Introduction. Liver cirrhosis is associated with hyperdynamic circulation which can result in heart failure. Transjugular intrahepatic portosystemic shunt (TIPS) due to increase of cardiac output is a stressful stimulus for cardiovascular system. Therefore, new methods for early detection of heart failure are needed. Transmitral flow is a marker of diastolic dysfunction.
Aim. To analyze short- and long-term effect of TIPS procedure on transmitral flow.
Material and Methods. 55 patients (38 men and 17 women, 55.6 ± 8.9 years) with liver cirrhosis treated with TIPS were enrolled in the study. Echocardiography was performed before, 24 h, 7, 30 and 180 days after the procedure. During 6 month follow up 22 patients died.
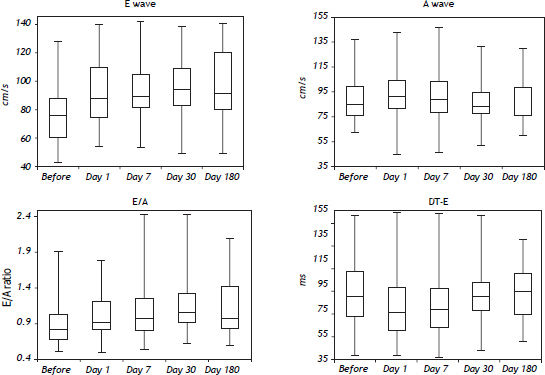
Results. Left ventricle end-diastolic diameter was increasing during the follow-up [baseline: 47 (44.7–51.2) mm, day 7: 50 (46.5–51.3) mm, p < 0.05; day 30: 49.5 (46.7–55.2) mm, p < 0.01; 6 months: 52.5 (48.3–55.2) mm, p < 0.01)]. The peak early filling velocity (E) was significantly increasing [before: 75.5 (60.5–87.3) cm/s, 24 h: 88 (74.3–109.7), p < 0.01; day 7: 89 (81.5–105) p < 0.01; 1 month: 94 (82.7–108.5) p < 0.01; 6 month: 91 (80.1–120.2) p < 0.01]. Peak late atrial filling velocity (A) significantly increased within 24 h after the procedure: 85.1 (76.2–99.5) vs. 91.2 (81.5–104.5) cm/s, p < 0.05. The E/A ratio was increasing during the follow up (baseline: 0.88, 24 h after: 0.89, 1 week: 1.0, 30 days: 1.13, 6 month: 1.06 p < 0.01).
Conclusion. Hemodynamic changes following TIPS procedure can be monitored using echocardiography. Transmitral flow analysis can serve as a useful tool for evaluating of diastolic function in these patients.
A transjugular intrahepatic portosystemic shunt (TIPS) is a percutaneous created connection within the liver between the portal and systemic circulation. A TIPS is placed to reduce portal pressure in patients with severe complications of portal hypertension (variceal bleeding, refractory ascites and hepatorenal syndrome).1,2 However, TIPS placement can be associated with potential complications and negative side effects.3 TIPS placement is associated with increased heart rate, cardiac output and plasma volume, and reduced systemic vascular resistance ad arterial blood pressure.4–6 Furthermore, liver cirrhosis per se is frequently associated with cardiac dysfunction and abnormalities in the central, splanchnic and peripheral circulation, and hemodynamic changes caused by humoral and nervous dysregulation.7–9 Transjugular intrahepatic portosystemic shunt (TIPS) insertion represents stressful situation in this group of patients. Thus, the prognosis of the patients after TIPS procedure can be affected by the development of heart failure, especially at those who have preexisting myocardial dysfunction.
It has been shown, that diastolic dysfunction is relatively frequent in patients with liver cirrhosis and is associated with increased mortality.10,11 The diastolic dysfunction can progress to severe heart failure.12–14 Therefore, early diagnosis of diastolic heart failure could help to identify risk patients. Diastolic function can be evaluated using Doppler analysis of transmitral flow.12 The aim of our study was to investigate short- and long-term effects of TIPS on transmitral flow parameters with the aim to identify risk patients.
Material And MethodsStudy populationThe study population consisted of fifty-five consecutive patients (38 men and 17 women, aged 55.6 ± 8.9 years, range 37–74) with liver cirrhosis treated with elective transjugular portosystemic shunting. The causes of liver disease were alcohol consumption in 36 (65%) pts., viral hepatitis in 7 (13%) pts., non-alcoholic steatohepatitis in 6 (11%) pts., autoimmune hepatitis in 2 (4%) pts., and un-known origin in 4 (7%) pts.
A standard procedure was used for TIPS insertion, which was performed under sedation. The conventional angioplasty and deployment of bare metal stents (Wallstent; Boston Scientific, Natick, Massachusetts), nondedicated ePTFE-covered stent grafts (Jostent; Jomed, Rangendingen, Germany; and Ella stent graft; Ella, Hradec Kralove, Czech Republic), and dedicated ePTFE-covered stent grafts (Viatorr; W. L. Gore and Associates) were performed. The decision on the type of intervention (conventional angioplasty, bare metal stent, nondedicated or dedicated ePTFE-covered stent graft) was solely that of the operator. The diameter of the balloon or stent used was 10 or 12 mm in all cases.
Patient demographic characteristics are shown in table 1. Patency of the shunt was evaluated with Doppler ultrasound of the liver tissue during regular follow-up. All patients were in a stable condition, with no gastrointestinal bleeding being recorded during the 15 days preceding TIPS insertion.
Patient demographic characteristics.
| Sex | |
| Men, n (%) | 38 (69) |
| Women, n (%) | 17 (31) |
| Age | |
| All patients (x ± SD) | 55.6 ± 8.9 |
| Women (x ± SD) | 55.1 ± 9.4 |
| Men (x ± SD) | 55.8 ± 8.7 |
| Etiology | |
| Alcohol, n (%) | 36 (65) |
| Viral, n (%) | 7 (13) |
| NASH, n (%) | 6 (11) |
| Autoimmune, n (%) | 2 (4) |
| Unknown, n (%) | 4 (7) |
| Child - Pugh score (points, x ± SD) | 8.7 ± 1.5 |
| Child classification | |
| A, n (%) | 7 (13) |
| B, n (%) | 32 (58) |
| C, n (%) | 16 (29) |
| Indications for TIPS | |
| Recurrent bleeding, n (%) | 13 (23) |
| Refractory ascites, n (%) | 40 (73) |
| PH hydrothorax, n (%) | 2 (4) |
| Stents used | |
| ePTFE covered stents n (%) | 29 (53) |
| Bare metallic stents n (%) | 26 (47) |
| Portosystemic gradient (mmHg) | |
| Before TIPS median (min; max) | 25 (5; 34) |
| After TIPS median (min; max) | 8,5 (3; 18) |
| TIPS dysfunction | |
| Up to 1 month, n (%) | 4 (7) |
| Up to 6 months, n (%) | 11 (20) |
NASH: non-alcoholic steatohepatitis.
Medication affecting hemodynamics, such as ß-blockers and vasodilators, was stopped for at least 10 days before TIPS. Diuretics were kept constant during the week before TIPS insertion.
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki, and was approved by Ethical committee of our institution. Written informed consent was obtained from each patient.
EchocardiographyAll echocardiography were taken by two experienced cardiologists who were unaware about the study protocol. The detailed M-mode, two-dimensional, color Doppler, and PW Doppler analyses were performed on resting subjects in regular setting by HP Sonos 5500 (Hewlett Packard, USA) imaging system with a 2.5 MHz transducer. Echocardiography was performed before TIPS procedure, 24 h, 7, 30 and 180 days after the procedure.
All parameters were taken on the basis of the American Society of Echocardiography standards.15 Data were obtained from the parasternal, apical and subcostal views. We measured left ventricular end-diastolic (LVEDD) and end-systolic diameters (LVESD), end-diastolic septal and posterior wall thickness, left atrium (LA) diameter, right ventricle (RV) diameter and inferior vena cava (IVC) diameter. Modified Simpson’s method was used to determine LV ejection fraction.
Mitral flow velocities were evaluated by PW Doppler from apical four-chamber view with the sample volume positioned at the tip of the mitral leaflets and at an angle as parallel to mitral flow as possible. The following parameters were measured: peak early filling velocity (E), peak late atrial filling velocity (A), E/A ratio and deceleration time of E velocity (DT-E). Isovolumic relaxation time (IVRT) was derived by placing the cursor of Doppler in the left ventricle outflow tract to simultaneously display the end of aortic ejection and the onset of mitral inflow. Impaired left ventricular relaxation was defined as reduction in the E/A ratio (< 1) and a prolongation of DT-E (> 220 ms).
Tricuspid regurgitation jet velocity was obtained using continuous-wave Doppler, and the systolic pressure gradient across the tricuspid valve was calculated using the modified Bernoulli equation.
All Doppler parameters were recorded at horizontal speed of 50 mm/s. The average values obtained at least three consecutive cardiac cycles were taken in to the consideration in statistical analysis.
Hemodynamic monitoringAll patients were monitored by using Swan-Ganz pulmonary catheter inserted in pulmonary circulation before TIPS procedure. Only local anesthesia using lidocaine was used. The hemodynamic parameters, i.e. heart rate, blood pressure, cardiac output (CO), cardiac index (CI), systemic vascular resistance (SVR), pulmonary vascular resistance (PVR), pulmonary artery pressure (PAP), pulmonary capillary wedge pressure (PCWP) were measured before and 24 h after the TIPS procedure. The baseline data were compared with the reference values of our laboratory.
Statistical AnalysisStatistical analysis was performed by Statistica 5 programme (Tulsa, USA). Normally distributed variables are expressed as means ± standard deviation, while non-normally distributed variables are expressed as median (interquartile range). Categorical variables are presented as percentages. Continuous variables were compared using Student’s t-or Mann-Whitney tests, where appropriate. For categorical variables, comparisons between the groups were made using χ2 test. Kaplan-Meier survival analysis was used to calculate the survival of the patients. A p value of < 0.05 was considered statistically significant.
ResultsPatient characteristicsPatient demographic characteristics are shown in table 1. The study group consisted of 55 patients (38 men, 17 women), the mean age was 55. 6 ± 8.9 years. All patients had sinus rhythm. The mean Child Pugh score was 8.7 ± 1.5 points.
Hemodynamic parameters (pulmonary artery catheterization)We found increase in pulmonary capillary wedge pressure (9.5 ± 1.9 vs. 10.7 ± 3.6 mmHg, p < 0.05), cardiac output (7.2 ± 1.7 vs. 9.5 ± 1.9 L/min, p < 0.001), cardiac index (4.3 ± 2.3 vs. 5.2 ± 1.1 L/min/m2, p < 0.05). Before the TIPS procedure, high cardiac index was present in 17 (31%) patients, 24 h after the procedure, 44 (84%) patients had higher cardiac index. Before the procedure, mean systemic vascular resistance was significantly decreased compare to normal values (908.2 ± 276.8 vs. 1,170 ± 270 dyn•sec•cm-5, p < 0.01), and after the procedure significantly decreased (908.2 ± 276.8 vs. 631 ± 132.7 dyn•sec•cm-5, p < 0.001). Pulmonary vascular resistance was without significant change (89.3 ± 51.6 vs. 82.3 ± 43.3 dyn•sec•cm-5, p ns.).
EchocardiographyLeft atrium diameter: there were no significant differences during the short-term follow-up [baseline: 39.5 (36.2 to 43.4) mm, 24 h: 38.5 (34.7 to 42.2) mm, p ns; day 7: 41 (39 to 44.2) mm, p ns)]. But during follow-up, the LA diameter increased [30 days after procedure: 40.0 (37 to 43.2) mm, p < 0.05; 180 days after TIPS: 40.5 (37.7 to 45.2) mm, p < 0.05)]. Similarly, left ventricle end-diastolic diameter was increasing during the follow-up period [baseline: 47 (44.7 to 51.2) mm; 7 days after the procedure: 50 (46.5 to 51.3) mm, p < 0.05; 30 days after the procedure: 49.5 (46.7 to 55.2) mm, p < 0.01; 180 days after the procedure: 52.5 (48.3 to 55.2) mm, p < 0.01)]. Vena cava inferior diameter increased during late phase of follow-up [baseline: 17 (15.5 to 19) mm; 30 days after the procedure: 18.7 (16.5 to 19.8) mm, p < 0.05; 180 days after the procedure: 19 (18.2 to 20.5), p < 0.05]. Right ventricle and left ventricle end-systolic diameters were without any significant change. Parameters of the function of the left ventricle (ejection fraction and fractional shortening) were without significant changes (Table 2).
Basic echocardiographic parameters in patients before and after the TIPS procedure during 6 month follow-up.
| Before | 24 h | 7 days | 30 days | 180 days | |
|---|---|---|---|---|---|
| Left atrium (mm) | 39.5 (36.2–43.4) | 38.5 (34.7–42.2) | 41 (39–44.2) | 40.0 (37–43.2)* | 40.5 (37.7–45.2) * |
| Right ventricle (mm) | 27 (25–29) | 28 (26–30) | 29 (27.5–30) | 30 (27–31) | 30 (27.3–31.2) |
| LVESD (mm) | 31.5 (30.1–34.3) | 30 (27.2–34.8) | 29 (27.5–30.8) | 31.2 (29.6–33.2) | 34 (31.5–36.9)* |
| LVEDD (mm) | 47 (44.7–51.2) | 46 (44.6–48.7) | 50 (46.5–51.3)* | 49.5 (46.7–55.2)** | 52.5 (48.3–55.2)** |
| EF(%) | 67 (61.8–70.1) | 65.5 (61.8–68.2) | 67.5 (62.4–71.3) | 67.25 (60.7–70.8) | 66.75 (61.8–70.1) |
| FS | 0.34 (0.31–0.37) | 0.34 (0.31–0.38) | 0.34 (0.28–0.37) | 0.36 (0.31–0.39) | 0.35 (0.28–0.40) |
| VCI diameter (mm) | 17 (15.5–19) | 17 (15.1–19.4) | 18 (16.3–19.4) | 18.7 (16.5–19.8)* | 19 (18.2–20.5)* |
| RV/LV | 0.56 (0.52–0.61) | 0.62 (0.58–0.6)* | 0.58 (0.55–0.63) | 0.57 (0.51–0.62) | 0.57 (0.53–0.61) |
The data are expressed as median (25–75 percentiles). LVESD: left ventricular end-systolic diameter. LVEDD: left ventricular end-diastolic diameter. EF: ejection fraction of the left ventricle. FS: fractional shortening; VCI: inferior vena cava diameter. RV/LV: right and left ventricle ratio.
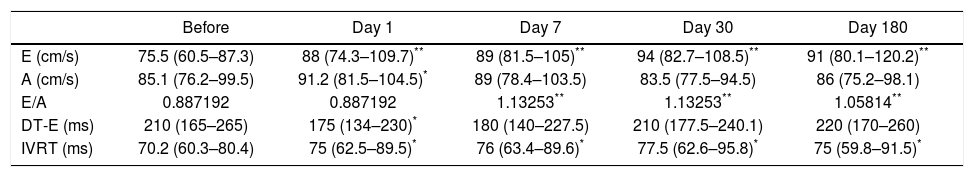
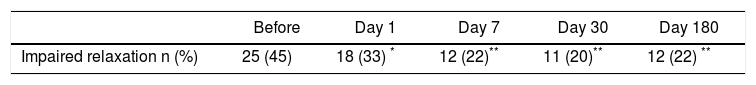
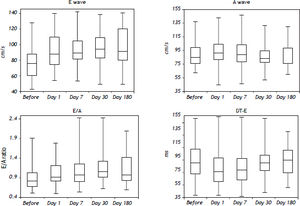
Table 3 shows mitral inflow parameters. During 6 month follow-up, the peak early filling velocity (E) was significantly increasing. Peak late atrial filling velocity (A) significantly increased within 24 h after the procedure [85.1 (76.2–99.5) vs. 91.2 (81.5–104.5) cm/s, p < 0.05]. The E/A ratio was abnormal at the beginning of the study. However, during the followup was significantly increasing and became normal (Figure 1). Isovolumic relaxation time was increasing during the follow-up period. Impaired left ventricular relaxation was present in 25 (45%) patients before the procedure. During the follow-up, the number of the patients with impaired left ventricular relaxation was significantly decreasing (Table 4). There were no patients with other types of mitral filling patterns (pseudonormalisation or restrictive filling pattern).
Doppler mitral inflow parameters.
| Before | Day 1 | Day 7 | Day 30 | Day 180 | |
|---|---|---|---|---|---|
| E (cm/s) | 75.5 (60.5–87.3) | 88 (74.3–109.7)** | 89 (81.5–105)** | 94 (82.7–108.5)** | 91 (80.1–120.2)** |
| A (cm/s) | 85.1 (76.2–99.5) | 91.2 (81.5–104.5)* | 89 (78.4–103.5) | 83.5 (77.5–94.5) | 86 (75.2–98.1) |
| E/A | 0.887192 | 0.887192 | 1.13253** | 1.13253** | 1.05814** |
| DT-E (ms) | 210 (165–265) | 175 (134–230)* | 180 (140–227.5) | 210 (177.5–240.1) | 220 (170–260) |
| IVRT (ms) | 70.2 (60.3–80.4) | 75 (62.5–89.5)* | 76 (63.4–89.6)* | 77.5 (62.6–95.8)* | 75 (59.8–91.5)* |
The data are expressed as median (25–75 percentiles). E: peak early filling velocity. A: peak late atrial filling velocity. DT-E: deceleration time of E velocity. IVRT: isovolumic relaxation time.
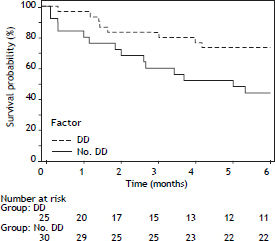
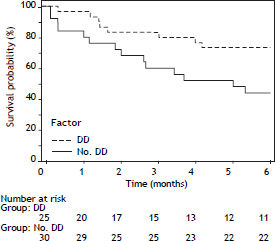
During 6 months follow-up 22 patients died. Out of 25 pts with diastolic dysfunction (DD), 14 pts (56%) died. Out of 30 patients without diastolic dysfunction (no DD), 8 (26%) died. The survival of the patients with diastolic dysfunction was significantly decreased (DD vs. no DD: HR: 0.54, 95% CI: 0.310.94, p 0.0149). Figure 2 shows Kaplan-Meier curves of survival.
DiscussionThe main findings of this study are:
- •
Hemodynamic abnormalities (e.g. hyperdynamic circulation, lower systemic vascular resistance, impaired relaxation of the left ventricle) are frequent in patients with liver cirrhosis.
- •
The transjugular portosystemic shunting is potent stimulus which can deteriorate hemocirculation (further increase of the cardiac output, increase of the pulmonary capillary wedge pressure, decrease of the systemic vascular resistance).
- •
TIPS procedure affects pulsed-wave Doppler parameters of mitral inflow (increase of peak early filling velocity and normalization of E/A ratio).
- •
The presence of diastolic dysfunction is associated with significantly increased mortality.
Liver cirrhosis is frequently associated with hyperdynamic syndrome which comprises increased heart rate, cardiac output and plasma volume, and reduced systemic vascular resistance and arterial blood pressure.16–18 We observed increased cardiac output in 17 (31 %) pts. and decreased systemic vascular resistance in 28 (51%) pts. before the procedure. High cardiac output is a result of increase in venous return, heart rate and contractility, which are controlled by the autonomic nervous system. Cardiac output can be increased also by vasodilatation, the presence of arteriovenous communications, expanded blood volume and increased sympathetic nervous activity.7,8
In our study, cardiac output and cardiac index increased and systemic vascular resistance decreased as a result of the creation of postosystemic shunt. This was accompanied with increase in pulmonary artery wedge pressure. All these findings indicate that TIPS procedure is a potent factor that may affect blood circulation.
Diastolic dysfunction is relatively frequent in patients with liver cirrhosis.11,13,18 Recent studies of left ventricular filling in cirrhosis support the presence of a subclinical myocardial disease with diastolic dysfunction.19 It has been shown that myocardial fibrosis and increased myocardial mass lead to increased stiffness of the myocardial wall resulting in impaired ventricular filling and diastolic dysfunction.8,10 Furthermore, the TIPS procedure provides additional burden on cardiovascular system.
In our study, the diastolic dysfunction (impaired relaxation) was present in 45 % of patients before the procedure. 24 h later, the E/A ratio increased and the number of patients with impaired relaxation decreased. Further increase was observed until the end of the follow-up. This was caused by the increase of peak early filling velocity (E). In contrast to E wave, the peak late atrial filling velocity (A) only slightly increased 24 h after the TIPS procedure. Deceleration time had biphasic response: a rise in the first 24 h was followed by a decrease to baseline value. It is not easy to explain these changes. In our study, major changes were observed in increasing velocity of the E wave. It is known that E velocity is influenced by left atrial pressure at mitral valve opening, the relative driving force between the left atrium and left ventricle, minimal left ventricular diastolic pressure, compliance of the left atrium and the rate of ventricular relaxation. Creation of the porto-systemic shunt leads to the changes in a number of parameters (increase in cardiac output, cardiac index, heart rate, etc.), but the most important seems to be a decrease in systemic vascular resistance.13,20
Furthermore, diastolic dysfunction is probably associated with poor outcome of these patients. Cazzaniga, et al. showed that diastolic dysfunction (E/A < 1) is associated with poor survival in patient undergoing TIPS procedure in a group of 32 pts. with liver cirrhosis.19
In our study, we confirmed the association of diastolic dysfunction and increased mortality in patients with liver cirrhosis. Diastolic dysfunction may be a significant factor in the development of heart failure, may precede systolic dysfunction in patients with cirrhosis, and may play a part in the pathogenesis of sodium fluid retention in cirrhosis.7,10
Study limitations: the diastolic dysfunction was evaluated using pulsed-Doppler techniques. The tissue Doppler analysis was not performed. During follow-up, the patients may develop TIPS dysfunction (decreased patency or closure of the porto-systemic shunt) that can affect the hemodynamic parameters. This factor was eliminated by Doppler ultrasound of the liver tissue during regular follow-up.
Despite the limitations, the study showed:
- •
Liver cirrhosis is associated with the increased number of the diastolic dysfunction.
- •
Diastolic dysfunction is associated with decreased survival.
- •
Transjugular porto-systemic shunt creation is associated with significant hemodynamic changes.
- •
The main change was in pulsed-wave Doppler mitral inflow analysis (increase in E velocity) and it was probably associated with decreased systemic vascular resistance.
Today, a great effort is placed on efforts to improve quality of life and survival of patients with liver cirrhosis treated with TIPS. Therefore, the risk stratification and proper selection of the patients for TIPS procedure are very important.21–23 It has been shown, that hemodynamic monitoring before and after the TIPS procedure is very helpful for selecting of the patients.24 Our study confirmed the need of hemodynamic monitoring of patients undergoing TIPS procedures and showed, that echocardiography as a non-invasive method can be very useful for this purpose.
Abbreviations- •
A wave: peak late atrial filling velocity.
- •
CI: cardiac index.
- •
CO: cardiac output.
- •
DT-E: deceleration time of E velocity.
- •
E wave: peak early filling velocity wave.
- •
EF: ejection fraction.
- •
FS: fractional shortening.
- •
IVC: inferior vena cava.
- •
IVRT: isovolumic relaxation time.
- •
LAD: left atrium diameter.
- •
LV: left ventricle.
- •
LVEDD: left ventricular end-diastolic diameter.
- •
LVESD: end-systolic diameter.
- •
PAP: pulmonary artery pressure.
- •
PCWP: pulmonary capillary wedge pressure.
- •
PVR: pulmonary vascular resistance.
- •
PW: pulsed wave Doppler.
- •
RV: right ventricle.
- •
SVR: systemic vascular resistance.
- •
TIPS: transjugular intrahepatic portosystemic shunt.
Supported by the programme PRVOUK P37/03.