Background and rationale. Non-alcoholic hepatic steatosis refers to the accumulation of triglycerides in the liver in the absence of alcohol consumption. Granulocyte colony-stimulating factor (G-CSF) has been reported to be an effective treatment for a variety of liver diseases. We examined the possible therapeutic effects of G-CSF on non-alcoholic hepatic steatosis in rats.
Material and methods. Thirty-week-old Otsuka Long Evans Tokushima Fatty (OLETF) rats received water containing 30% sucrose for 8 weeks to promote the development of non-alcoholic hepatic steatosis. After development of the model, the rats were injected with G-CSF (100 Mg/kg/day) or saline for 5 days. Four weeks after this treatment, serum levels of glucose, total cholesterol (TC), triglyceride (TG), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and free fatty acids (FFA) were measured. Histology was examined by hematoxylin and eosin (H-E) and periodic acid Schiff (PAS) staining, and levels of expression of hepatic lipogenic enzymes were determined by RT-PCR.
Results. The G-CSF-treated rats displayed significantly fewer lipid droplets than the saline-treated rats (P < 0.01), and their levels of sterol regulatory element-binding protein (SREBP)-1c, fatty acid synthase (FAS), and acetyl-CoA carboxylase (ACC) mRNAs were also lower (P < 0.01), as were their liver weight and serum levels of TG and FFA (P < 0.05).
Conclusion. Our results indicate that G-CSF ameliorated non-alcoholic hepatic steatosis in the OLETF rat, and this therapeutic effect involved a reduction of SREBP-1c expression. Therefore, G-CSF deserves further study as a potential treatment for non-alcoholic hepatic steatosis.
Non-alcoholic hepatic steatosis refers to the accumulation of triglycerides in the liver in the absence of alcohol consumption.1 It is an early stage of non-alcoholic fatty liver disease (NAFLD).2 NAFLD is a major health problem affecting about 20 percent of the general population,3,4 and hepatic steatosis has been reported in 48.7% of morbidly obese patients.5 Non-alcoholic hepatic steatosis is frequently associated with obesity and insulin resistance.6,7 When accompanied by other metabolic disorders, it can progress to severe NAFLD, nonalcoholic steatohepatitis, fibrosis, and ultimately cirrhosis.8–10 Steatosis has been considered the “first hit” in a “two-hit hypothesis” for the development of NAFLD.11 At present there is no effective treatment for non-alcoholic hepatic steatosis. Lifestyle changes, similar to those recommended for obesity, are the best therapeutic option,12 but they are hard to achieve. Therefore effective drug treatments are needed.
Granulocyte colony-stimulating factor (G-CSF) is widely used to mobilize hematopoietic stem cells,13,14 and has been reported to be an effective treatment for a variety liver diseases. For example, one study demonstrated that G-CSF ameliorated acute hepatic failure by enhancing the homing of transplanted bone marrow mononuclear cells to the liver.15 Another study showed that G-CSF protected liver tissue from collagen deposition in carbon tetrachloride (CCl4)-induced liver fibrosis in mice.16 However the therapeutic effect of G-CSF on non-alcoholic hepatic steatosis, the first step in NAFLD, is unknown, although the authors have previously studied the effect of G-CSF on diabetic cardiomyopathy in rats.17
We therefore have investigated the effect of G-CSF administration on non-alcoholic hepatic steatosis and on the mRNAs encoding hepatic lipogenic enzymes sterol regulatory element-binding protein-1c (SREBP-1c), fatty acid synthase (FAS), and acetyl-CoA carboxylase (ACC).
Material and MethodsAnimalsThis study was performed in compliance with the ARRIVE guidelines on animal research,18 and the Hanyang University Institutional Animal Care and Use Committee approved all protocols. We used male Otsuka Long Evans Tokushima Fatty (OLETF) rats and control Long-Evans Tokushima Otsuka (LETO) rats, supplied by the Tokushima Research Institute, Otsuka Pharmaceutical (Tokushima, Japan). We used the OLETF rats as an animal model of non-alcoholic hepatic steatosis. OLETF rats are well-established animal models of obesity,19 type 2 diabetes mellitus,20 and NAFLD21,22 as well as hepatic steatosis.23,24 The animals were maintained in the Hanyang University Medical School Animal Experiment Center and were kept in a specific pathogen-free facility at controlled temperature (23 ± 2 oC) and humidity (55 ± 5%) with a 12-h artificial light and dark cycle.
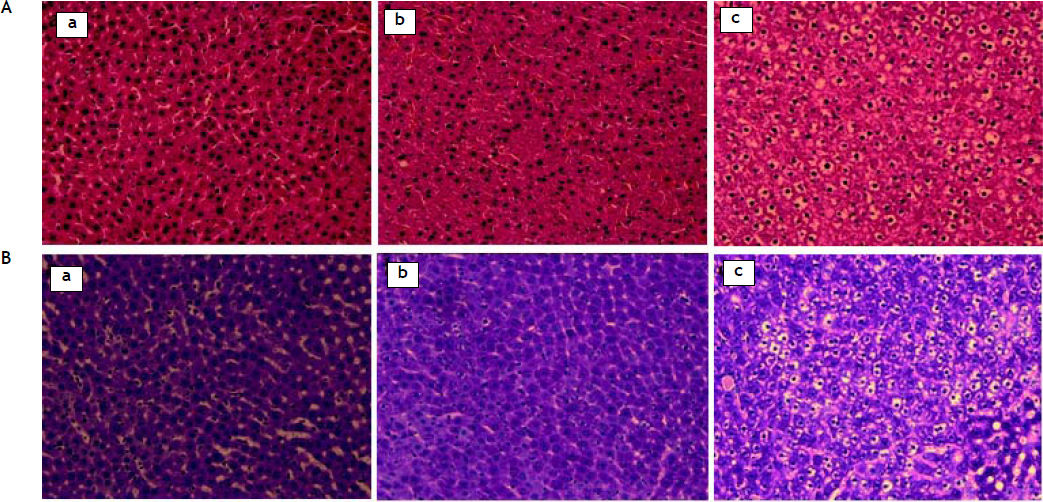
Animal model and experimental protocolStarting at 30 weeks of age, all the OLETF rats received water containing 30% sucrose for 8 weeks to facilitate the development of non-alcoholic hepatic steatosis. After steatosis had been induced, the rats received no more sucrose water. The LETO rats, as normal controls, received water without sucrose. The development of non-alcoholic hepatic steatosis was confirmed by hematoxylin and eosin (H-E) and periodic acid Schiff (PAS) staining. At 38 weeks the OLETF rats were randomly divided into a G-CSF-treated group (G-CSF 100 μg/kg/day intraperitoneally for 5 days; Leucostim, Dong-A Pharmaceutical, Korea, n = 5) and a saline-treated group (n = 4). The LETO rats (n = 4) were injected only with saline. Four weeks after the treatment, blood was collected for biochemical analysis, and the animals were killed under anesthesia. Immediately after death, the abdomens were opened and the livers were quickly removed for histopathological examination.
Biochemical analysisBlood samples were taken from the tail vein after 8 h of fasting. Serums were obtained by centrifugation, and stored at −70 oC. Serum glucose, total cholesterol (TC), triglyceride (TG), alanine aminotransferase (ALT), aspartate aminotrans (AST) and free fatty acids (FFA) were measured with an Olympus AU400 auto analyzer (Olympus GmbH, Germany).25
Histological examinationTo examine liver morphology, 4% paraformaldehyde-fixed paraffin-embedded liver sections of 3 μm thickness were stained with H-E and PAS. Degrees of hepatic steatosis were estimated by oil red O staining of frozen 3 μm sections.26 Three areas of digitized images of the oil red O-stained liver sections were selected at random from the individual sections and analyzed with Image-Pro Plus software (Media Cybernetics, MD, USA). Steatosis was calculated as a percentage of the ratio of the area of oil red O stained lipid droplets to total tissue area.23,24
Quantitative real-time polymerase chain reaction (PCR)Total RNA was extracted from 20 mg samples of liver tissue using Qiazol reagent (Qiagen, Valencia, CA) following the manufacturer’s instructions. RNA concentrations were measured with a Nanodrop ND-2000 spectrophotometer (Thermo Fisher Scientific Inc., DE, USA), and purity was determined by measuring ratios of A260 and A280, which ranged from 1.8 to 2.0.
For the real-time PCR, 3 μm RNA samples were reverse-transcribed with Moloney murine leukemia virus reverse transcriptase (Invitrogen Co., CA, USA). The mRNA expression was quantified using real-time PCR (Roche, Basel, Switzerland) with the LightCycler® FastStart DNA Master SYBR Green I kit (Roche Diagnostics, IN, USA).
The primers used were:
- •
SREBP-1c:
- °
81 bp, sense: 5’-GCT ACC GTT CCT CTA TCA ATG ACA A-3’.
- °
Anti-sense: 5’-CAG ATT TAT TCA GCT TTG CCT CAG T-3’.
- °
- •
FAS:
- °
99 bp, sense: 5’-TCC ACA GCT CTT ACA GTG AGA ATC A-3’.
- °
Anti-sense: 5’-CTT CTC CAG GGT GGG GAC CAG-3’.
- °
- •
ACC:
- °
97 bp, sense: 5’-AGA GTG AGT GCT CTC AAT TCT GTC C-3’.
- °
Anti-sense: 5’-GTC CTT CTT CTT TCC CGA TAA TGT C-3’.
- °
- •
GAPDH:
- °
96 bp, sense: 5’-CCT TCT CTT GTG ACA AAG TGG ACA T-3’.
- °
Anti-sense: 5’-CGT GGG TAG AGT CAT ACT GGA ACA T-3’.
- °
We performed PCR using the following steps: incubation for 10 min at 95 °C followed by 45 cycles of 10 s at 95 °C, 10 s at 60 °C, and 8 s at 72 °C and a final dissociation curve step at 65 °C for 15 s. The crossing point (Cp) of each PCR was automatically determined by the LightCycler® program. PCR reactions for all samples were each run in duplicate. The measured transcript levels were normalized against those of GAPDH.
Statistical analysisAll data were analyzed with SPSS statistics 17.0 software. The results are presented as means ± SD, while the oil red O staining using image analysis system are presented as means ± SE. Comparisons between groups were made using one-way analysis of variance (ANOVA) followed by the post-hoc LSD test. Side-to-side comparisons within the same group were made with Student’s t test for paired data. Values of P < 0.05 were considered statistically significant.
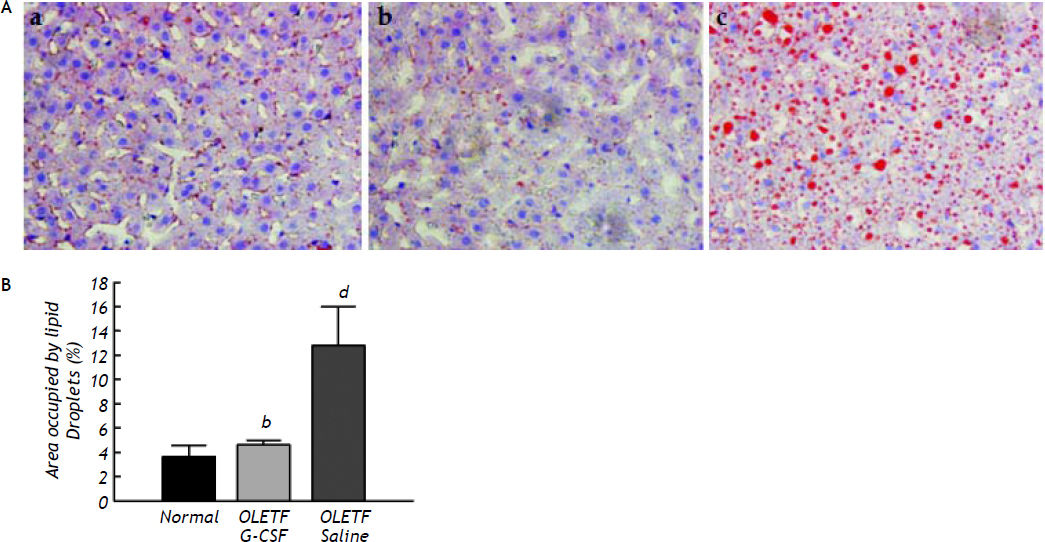
ResultsHistologyThe development of non-alcoholic hepatic steatosis was confirmed by H-E and PAS staining. All histological data were evaluated by a separate blinded investigator. Fibrosis, collagen deposition and inflammatory cell infiltration did not evident, whereas lipid vacuoles and mild ballooning in the hepatocytes were observed (data not shown). Four weeks after treatment, numerous cytoplasmic lipid vacuoles and mild ballooning were evident in the hepatocytes of the saline-treated group. In contrast, no fat accumulation could be seen in the livers of the G-CSF-treated group, which were no different from those of the normal control group (Figure 1). The area of lipid droplets as indicated by oil red O staining was much lower in the G-CSF-treated group than in the saline-treated group (4.62 ± 0.38% vs. 12.80 ± 3.23%, P < 0.01) and not significantly different from that in the normal control group (3.65 ± 0.94%) (Figure 2).
Histological changes in liver tissue seen by oil red O staining (magnification x 200). In oil red O-stained sections, red vesicles indicate lipid droplets. A. Liver of normal control rat (a), G-CSF treated OLETF rat (b) and saline-treated OLETF rat (c). B. Quantitative analysis of images of oil red O-stained liver sections. The mean percent area occupied by oil red O-stained lipid droplets was calculated for 3 randomly selected fields of each liver section. All data are expressed as means ± SD. bP < 0.01 vs. saline treated OLETF rats. d P < 0.01 vs. normal control rats.
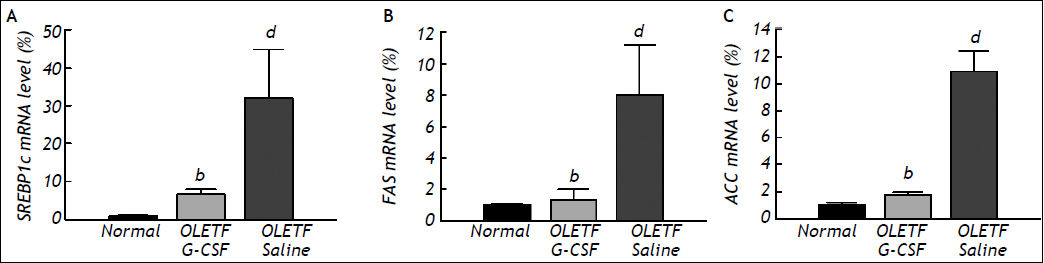
Levels of SREBP-1c, FAS, and ACC mRNA in the liver were measured by real-time PCR. The level of SREBP-1c mRNA was significantly lower in the G-CSF-treated group than in the saline-treated group (6.69 ± 1.28 vs. 32.06 ± 12.77, P < 0.01), and was equal to that in the normal control group (1.00 ± 0.20) (Figure 3A). The level of FAS mRNA in the G-CSF-treated group was also significantly lower than in the saline-treated group (1.33 ± 0.66 vs. 8.02 ± 3.20, P < 0.01), and was equal to that in the normal control group (1.00 ± 0.09) (Figure 3B). Furthermore, the level of ACC mRNA was significantly lower in the G-CSF-treated group than in the saline-treated group (1.77 ± 0.18 vs. 10.93 ± 1.46, P < 0.01), and was equal to that in the normal control group (1.00 ± 0.23) (Figure 3C).
Levels of mRNA in liver tissue after treatment as determined by real-time polymerase chain reaction (PCR) analysis. A-C. Sterol regulatory element-binding protein (SREBP)-1c, fatty acid synthase (FAS), and acetyl-CoA carboxylase mRNA levels, respectively. Mean values were obtained from the livers of four separate animals. PCR reactions were performed in duplicate. The transcript levels were normalized by comparison with GAPDH expression. All data are expressed as means ± SD. bP < 0.01 vs. saline treated OLETF rats. dP < 0.01 vs. normal control rats.
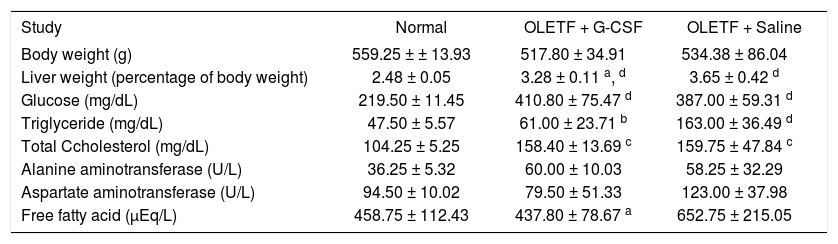
At the end of the experiment, liver weight relative to body weight was lower in the G-CSF-treated group than in the saline-treated group (3.28 ± 0.11% vs. 3.65 ± 0.42%, P < 0.05) but still higher than in the normal control group (2.48 ± 0.05%, P < 0.01). Levels of circulating TG and FFA were lower in the G-CSF-treated group than in the saline-treated group (61.00 ± 23.71 mg/dL vs. 163.00 ± 36.49 mg/dL, P < 0.01 and 437.80 ± 78.67 μEq/L vs. 652.75 ± 215.05 μEq/L, P < 0.05, respectively). Circulating glucose and TC were similar in the G-CSF-treated and saline-treated groups, but appeared higher than in the normal control group. Body weight and circulating ALT and AST were similar in the three groups (Table 1).
Levels of metabolic parameters in the three groups.
| Study | Normal | OLETF + G-CSF | OLETF + Saline |
|---|---|---|---|
| Body weight (g) | 559.25 ± ± 13.93 | 517.80 ± 34.91 | 534.38 ± 86.04 |
| Liver weight (percentage of body weight) | 2.48 ± 0.05 | 3.28 ± 0.11 a, d | 3.65 ± 0.42 d |
| Glucose (mg/dL) | 219.50 ± 11.45 | 410.80 ± 75.47 d | 387.00 ± 59.31 d |
| Triglyceride (mg/dL) | 47.50 ± 5.57 | 61.00 ± 23.71 b | 163.00 ± 36.49 d |
| Total Ccholesterol (mg/dL) | 104.25 ± 5.25 | 158.40 ± 13.69 c | 159.75 ± 47.84 c |
| Alanine aminotransferase (U/L) | 36.25 ± 5.32 | 60.00 ± 10.03 | 58.25 ± 32.29 |
| Aspartate aminotransferase (U/L) | 94.50 ± 10.02 | 79.50 ± 51.33 | 123.00 ± 37.98 |
| Free fatty acid (μEq/L) | 458.75 ± 112.43 | 437.80 ± 78.67 a | 652.75 ± 215.05 |
All data are expressed as means ± SD.
In this study we showed that G-CSF ameliorates non-alcoholic hepatic steatosis in the OLETF rat, and reduces expression of the hepatic lipogenic genes SREBP-1c, FAS, and ACC in the liver.
Histologically, we observed that G-CSF clearly decreased lipid droplets and ballooning in hepatocytes (Figure 1); the administration of G-CSF decreased by about three fold the area of lipid droplets in the liver sections stained with oil red O (Figure 2), which is a well-established method of assessing the extent of steatosis.23,27 Also, liver weight relative to body weight was reduced by G-CSF without any change in body weight (Table 1).
Recently, several possible mechanisms of the general effect of G-CSF on various liver diseases have been suggested. Enhanced bone marrow cell homing towards damaged liver cells may induce trans-differentiation or a paracrine effect, contributing to the regeneration of the damaged liver.15 In addition, G-CSF may act directly on the liver cells through G-CSF receptors.28 In this study, we only determined the effect of G-CSF on non-alcoholic hepatic steatosis, which was found to be associated with down-regulation of SREBP-1c, FAS, and ACC. Further study is required to confirm the mechanisms underlying the effect of G-CSF on the liver.
Zhiyong Guo, et al. demonstrated that administration of diazoxide for 22 weeks reduced fat deposition in the liver,29 and several groups have shown that long-term exercise training has a beneficial effect on non-alcoholic hepatic steatosis.30,31 Our findings suggest that G-CSF is effective with only a short-term treatment. In previous studies, the beneficial effects of treatment for non-alcoholic hepatic steatosis were due to weight reduction,21,29 whereas in our hands G-CSF ameliorated steatosis without weight reduction. Clinical studies of G-CSF have shown that it does not have any severe side effects, though it may cause transient bone pain.32
According to previous studies, hepatic lipogenesis is responsible for the development of hepatic steatosis. Hepatic lipogenesis is caused by an imbalance between input (uptake and synthesis) and output (oxidation and degradation) of FFA.12,33 In our study, G-CSF reduced circulating FFA down to the level in the normal control group (Table 1).
SREBP-1c plays an important role in hepatic lipogenesis,33,34 and increased expression of SREBP-1c accelerates hepatic lipogenesis by activating lipogenic enzymes such as FAS, acetyl-CoA carboxylase (ACC), and stearoyl-CoA desaturase (SCD) (Figure 3).6,34,35 Also over-expression of SREBP-1c leads to the development of hepatic steatosis in mice,36 and SREBP-1c synergistically regulates expression of lipogenic genes such as FAS, ACC and SCD.35,37 Many genes are associated with hepatic steatosis, and SREBP-1c, FAS, and ACC are comparatively known as a key regulator of hepatic steatosis.35,38 In the present study, we showed that G-CSF decreased the mRNA levels of SREBP-1c, FAS, and ACC mRNAs (Figures 3).
In other studies, hepatic fat accumulation closely correlated with the oxidative stress.39,40 Oxidative stress results from an imbalance between oxidant and antioxidant that leads to oxidative damage in the liver.41 Thus, this study had limitations in that the fatty acid oxidation in the liver and the hepatic mitochondrial enzymes were not analyzed. It is necessary to evaluate the changes in oxidative stress in future studies to clarify the mechanism by which G-CSF ameliorates hepatic steatosis.
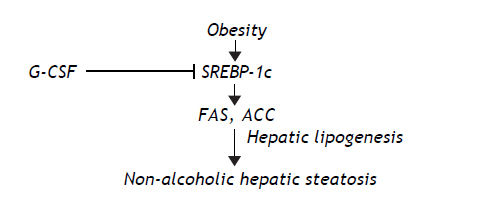
Taken together our findings suggest that G-CSF reduces hepatic lipogenesis by inhibiting SREBP-1c production, which in turn reduces the accumulation of lipogenic enzymes such as FAS and ACC (Figure 4).
A reduction in circulating TG and FFA reduces the input of fatty acid to hepatocytes. SREBPs are well known as enhancers of FFA biosynthesis and FFA uptake.42 We showed that administration of G-CSF reduced the expression of SREBP-1c and circulating TG and FFA (Figure 3A and Table 1). Our analysis indicates that G-CSF reduces expression of SREBP-1c, resulting in less circulating TG and FFA. Further study is required to establish whether SREBP-1c is the main target of G-CSF, and to examine the expression of lipogenic enzymes other than FAS and ACC.
In summary, we have demonstrated that G-CSF ameliorates non-alcoholic hepatic steatosis in the OLETF rat model, and reduces the expression of SREBP-1c, which plays a key role in the development of hepatic steatosis.33 We speculate that the reduction of SREBP-1c expression by G-CSF is related to the improvement of the non-alcoholic hepatic steatosis. Despite the fact that the underlying mechanism is not known, our findings indicate that administration of G-CSF dramatically ameliorates non-alcoholic hepatic steatosis. To our knowledge, this is first report of the effect of G-CSF on non-alcoholic hepatic steatosis. In addition, we present evidence that the beneficial effect of G-CSF is associated with down-regulation of SREBP-1c. Therefore, our findings suggest that G-CSF is a novel approach to the treatment of non-alcoholic hepatic steatosis.
Abbreviations- •
NAFLD: non-alcoholic fatty liver disease.
- •
G-CSF: granulocyte colony-stimulating factor.
- •
SREBP: sterol regulatory element-binding protein.
- •
FAS: fatty acid synthase.
- •
ACC: acetyl-CoA carboxylase.
- •
OLETF: Otsuka Long Evans Tokushima Fatty.
- •
LETO: Long-Evans Tokushima Otsuka.
- •
H-E: hematoxylin and eosin.
- •
PAS: periodic acid Schiff.
- •
TC: total cholesterol.
- •
TG: triglyceride.
- •
ALT: alanine aminotransferase.
- •
AST: aspartate aminotrans.
- •
FFA: free fatty acids.
- •
PCR: polymerase chain reaction.
- •
SCD: stearoyl-CoA desaturase.
This work was supported by the grant for the Medical Research Center (2011-0028261) funded by the National Research Foundation of Korea (NRF) of the Ministry of Education, Science and Technology (MEST), Republic of Korea.