C, ursodeoxycholic acid, ferrum, interferon, tamoxifene, amiodarone, biologic agents, folate or vitamin B supplements, antibiotic, any medication against tuberculosis, epilepsy or viruses, or any medication affecting hemostasis, such as antiplatelet agents, aspirin or oral anticoagulants.
- •
Use of intravenous glucose administration or parenteral nutrition within a 1-month period before screening.
Morning (8-9 am) fasting blood samples were collected 1–2 h prior to liver biopsy; the latter was performed under computed tomography-guidance by an experienced radiologist and was interpreted by two experienced pathologists. Serum AST, ALT, GGT, ALP, creatinine, triglycerides, high-density lipoprotein cholesterol (HDL-C), and glucose were measured within 1 h after blood drawing, with standard methods on an automated analyzer (Olympus AU2700; Olympus, Hamburg, Germany). Erythrocyte sedimentation rate (ESR) was also measured within 1h after blood drawing, on another automated analyzer (Ves Matic 20; Menarini Diagnostics, Rungis, France). Sera were also immediately frozen at −30°C for the measurement of high-sensitivity C-reactive protein (hsCRP), insulin, vaspin, resistin, RBP-4, IL-1α and IL-6, measured in one batch at the end of the study. hsCRP was measured with latex-enhanced immunonephelometry on a BNII analyzer (Siemens Healthcare Diagnostics, Deerfield, IL, USA). Insulin was measured with immuno-chemiluminescence on an Immulite 2500 immunoassay system (Siemens Healthcare Diagnostics, Deerfield, IL, USA). IL-6 was measured with electrochemiluminescence on a Modular Analytics E170 (Roche Diagnostics, Basel, Switzerland; sensitivity 0.7 pg/mL). Vaspin (sensitivity 26 pg/mL), resistin (sensitivity 2 pg/mL), RBP-4 (sensitivity 0.1 ng/mL), IL-1α (sensitivity 0.2 pg/mL) were measured with enzyme-linked immunosorbent assay (ELISA; RayBiotech, Norcross, GA, USA): vaspin (intra-assay coefficient of variation (CV)< 10% ; inter-assay CV < 15%); resistin (intra-assay CV < 10%; inter-assay CV < 12%); RBP-4 (intra-assay CV < 10%; inter-assay CV < 15%); IL-1α (intra-assay CV < 10%; inter-assay CV < 12%).
BMI was calculated by the formula:
IR was quantified by homeostasis model of assessment insulin resistance (HOMA-IR) using the formula:
(AST/ALT) ratio was also calculated.
Statistical analysisContinuous variables are presented as mean ± standard error of the mean (SEM). Categorical variables are presented as frequencies. Kolmogorov-Smirnov test was used to check the normality of distributions of continuous variables. χ2 test was used for between group comparisons, in case of categorical variables. Spearman’s coefficient (rs) was used for binary correlations. In case of continuous variables, between groups comparisons were performed with independent samples T-test or Mann-Whitney test, when the compared groups were two, or one-way analysis of variance (ANOVA) or Kruskal-Wallis test, when the compared groups were more than two. In case of statistically significant difference in ANOVA or Kruskal-Wallis test, Bonferroni post-hoc correction was used for multiple pairwise comparisons. Analysis of covariance (ANCOVA) was used to adjust between group comparisons for covariates. Multiple logistic regression analysis (method “enter”) was used within NAFLD patients to identify whether the studies adipokines or interleukins were independently associated with NASH (dependent variable SS = 0 or NASH = 1) or specific histological lesions (e.g., dependent variable for steatosis grade: ≤ 33% = 0 or > 33% = 1). For the need of logistic regression analysis, included variables that did not follow normal distribution were logarithmically transformed. Statistical analysis was performed with SPSS 21 for Macintosh (IBM Corporation, Armonk, NY, USA). Significance was set at p<0.05 (two-tailed) in all tests.
ResultsComparisons between groupsTwenty-nine patients with biopsy-proven NAFLD (15 with SS and 14 with borderline or definite NASH) and 25 controls were included in this study. Serum vaspin levels were lower in NAFLD patients (n = 29) compared with the controls (586.8 ± 42.2 vs. 728.5 ± 39.3 pg/mL, respectively; P = 0.018) and IL-6 levels higher (2.7 ± 0.4 vs. 1.5 ± 0.2 pg/mL, respectively; P = 0.009). Resistin, RBP-4 and IL-1α levels were similar between NAFLD patients and controls.
Subsequently, comparisons among controls (n = 25), SS (n = 15) and NASH (n = 14) patients were performed. Comparative data of the study groups are presented in Table 1. Sex, age, BMI and WC were similar between groups. There was a statistically significant trend towards lower vaspin levels and higher IL-6 levels, from controls to SS patients and to NASH patients (Table 1). In pair-wise comparisons, serum vaspin levels were statistically lower in NASH patients than controls (p = 0.025), but similar between SS patients and controls, or SS and NASH patients (Figure 1A). Conversely, serum IL-6 levels were statistically higher in NASH patients than controls (p = 0.046), but similar between SS patients and controls, or SS and NASH patients (Figure 1B). There was not a statistically significant trend regarding resistin, RBP-4 and IL-1α. As expected, there were statistically significant trends in ALT, AST, ALT/AST ratio, GGT, ALP, triglycerides, HDL-C, glucose, insulin and HOMA-IR between groups, with NASH generally being the group with the higher metabolic burden (Table 1). ESR was higher in NASH patients than either controls or SS patients. Despite a similar trend in hsCRP, the difference did not reach the level of statistical significance.
Comparative data of the study groups.
| Control group | SS patients | NASH patients | p-value for trend* | Reference range | |
|---|---|---|---|---|---|
| Patients/Women (N) | 25/20 | 15/10 | 14/12 | 0.438 | - |
| Age (years) | 53.6 ± 1.8 | 53.9 ± 2.6 | 54.8 ± 1.6 | 0.921 | - |
| BMI (kg/m2) | 30.5 ± 0.8 | 31.9 ± 1.3 | 33.9 ± 1.6 | 0.109 | 20-25 |
| WC (cm) | 99.8 ± 1.7 | 105.1 ± 3.3 | 107.3 ± 2.7 | 0.083 | Male < 94 Female < 80 |
| AST (U/L) | 20.0 ± 0.8 | 27.4 ± 2.0a | 41.5 ± 3.9a,b | <0.001 | 10-31 |
| ALT (U/L) | 19.4 ± 1.7 | 41.7 ± 5.5 | 65.0 ± 15.0a | <0.001 | 10-34 |
| AST/ALT ratio | 1.14 ± 0.06 | 0.76 ± 0.07a | 0.79 ± 0.07a | <0.001 | - |
| GGT (U/L) | 19.4 ± 3.3 | 46.5 ± 11.7 | 60.9 ± 13.9a | <0.001 | 0-38 |
| ALP (U/L) | 62.8 ± 2.8 | 83.8 ± 7.5a | 79.0 ± 7.1 | 0.012 | 30-120 |
| Creatinine (mg/dL) | 0.90 ± 0.02 | 0.94 ± 0.04 | 0.83 ± 0.03 | 0.066 | 0.6-1.1 |
| Triglycerides (mg/dL) | 119.5 ± 11.6 | 162.3 ± 21.9 | 210.8 ± 29.3a | 0.006 | < 150 |
| HDL-C (mg/dL) | 58.2 ± 3.0 | 50.7 ± 2.8 | 47.2 ± 1.6a | 0.022 | Male > 40 Female > 50 |
| Glucose (mg/dL) | 89.0 ± 2.3 | 99.9 ± 7.3 | 110.6 ± 5.3a | 0.007 | 60-100 |
| Insulin (μU/mL) | 5.2 ± 3.6 | 10.2 ± 1.8 | 15.1 ± 2.8a | 0.002 | 6-27 |
| HOMA-IR | 1.20 ± 0.21 | 2.49 ± 0.45 | 4.48 ± 0.94a,b | 0.001 | na |
| ESR 1h (mm) | 17.7 ± 2.1 | 14.8 ± 3.0 | 35.4 ± 8.4a,b | 0.020 | |
| hsCRP (mg/L) | 3.8 ± 0.4 | 3.5 ± 0.4 | 6.0 ± 1.4 | 0.096 | |
| Vaspin (pg/mL) | 728.5 ± 39.3 | 634.6 ± 63.7 | 531.5 ± 52.0a | 0.028 | na |
| Resistin (pg/mL) | 72.6 ± 12.6 | 48.3 ± 12.9 | 44.6 ± 10.5 | 0.235 | na |
| RBP-4 (ng/mL) | 15.9 ± 2.2 | 13.9 ± 2.7 | 8.3 ± 1.9 | 0.076 | na |
| IL-1α (pg/mL) | 2.8 ± 0.6 | 1.5 ± 0.5 | 1.4 ± 0.6 | 0.203 | na |
| IL-6 (pg/mL) | 1.5 ± 0.2 | 2.5 ± 0.6 | 3.0 ± 0.6a | 0.032 | na |
Data are presented in mean ± standard error of the mean (SEM).
p<0.05 compared to SS patients (Bonferroni post-hoc correction). ALP: alkaline phosphatase. AST: aspartate transaminase. ALT: alanine transaminase. BMI: body mass index. ESR: erythrocyte sedimentation rate. HDL-C: high-density lipoprotein cholesterol. HOMA-IR: homeostasis model of assessment insulin resistance. hsCRP: high-sensitivity C-reactive protein. IL: interleukin. GGT: gamma glutamyl transferase. NAFLD: nonalcoholic fatty liver disease. NASH: nonalcoholic steatohepatitis. RBP: retinol-binding protein. SS: simple steatosis. WC: waist circumference.
Serum vaspin (A) and interleukin-6 (B) levels (mean ± standard error of the mean) in patients with simple steatosis (SS), nonalcoholic steatohepatitis (NASH) and controls. Serum vaspin levels was significantly higher, whereas interleukin-6 lower in NASH patients than controls. * p < 0.05 compared to control group.
After adjustment for BMI, both vaspin (estimated marginal means; controls: 728.4 ± 43.3; SS: 634.6 ± 55.2; NASH: 541.0 ± 62.8 pg/mL; p = 0.057) and IL-6 (estimated marginal means; controls: 1.5 ± 0.3; SS patients: 2.5 ± 0.4; NASH patients: 2.5 ± 0.5 pg/mL; p = 0.138) did not remain significantly different between groups. Non-significant comparative results between groups did not change for resistin, RBP-4 and IL-1α, after adjustment for BMI. Similar results were retrieved after adjustment for WC instead of BMI.
In logistic regression analysis within NAFLD patients (n = 29), the presence or not of NASH was selected as dependent variable. None of the studied adipokines or interleukins was associated with NASH independently from BMI (or WC) (model 1), or BMI (or WC), age, log(ALT) and log(HOMA-IR) (model 2).
Comparisons within specific histological lesionsCirculating adipokines and interleukins were compared within specific histological lesion of NAFLD patients (n = 29). No statistically significant difference was observed in vaspin, resistin, RBP-4, IL-1α and IL-6 between groups of low vs. high grade of steatosis, fibrosis, lobular inflammation, portal inflammation or ballooning (Table 2). These results did not change for any histological lesion after adjustment for each adipokine or interleukin for BMI.
Vaspin, resistin, RBP-4, IL-1α and IL-6 levels between groups of specific histological lesions in NAFLD patients (n = 29).
| Histological lesion | Patients (N) | Vaspin (pg/mL) | Resistin (pg/mL) | RBP-4 (ng/mL) | IL-1α (pg/mL) | IL-6 (pg/mL) |
|---|---|---|---|---|---|---|
| Steatosis grade (p-value)* | 0.249 | 0.492 | 0.272 | 0.669 | 0.903 | |
| < 33% | 18 | 623.6 ± 54.4 | 44.8 ± 10.9 | 12.8 ± 2.3 | 1.3 ± 0.5 | 2.7 ± 0.6 |
| > 33% | 11 | 520.4 ± 64.6 | 49.3 ± 12.9 | 8.8 ± 2.3 | 1.7 ± 0.7 | 2.8 ± 0.6 |
| Fibrosis stage (p-value)* | 0.710 | 0.730 | 0.731 | 0.206 | 0.725 | |
| Absent | 10 | 565.1 ± 68.7 | 43.2 ± 18.8 | 10.4 ± 3.1 | 0.9 ± 0.4 | 2.5 ± 0.8 |
| Present | 19 | 598.8 ± 54.8 | 48.3 ± 8.2 | 11.7 ± 2.1 | 1.8 ± 0.6 | 2.8 ± 0.5 |
| Lobular inflammation (p-value)* | 0.670.274 | 0.1050.550 | 0.151 | |||
| Absent | 18 | 644.6 ± 58.4 | 53.7 ± 12.7 | 13.4 ± 2.4 | 1.6 ± 0.5 | 2.4 ± 0.6 |
| Present | 11 | 497.4 ± 50.1 | 34.8 ± 5.5 | 7.7 ± 2.0 | 1.2 ± 0.6 | 3.3 ± 0.7 |
| Portal inflammation (p-value)* | 0.141 | 0.098 | 0.108 | 0.776 | 0.465 | |
| None to minimal | 18 | 637.1 ± 58.4 | 55.2 ± 12.8 | 13.4 ± 2.4 | 1.4 ± 0.5 | 2.4 ± 0.5 |
| Greater than minimal | 11 | 508.9 ± 53.1 | 32.3 ± 3.1 | 7.7 ± 2.0 | 1.6 ± 0.7 | 3.3 ± 0.8 |
| Ballooning (p-value)* | 0.226 | 0.246 | 0.255 | 0.545 | 0.228 | |
| Absent | 6 | 686.0 ± 117.5 | 28.7 ± 5.7 | 15.1 ± 4.0 | 1.3 ± 0.6 | 1.9 ± 0.8 |
| Present | 23 | 559.7 ± 43.1 | 51.2 ± 10.1 | 10.2 ± 1.9 | 1.5 ± 0.5 | 3.0 ± 0.5 |
Data are presented in mean ± standard error of the mean (SEM).
In logistic regression analysis within each histological lesion (i.e., steatosis, fibrosis, lobular inflammation, portal inflammation and ballooning), none of the studied adipokines or interleukins was associated with none histological lesion independently from BMI (or WC) (model 1), or BMI (or WC), age, log(ALT) and log(HOMA-IR) (model 2).
Correlations between adipokines, interleukins and other parametersCorrelations between adipokines, interleukins and other parameters of the study are presented in table 3. Vaspin was positively correlated with resistin, RBP-4 and HDL-C, whereas negatively with AST and NAS. Resistin was positively correlated with RBP-4, IL-1 α and HDL-C. RBP-4 was positively correlated with IL-1 α and HDL-C. IL-6 was positively correlated with WC, AST, glucose, insulin, HOMA-IR, ESR and hsCRP. Apart from the aforementioned positive correlations with resistin and RBP-4, no other correlation was observed for IL-1α.
Correlations between adipokines, interleukins and other study parameters.
| Vaspin (pg/mL) | Resistin (pg/mL) | RBP-4 (ng/mL) | IL-1α (pg/mL) | IL-6 (pg/mL) | |
|---|---|---|---|---|---|
| Age (years) | 0.009 (0.954) | 0.038 (0.783) | 0.110 (0.428) | -0.174 (0.222) | 0.178 (0.202) |
| BMI (kg/m2) | -0.214 (0.128) | -0.207 (0.138) | -0.056 (0.688) | -0.172 (0.233) | 0.261 (0.062) |
| WC (cm) | -0.205 (0.149) | -0.132 (0.352) | -0.099 (0.486) | -0.053 (0.717) | 0.296 (0.035) |
| AST (U/L) | -0.358 (0.009) | -0.023 (0.867) | 0.004 (0.980) | 0.111 (0.440) | 0.297 (0.031) |
| ALT (U/L) | -0.260 (0.060) | -0.044 (0.752) | -0.067 (0.630) | 0.087 (0.544) | 0.233 (0.093) |
| AST/ALT ratio | 0.065 (0.646) | 0.027 (0.845) | 0.135 (0.332) | 0.023 (0.873) | 0.042 (0.759) |
| GGT (U/L) | -0.159 (0.255) | -0.108 (0.436) | 0.040 (0.775) | 0.018 (0.902) | 0.148 (0.289) |
| ALP (U/L) | -0.113 (0.419) | -0.051 (0.716) | -0.010 (0.940) | 0.155 (0.422) | -0.020 (0.885) |
| Creatinine (mg/dL) | 0.048 (0.733) | 0.072 (0.604) | -0.078 (0.577) | 0.068 (0.636) | -0.225 (0.102) |
| Triglycerides (mg/dL) | -0.250 (0.071) | -0.065 (0.641) | -0.132 (0.342) | -0.073 (0.610) | 0.098 (0.485) |
| HDL-C (mg/dL) | 0.339 (0.013) | 0.327 (0.016) | 0.292 (0.032) | -0.020 (0.887) | -0.012 (0.929) |
| Glucose (mg/dL) | -0.122 (0.383) | 0.015 (0.915) | 0.140 (0.311) | 0.037 (0.797) | 0.358 (0.008) |
| Insulin (μ/mL) | -0.174 (0.217) | -0.133 (0.341) | 0.055 (0.698) | -0.030 (0.835) | 0.470 (<0.001) |
| HOMA-IR | -0.176 (0.213) | -0.115 (0.412) | 0.083 (0.554) | -0.027 (0.851) | 0.490 (<0.001) |
| ESR 1h (mm) | -0.198 (0.155) | -0.031 (0.823) | 0.071 (0.610) | -0.174 (0.222) | 0.557 (<0.001) |
| hsCRP (mg/L) | -0.104 (0.457) | -0.027 (0.847) | 0.134 (0.335) | 0.025 (0.862) | 0.393 (0.004) |
| NAS | -0.391 (0.039) | 0.088 (0.651) | -0.325 (0.085) | -0.055 (0.778) | 0.258 (0.185) |
| IL-6 (pg/mL) | -0.171 (0.122) | 0.001 (0.998) | 0.003 (0.983) | 0.051 (0.725) | - |
| RBP-4 (ng/mL) | 0.522 (<0.001) | 0.389 (0.004) | - | - | - |
| Resistin (pg/mL) | 0.364 (0.007) | - | - | - | - |
Data are presented as Spearman’s coefficient of correlation (p-value). ALP: alkaline phosphatase. AST: aspartate transaminase. ALT: alanine transaminase. BMI: body mass index. ESR: erythrocyte sedimentation rate. HDL-C: high-density lipoprotein cholesterol. HOMA-IR: homeostasis model of assessment insulin resistance. hsCRP: high-sensitivity C-reactive protein. IL: interleukin. GGT: gamma glutamyl transferase. NAS: NAFLD activity score. RBP: retinol-binding protein. SS: simple steatosis. WC: waist circumference.
In this series, lower circulating vaspin, but higher IL-6 levels were observed in NASH patients than controls; however, both vaspin and IL-6 levels were similar in SS and NASH patients and were not independently associated with NASH. Resistin, RBP-4 and IL-Ια levels were similar between groups and were also not independently associated with NASH. None of the studied adipokines or interleukins showed a distinct distribution within specific hepatic histological lesions (i.e., steatosis, fibrosis, lobular and portal inflammation, and ballooning).
Data on the selected adipokines and interleukins are limited and/or inconclusive in NAFLD populations, as we have recently reviewed.3 Regarding the pleiotropic proinflammatory cytokine, IL-6, it is produced by various tissues, including the spleen,8 visceral adipose tissue and the liver;9 its primary physiological function involves induction of acute phase reactions by stimulating hepatocytes. In studies with histologically confirmed NAFLD, most authors reported higher IL-6 levels in NAFLD10-13 or NASH11–17 patients than controls, whereas few authors reported similar levels between NASH patients and controls.18,19 In relative comparisons between SS and NASH patients, higher IL-6 levels in NASH than SS patients were observed in some studies,15–17,20,21 whereas similar IL-6 levels in other studies.10,12–14,19,22 Although there was a trend towards higher IL-6 levels in NASH compared with SS in our study (Table 1; Figure 1), this did not reach the level of statistical significance, possibly owing to small sample size and histological classification. Regarding specific hepatic histological lesions, most authors did not report an association with IL-6, whereas a few authors reported positive association between IL-6 and steatosis,16,21 lobular inflammation16,20 or fibrosis.16,19 However, a limitation of most observational studies is the fact that the controls were of lower BMI than NAFLD patients, and adjustment for adiposity was rarely performed. Notably, in a 3-year prospective cohort study, neither IL-6 levels nor their change (baseline to month 36) was associated with NAFLD or fibrosis progression.23 Currently, the proinflammatory IL-6 is associated with the development of IR, but its exact role in the pathogenesis of NAFLD is still remaining to be determined.
Vaspin, regarded as an insulin sensitizing and anti-orexigenic adipokine, is expressed in human adipose tissue (predominantly visceral) and the liver;24 it is related to obesity and insulin sensitivity. In studies with histologically confirmed NAFLD, some authors reported higher circulating vaspin levels in NAFLD25,26 or NASH27 patients than controls, whereas other authors reported similar vaspin levels between NAFLD patients and controls.28 This controversy may be attributed to the fact that the controls were of lower BMI than patients in all previous studies,25–27 contrary to our study, in which BMI and WC were similar between groups. Indeed, when we adjusted vaspin levels for BMI (or WC), between group differences were not different. In the comparison between SS and NASH patients, similar vaspin levels were observed in two studies,25,28 similarly to our findings.
Resistin, regarded as a proinflammatory adipokine, is produced by macrophages infiltrating human adipose tissue rather than adipocytes.29 Peripheral blood mononuclear cells are also main producers of human resistin.30 Although a positive association of resistin with IR, inflammation and NAFLD are documented in rodents, the topic remains inconclusive in human NAFLD.3 In histologically confirmed NAFLD, some authors have reported higher circulating resistin levels in NAFLD,31,32 SS,33,34 or NASH34 patients than controls (of lower BMI than NAFLD patients in most studies). However, other authors reported similar resistin levels between NAFLD,12,35–37 SS,12,37,38 or NASH12,37–39 patients and controls, similarly to our findings. In the comparison between NASH and SS patients, some authors reported higher resistin levels in NASH,31,40 whereas others reported similar levels.12,34,36–38 There is also one study with pediatric NAFLD, in which lower resistin levels were observed in NASH than SS patients.41 An inverse correlation between resistin and steatosis was also shown in another study with ultrasonographically confirmed NAFLD.42
RBP-4, regarded as a proinflammatory adipokine, is predominantly expressed in visceral rather than subcutaneous adipose tissue43 and the liver;44 RBP-4 levels were found to be connected with the inflammatory response in obese individuals. Data regarding RBP-4 in studies with histologically confirmed NAFLD are inconclusive.3 Some authors reported higher circulating RBP-4 levels in SS,33,45 or NASH45 patients than controls. In the comparison between NASH and SS patients, some authors reported similar levels,45–48 whereas others reported lower levels in NASH than SS.49,50 We previously published similar RBP-4 levels between NAFLD patients and obese controls, but also a trend towards higher levels in either NAFLD patients or obese controls compared with lean controls; notably, this difference became robustly significant after adjustment for BMI.48 Although we measured RBP-4 with another kit in this study that the previous one (Biovendor Research and Diagnostic Products, Candler, NC, USA),48 this study replicated the same results regarding RBP-4 between patients with SS, NASH and controls of similar BMI.
IL-1α, regarded as a proinflammatory cytokine, for which information about its distinct role in various diseases, including NAFLD, is limited, since most clinical studies have focused on the role of IL-1β.51 To the best of our knowledge, there is only one previous study in morbidly obese patients with NAFLD, in which, as in our study, similar IL-1 a levels were observed in patients with SS, NASH and controls (of normal BMI).52 However, when peripheral blood cells were stimulated with lipopolysac-charide, IL-1 a production was higher in SS and NASH patients than controls.52 Another experimental study showed a critical role of IL-1α (and IL-1β) in the transformation of SS to NASH and liver fibrosis in hypercholes-terolemic mice. Therefore, apart from IL-1β, the possible neutralizing of IL-1α to inhibit the development of NASH should be explored.53
In this series, circulating levels of resistin, RBP-4 and IL-1 a were positively associated each other. Furthermore, vaspin was positively associated with resistin and RBP-4. Although correlations cannot prove causality, we could speculate that resistin, RBP-4 and IL-1 a increase each other in a vicious cycle of inflammation in NAFLD, whereas vaspin is increased in response to resistin and RBP-4, as a counterbalancing factor targeting to limit inflammation. The inverse association between vaspin levels and NAS, possibly implying a protective effect of vaspin in the liver, also needs further research. Finally, the positive association between IL-6 and other markers of inflammation (ESR, hsCRP) or glucose metabolism (glucose, insulin, HOMAIR) are expected, since IL-6 is a key player in liver inflammation, but also inhibits insulin receptor.9
Based on the design of this study, we could hardly propose the use of a single adipokine or a combination of them for the noninvasive diagnosis or treatment of NAFLD. As we have proposed elsewhere,3 a certain profile of adipokines/cytokines (e.g, a combination of high leptin, resistin, RBP-4, IL-6, and low adiponectin) would favour NASH over SS, but its diagnostic value remains to be elucidated. Furthermore, the deeper knowledge on the interplay between adipokines/cytokines and NAFLD may lead to clinical trials investigating the effect of adipokine-targeted interventions on NAFLD treatment, which is currently an unmet medical need.54
Strength of this study was that patients with SS and NASH and controls were of similar BMI and WC; despite this, we further adjusted the crude results for BMI or WC. This is considered to be of importance, because we consider that adiposity is the most important confounding when evaluating adipokine levels. Nevertheless, in most relevant studies, the controls were of lower BMI and WC, and adjustment for BMI or WC was rarely performed. Furthermore, NAFLD was histologically confirmed in our patients; despite its limitations, including sampling error, intra-and inter-individual variability, liver biopsy is currently considered to be the best available method for the diagnosis of NAFLD. However, this study has also certain limitations. The sample size was small, but it was sufficient to accurately replicate prior established associations of NAFLD with HOMA-IR and liver function tests, and to demonstrate significant associations with IL-6 and vaspin. Furthermore, the cross-sectional design of the study cannot prove causality, but can certainly raise credible hypotheses to be confirmed and extended by future prospective cohort as well as mechanistic studies. Moreover, the controls were not subjected to liver biopsy, due to obvious ethical considerations.
In conclusion, in this series, lower circulating vaspin, but higher IL-6 levels were observed in NASH patients than controls, whereas resistin, RBP-4 and IL-1 a levels were similar between the groups. Although none of the studied adipokines or interleukins could independently differentiate SS from NASH patients, or patients with more severe than less severe hepatic histological lesions, large-scale prospective cohort studies are needed, mainly to elucidate the role of IL-6, which possibly promotes inflammation, and vaspin, being possibly beneficial, in NAFLD. The association between IL-6 and NASH, if validated, may also have a therapeutic potential in NASH, since antibodies against IL-6 or its receptors have been approved for the treatment of inflammatory diseases, other than NASH.
Abbreviations- •
ALP: alkaline phosphatase.
- •
ALT: alanine transaminase.
- •
AST: aspartate transaminase.
- •
BMI: body mass index.
- •
ELISA: enzyme-linked immunosorbent assay.
- •
ESR: erythrocyte sedimentation rate.
- •
GGT: gamma-glutamyl transferase.
- •
HDL-C: high-density lipoprotein cholesterol.
- •
HOMA-IR: homeostatic model of assessment insulin resistance.
- •
hsCRP: high-sensitivity C-reactive protein.
- •
IL: interleukin.
- •
IR: insulin resistance.
- •
NAFLD: non-alcoholic fatty liver disease.
- •
NAS: NAFLD Activity Score.
- •
NASH: nonalcoholic steatohepatitis.
- •
RBP: retinol-binding protein.
- •
SS: simple steatosis.
- •
WC: waist circumference.
No grant or financial support for this study.
Disclosure StatementNo potential conflict of interest relevant to this article is declared.
AcknowledgmentsThe authors feel more than grateful to Dr Efthimia Zafeiriadou (experienced radiologist), who performed the liver biopsies under computed tomography-guidance, and to Dr Kalliopi Patsiaoura and Dr Evangelia Katsiki (experienced pathologists), who interpreted the histological specimens.