Primary biliary cholangitis is a rare disease with scarce epidemiological data in Southern Europe. The authors aimed to evaluate treatment response in a cohort of patients.
Materials and methodsThis retrospective observational single-centre study included patients with diagnostic criteria of primary biliary cholangitis. Data on disease presentation, laboratory results, treatment and clinical endpoints were collected and analyzed.
ResultsFifty-three patients were included, 89% women, with mean age of 62±15 years at diagnosis. The majority was asymptomatic (49%), tested positive for antimitochondrial antibodies (96%) and had increased alkaline phosphatase (median=214U/L). 75% of the patients had liver histology and the majority were in Ludwig's stage I (42%). Autoimmune hepatitis (AIH) features were found in seven patients (13%). All were treated with ursodeoxycholic acid (UDCA) and 56% achieved biochemical response at one year; patients with AIH features exhibited steeper decreases in alkaline phosphatase (p=0.007) and reached the endpoint of 40% decrease in alkaline phosphatase more frequently (p=0.017).
ConclusionIn conclusion a significant proportion of patients failed to achieve an adequate response to UDCA treatment. The response rate of patients with AIH features was better, which could be related to a different phenotype or to the potential impact of immunosuppressive agents.
Primary biliary cholangitis (PBC), previously named primary biliary cirrhosis, is a rare disease (0.3–5.8 per 100,000inhabitants/year), that predominantly affects women [1]; it is characterized by progressive destruction of small intrahepatic bile ducts with periportal inflammation, fibrosis, and potential cirrhosis [2].
Clinical manifestations related to cholestasis include fatigue and pruritus, which can significantly impact quality of life [3]. Furthermore, chronic cholestasis can lead to osteopenia and osteoporosis, hyperlipidaemia and liposoluble vitamin deficiencies. Although cirrhosis is infrequent, patients are at risk for developing end-stage hepatic disease complications like portal hypertension and hepatocellular carcinoma [2].
It is characterized by a progressive T cell predominant lymphocytic cholangitis and a serologic pattern of reactivity to antimitochondrial antibodies (AMA) – densely localized to the apical surface of biliary epithelial cells and associated with apoptosis [4]. In addition, half of the patients have, at least, one concurrent autoimmune disorder [5].
The diagnosis of PBC according to the European Association for the Study of the Liver (EASL) relies on the combination of elevation of alkaline phosphatase (AP) of liver origin for at least six months and presence of AMA positivity (≥1:40). Liver biopsy is not recommended for the diagnosis, except when PBC-specific antibodies are absent, coexistent AIH or NASH is suspected, or other co-morbidities are present. EASL also recommends that in the correct context, a diagnosis of AMA negative PBC can be made in patients with cholestasis and specific ANA immunofluorescence (nuclear dots or perinuclear rims) or ELISA results (sp100, gp210) [6].
For several years, PBC treatment has been centred on ursodeoxycholic acid (UDCA). A favourable long-term outcome is expected in patients with a good biochemical response after one year — serum bilirubin ≤1mg/dL (17μmol/L), AP≤3× ULN and AST≤2× ULN (Paris criteria) or by a decrease of 40% or normalization of serum AP (Barcelona criteria) [7]. However, a significant portion of patients (up to 40%) have suboptimal responses [8].
New therapies are emerging and, recently, obeticholic acid, a farnesoid×receptor, showed effective biochemical response in adults with primary biliary cholangitis, and is now an approved alternative, recommended in combination with UDCA in patients who have an inadequate response to UDCA or as monotherapy in those who are unable to take UDCA because of side effects [6,9]. Budesonide and fibrates may play a role in the treatment of PBC, but they are not yet recommended because data from phase III randomized trials are still pending [6].
Most of the patients have typical features, but some present with characteristics of both PBC and autoimmune hepatitis (PBC with AIH features, commonly termed overlap syndrome). Features of AIH may develop in a sequential and unpredictable manner or occur as a concomitant distinct disorder. Moreover, combination of UDCA and corticosteroids is required in most patients to obtain a complete biochemical response. This syndrome may represent an important unrecognized cause of treatment resistance in PBC patients [10,11].
Large-scale studies from the Global PBC Study Group and the UK-PBC consortium have shed some light in this highly heterogeneous disease and have led to the development of new risk scores [12,13].
Nevertheless, clear predictive factors of response to UDCA treatment are lacking. The aim of this study is to characterize a cohort of PBC patients, in order to access demographic, clinical and immunological features related to treatment response.
2Materials and methods2.1ParticipantsThis retrospective observational study included PBC patients, diagnosed according to EASL Clinical Practical Guidelines from July 2002 to April 2015 at Centro Hospitalar São João, a tertiary non-transplant centre in the North of Portugal. Exclusion criteria included viral hepatitis serology uncovering active/previous infection; imagological studies (abdominal ultrasound, endoscopic retrograde cholangiopancreatography or magnetic resonance cholangiopancreatography) compatible with extrahepatic causes of chronic cholestasis. Patients under specific circumstances that may cause cholestasis (e.g., pregnancy, childhood, HIV-infection) were also excluded. Ultimately, patients lacking sufficient clinical or laboratory data were also excluded.
2.2Data collectionData was collected from medical records of patients and the following variables were evaluated: age at diagnosis, gender and follow-up period; presentation and diagnostic tests. Data on comorbid illnesses, including concurrent autoimmune diseases, and on PBC complications, including development of cirrhosis, ascites, variceal bleeding, hepatic encephalopathy and hepatocellular carcinoma (HCC), and progression to liver transplantation were also collected.
Immunological and histopathological data were also obtained. AMA, antinuclear antibodies (ANA) and anti-smooth-muscle antibodies (ASMA) were determined by indirect immunofluorescence. Anti-M2 antibodies were determined by immunoblotting, according to the manufacturer's instructions. Transient elastography parameters (Fibroscan, Echosens, Paris) were also collected.
Information regarding treatment including UDCA, corticosteroid and other immunosuppressive drugs was recorded.
PBC was staged according to Ludwig's staging system[14] and the Barcelona Criteria were applied to evaluate treatment response.
2.3Statistical analysisAll data were analyzed by descriptive statistics and Fisher's exact test for categorical variables. Continuous variables were analyzed with independent samples and paired samples T-test, if normal distribution, or Mann–Whitney and Wilcoxon test for non-parametric distribution. Logistic regression was performed including the variables significant in univariate analysis. Statistical significance was established at a p<0.05 for all tests. Statistical analysis was performed using SPSS, version 23.
2.4Ethical considerationsThis study was conducted according to the Declaration of Helsinki. The study protocols were approved by the Ethics Committee of Centro Hospitalar São João, Porto, Portugal.
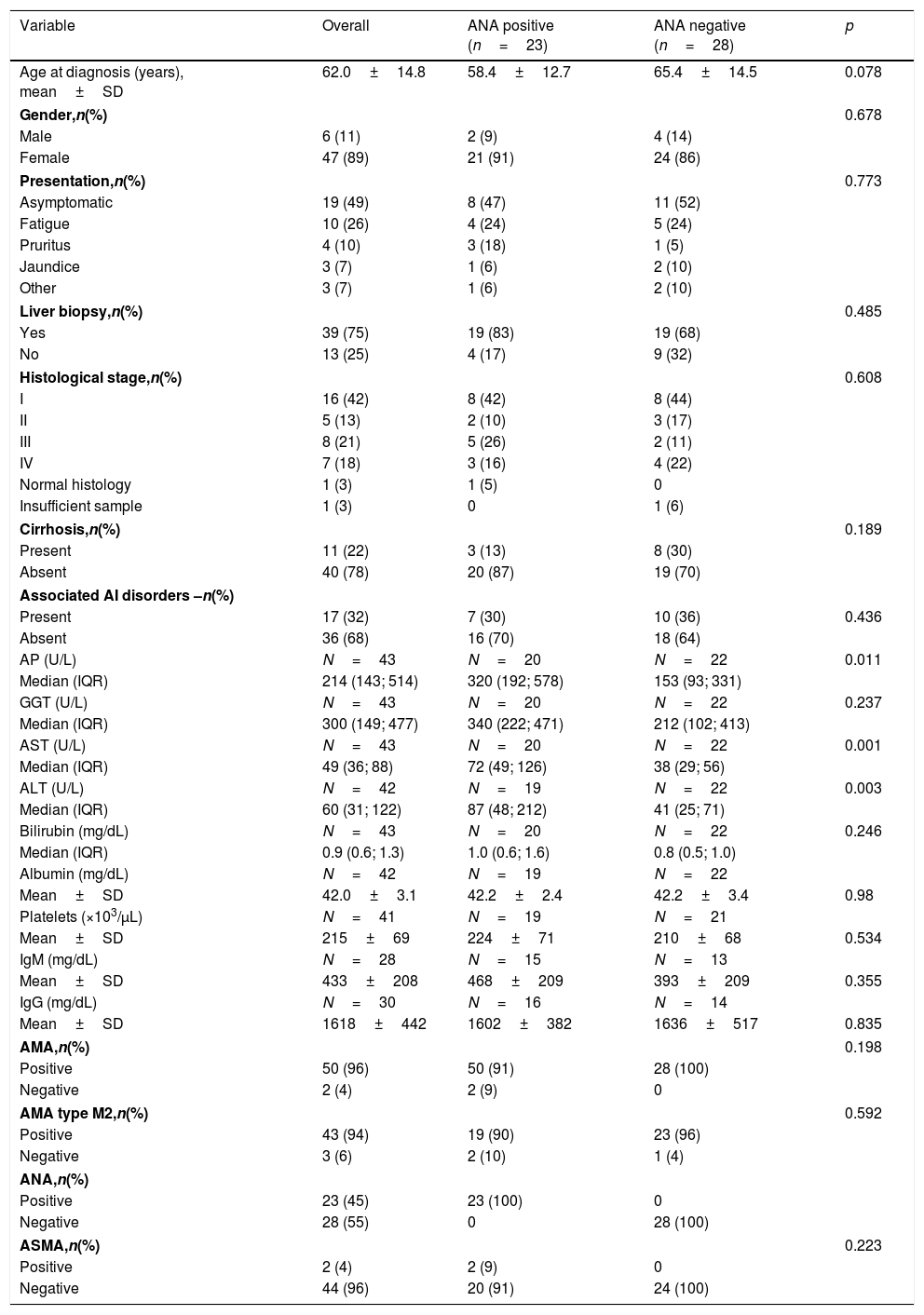
3Results3.1Patient's baseline characteristicsThe study included 53 patients with a mean follow-up period of 7±4 years. The majority were women (89%) with a mean age at diagnosis of 62 years.
At diagnosis, nearly half of patients were asymptomatic with abnormal liver function tests. The most commonly referred symptoms or signs were fatigue (26%), pruritus (10%) and jaundice (8%). Abnormal laboratory findings are summarized in Table 1. The vast majority of patients tested positive for AMA (96%) and AMA type M2 (94%) and a significant proportion (45%) were ANA-positive, with predominantly nuclear dot pattern (35%) at indirect immunofluorescence. Diagnosis in AMA negative patients was confirmed by liver biopsy. ASMA was positive in 4% of patients.
Patient's baseline characteristics.
| Variable | Overall | ANA positive (n=23) | ANA negative (n=28) | p |
|---|---|---|---|---|
| Age at diagnosis (years), mean±SD | 62.0±14.8 | 58.4±12.7 | 65.4±14.5 | 0.078 |
| Gender,n(%) | 0.678 | |||
| Male | 6 (11) | 2 (9) | 4 (14) | |
| Female | 47 (89) | 21 (91) | 24 (86) | |
| Presentation,n(%) | 0.773 | |||
| Asymptomatic | 19 (49) | 8 (47) | 11 (52) | |
| Fatigue | 10 (26) | 4 (24) | 5 (24) | |
| Pruritus | 4 (10) | 3 (18) | 1 (5) | |
| Jaundice | 3 (7) | 1 (6) | 2 (10) | |
| Other | 3 (7) | 1 (6) | 2 (10) | |
| Liver biopsy,n(%) | 0.485 | |||
| Yes | 39 (75) | 19 (83) | 19 (68) | |
| No | 13 (25) | 4 (17) | 9 (32) | |
| Histological stage,n(%) | 0.608 | |||
| I | 16 (42) | 8 (42) | 8 (44) | |
| II | 5 (13) | 2 (10) | 3 (17) | |
| III | 8 (21) | 5 (26) | 2 (11) | |
| IV | 7 (18) | 3 (16) | 4 (22) | |
| Normal histology | 1 (3) | 1 (5) | 0 | |
| Insufficient sample | 1 (3) | 0 | 1 (6) | |
| Cirrhosis,n(%) | 0.189 | |||
| Present | 11 (22) | 3 (13) | 8 (30) | |
| Absent | 40 (78) | 20 (87) | 19 (70) | |
| Associated AI disorders –n(%) | ||||
| Present | 17 (32) | 7 (30) | 10 (36) | 0.436 |
| Absent | 36 (68) | 16 (70) | 18 (64) | |
| AP (U/L) | N=43 | N=20 | N=22 | 0.011 |
| Median (IQR) | 214 (143; 514) | 320 (192; 578) | 153 (93; 331) | |
| GGT (U/L) | N=43 | N=20 | N=22 | 0.237 |
| Median (IQR) | 300 (149; 477) | 340 (222; 471) | 212 (102; 413) | |
| AST (U/L) | N=43 | N=20 | N=22 | 0.001 |
| Median (IQR) | 49 (36; 88) | 72 (49; 126) | 38 (29; 56) | |
| ALT (U/L) | N=42 | N=19 | N=22 | 0.003 |
| Median (IQR) | 60 (31; 122) | 87 (48; 212) | 41 (25; 71) | |
| Bilirubin (mg/dL) | N=43 | N=20 | N=22 | 0.246 |
| Median (IQR) | 0.9 (0.6; 1.3) | 1.0 (0.6; 1.6) | 0.8 (0.5; 1.0) | |
| Albumin (mg/dL) | N=42 | N=19 | N=22 | |
| Mean±SD | 42.0±3.1 | 42.2±2.4 | 42.2±3.4 | 0.98 |
| Platelets (×103/μL) | N=41 | N=19 | N=21 | |
| Mean±SD | 215±69 | 224±71 | 210±68 | 0.534 |
| IgM (mg/dL) | N=28 | N=15 | N=13 | |
| Mean±SD | 433±208 | 468±209 | 393±209 | 0.355 |
| IgG (mg/dL) | N=30 | N=16 | N=14 | |
| Mean±SD | 1618±442 | 1602±382 | 1636±517 | 0.835 |
| AMA,n(%) | 0.198 | |||
| Positive | 50 (96) | 50 (91) | 28 (100) | |
| Negative | 2 (4) | 2 (9) | 0 | |
| AMA type M2,n(%) | 0.592 | |||
| Positive | 43 (94) | 19 (90) | 23 (96) | |
| Negative | 3 (6) | 2 (10) | 1 (4) | |
| ANA,n(%) | ||||
| Positive | 23 (45) | 23 (100) | 0 | |
| Negative | 28 (55) | 0 | 28 (100) | |
| ASMA,n(%) | 0.223 | |||
| Positive | 2 (4) | 2 (9) | 0 | |
| Negative | 44 (96) | 20 (91) | 24 (100) | |
Abbreviations: AI, autoimmune; AMA, antimitochondrial antibodies; ANA, antinuclear antibodies; ALT, alanine aminotransferase; AP, alkaline phosphatase; ASMA, anti-smooth-muscle antibodies; AST, aspartate aminotransferase; GGT, γ-glutamyltransferase; Ig, Immunoglobulin.
Histological samples were available in 75% of the patients, and the bulk of them presented with Ludwig's stage I at diagnosis (42%). Stage 2 was observed in 13%, stage 3 in 21% and stage 4 in 18%. The remaining patients had normal histology (3%) or inadequate biopsy specimens (3%).
Roughly one-third of the patients exhibited a concomitant autoimmune condition and Sjogren's syndrome was the most common.
Regarding the AIH features observed in this cohort, five patients (9%) presented with PBC and AIH features simultaneously (ALT>5× ULN and biopsy specimens revealing moderate interface hepatitis). These patients were treated with combination therapy of UDCA plus established treatment for AIH (induction treatment with prednisolone and maintenance with AZA in the absence of contraindications). Additionally, two patients (4%) exhibited ALT>5× ULN: one of them had ASMA positivity and both were treated with immunosuppressive treatment plus UDCA. In these two cases, signs of moderate to severe interface hepatitis, mandatory for definitive diagnosis, were not detected. None of the other patients developed sequential AIH features during the follow-up period.
3.2Follow-up3.2.1TreatmentAll patients were treated with UDCA (13mg/kg/day) for at least one year, 28% of the patients were further managed with the addition of steroids or/and immunosuppressive agent. Corticosteroids were used with induction dosage of prednisolone 40mg followed by tapering until maintenance dosage of 5–15mg; azathioprine was started at 50mg and increased to maintenance dosage of 1–2mg/kg according to biochemical response. Others were further treated with off-label second line treatments such as budesonide and mycophenolate mofetil.
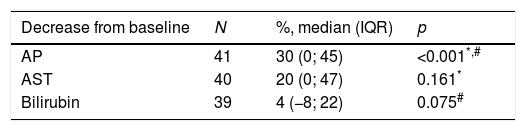
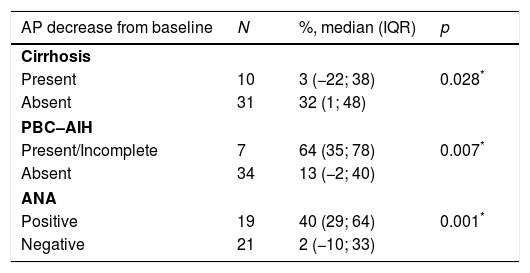
The authors analyzed the biochemical response to treatment. Serum AP exhibited a significant decrease from baseline after one year (median decrease from baseline=30% (IQR 0–45); p<0.001) (Table 2). Patients without cirrhosis (p=0.028), with AIH features (p=0.007) and positivity for ANA (p=0.001) featured a more robust decrease in serum AP. (Table 3).
AP decrease (%) from baseline assessed after one year of treatment based on patient's baseline characteristics.
After one year of treatment, only 32% (13/41) displayed a decrease of 40% in serum AP and, according to the Barcelona Criteria, 56% of the patients exhibited a good biochemical response to treatment with UDCA. Regarding the 34 patients with PBC without overlap syndrome, 50% revealed good biochemical response to treatment. On the other hand, 86% achieved the endpoint of good biochemical response among the seven patients with definite and incomplete PBC–AIH overlap syndrome.
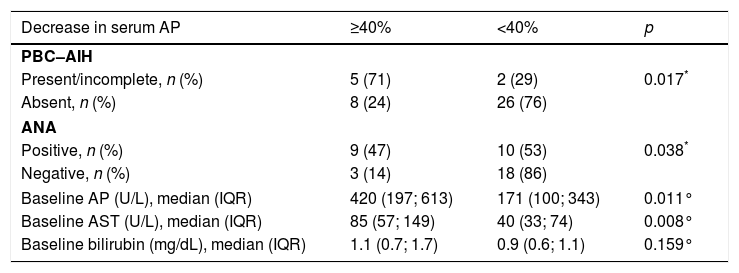
Furthermore, patient characteristics that were associated with at least 40% decrease in serum AP included: PBC with AIH features (p=0.017), ANA positivity (p=0.038), higher baseline serum AP (p=0.011) and higher baseline serum AST (p=0.008) (Table 4).
Evaluation of the endpoint of biochemical response to treatment – decrease of at least 40% in serum AP - based on patient's baseline characteristics.
| Decrease in serum AP | ≥40% | <40% | p |
|---|---|---|---|
| PBC–AIH | |||
| Present/incomplete, n (%) | 5 (71) | 2 (29) | 0.017* |
| Absent, n (%) | 8 (24) | 26 (76) | |
| ANA | |||
| Positive, n (%) | 9 (47) | 10 (53) | 0.038* |
| Negative, n (%) | 3 (14) | 18 (86) | |
| Baseline AP (U/L), median (IQR) | 420 (197; 613) | 171 (100; 343) | 0.011° |
| Baseline AST (U/L), median (IQR) | 85 (57; 149) | 40 (33; 74) | 0.008° |
| Baseline bilirubin (mg/dL), median (IQR) | 1.1 (0.7; 1.7) | 0.9 (0.6; 1.1) | 0.159° |
Ultimately, according to the logistic regression, the presence of complete/incomplete criteria for PBC–AIH overlap syndrome was independently associated with a decrease of 40% in serum AP (OR 9.286, p=0.018 (95% CI 1.475–58.467)).
3.2.2ComplicationsDuring the follow-up period, 45% of the patients were diagnosed with osteopenia. Hyperlipidaemia was diagnosed in 51% of the patients. Interestingly, no patient developed cirrhosis during the follow-up period.
More severe complications were rare. Two patients presented variceal bleeding, one developed hepatic encephalopathy associated with ascites and one patient with overlap syndrome presented acute hepatitis with coagulopathy. Two patients required liver transplantation for decompensated cirrhosis. There were no documented cases of hepatobiliary neoplasia. Three patients died due to non-hepatic causes (haemorrhagic stroke, heart failure and hypoglycaemic coma).
4DiscussionThis is the first study on this subject in Portugal and the documented baseline characteristics of the cohort of patients are similar to those of other regions, as the majority of them were middle-aged women [15]. Patient presentation can be diverse which makes it challenging for practitioners to guarantee adequate management. Moreover, as biochemical response to UDCA is not ideal, treatment failure must be properly assessed with validated scoring systems and alternative treatments are warranted [6]. Additionally, identification of AIH features and characterization of these patients is remarkably important because an erroneous diagnosis can lead to improper management.
At diagnosis, the majority were asymptomatic, reflecting the preclinical asymptomatic stages described for PBC that can last for decades [16]. Given the indolent natural history frequently exhibited by PBC patients, the diagnosis strongly relies on incidental findings such as abnormal laboratory tests. As expected, cholestasis indices presented median values above normal (median AP=214U/L (IQR 143–514)), with lesser degrees of cytolytic damage. In the same way, AMA antibodies play a key role in the diagnosis of these patients. Thus, the absence of AMA antibodies is thought to be associated with underdiagnosis, thereby reducing incidence and prevalence rates [15]. In this cohort, 75% of the patients underwent liver biopsy. Although biopsy is not currently recommended for AMA-positive PBC patients, advanced histological stages are consistently associated with poor prognosis [17] (as observed in this cohort, patients with cirrhosis at diagnosis exhibited significantly less robust decreases in AP (p=0.028) in response to UDCA). Therefore, histological grading has been widely used to stratify risk. As more recent noninvasive methods are being proposed for assessing hepatic fibrosis in patients with chronic liver diseases, liver biopsy may appear of limited relevance to assess risk. Currently, transient elastography is the most widely used and validated technique, however two-dimensional shear wave elastography is the latest elastography technology and recent data shows that it is a valid, simple, rapid and reproducible, with advantages including its low cost and more extensive applicability [18]. Magnetic resonance imaging of the abdomen can also emerge as an valid alternative to biopsy, as the usefulness of apparent diffusion coefficient in grading of hepatic fibrosis and predicting esophageal varices in cirrhotic patients has been disclosed [19,20]. Nonetheless, histological features may continue to have a role in patients with poor biochemical response to treatment [6]. Thus, the authors contemplate its use becoming limited to those who do not respond to treatment, including patients with AIH features.
Until recently, UDCA was the only therapy broadly recommended for PBC. Every included patient was treated accordingly and none exhibited significant adverse effects leading to treatment interruption. Interestingly, none of the patients developed cirrhosis during the follow-up period. Although this could be related to the sample size, it may translate the beneficial effect of UDCA therapy in hindering PBC clinical course. Nonetheless previous studies documented some degree of progression to cirrhosis [21–23]. The authors observed, as described in previous studies [24], an incomplete biochemical response to UDCA in 44% of the patients. There was a significant paired reduction of AP, but paired AST and bilirubin decrease after one year of treatment was not significant. Inappropriate biochemical response is rather relevant because it is associated with development of liver-dependent symptoms and poorer outcomes [25–27] and higher doses is not associated with better responses [28]. Consequently, the need for new therapies is evident. Obeticholic acid showed effective biochemical response in adults with primary biliary cholangitis and is now an approved alternative, recommended in combination with UDCA, in patients who have an inadequate response to UDCA, or as monotherapy, in those who are unable to take UDCA because of side effects [6,9]. Other therapeutic approaches are being investigated and a better understanding of pathogenesis can further contribute to an improved management of PBC patients in the future [29].
AIH features were identified in 13% of the patients. PBC–AIH can be difficult to diagnose, as characteristics from either PBC or AIH may dominate the clinical picture and develop simultaneously or in a sequential form. Moreover, the defined criteria for diagnosing PBC–AIH can be difficult to meet, as observed in this cohort with discordant histological and clinical findings. Consequently, underdiagnosis of patients baring this syndrome can lead to inappropriate management of patients associated with previously observed poorer outcomes [30]. Also, some studies of PBC patients exclude those with overlap features, which prevents an adequate comparison of the natural history and assessment of treatment efficacy. EASL suggests immunossupressive treatment in patients with severe interface hepatitis, and consideration in patients with moderate interface hepatitis. However, the role of corticosteroids and other forms of immunosuppression is yet to be defined as controlled clinical trials have not been performed [6]. Likewise, additional studies are needed to evaluate the role of supplementary PBC treatments, such as obeticholic acid and budesonide, when AIH features are present. Remarkably, in this cohort patients those with AIH features exhibited steeper decreases in serum AP (p=0.007) and obtained the endpoint of 40% decrease in serum AP more frequently than others (p=0.017). It is not clear if the improved response was due to a different phenotype of this subgroup, to an enhanced effect of UDCA or to the impact of the immunosuppressive agents. However, given the high percentage of patients further managed with immunosuppressive agents (28%), the authors consider that by treating patients without definite biopsy criteria, giving more preponderance to the clinical features of PBC/AIH, less patients that needed immunosuppression were left untreated. Nevertheless, the only variable independently associated with the evaluated response to UDCA was the presence of AIH features, despite the limitations of the sample.
As a retrospective observational study, some limitations have to be considered. In fact, the data was limited to available records and complete data was not available for all of the patients. Furthermore, as a single-centre study of a rare disease, the number of patients was also limited, so prevalence and incidence data could not be adequately analyzed. Additionally, due to the lack of adverse events in our cohort, survival analyses were unfeasible.
Although it is rare, PBC can result in serious adverse outcomes and therapy is not always effective. Subsequently, PBC should not be overlooked or underestimated by physicians. Further studies are needed to better characterize the complex phenotypes of PBC patients with autoimmune features, for whom clear management strategies are not clearly defined.
AbbreviationsPBC
primary biliary cholangitis
AMAantimitochondrial antibodies
EASLEuropean Association for the Study of Liver Diseases
APalkaline phosphatase
AIHautoimmune hepatitis
NASHnon-alcoholic steatohepatitis
ANAantinuclear antibodies
ELISAenzyme-linked immunosorbent assay
UDCAursodeoxycholic acid
ULNupper limit of normal
ASTaspartate transaminase
HCChepatocellular carcinoma
ANAantinuclear antibodies
ASMAanti-smooth muscle antibodies
SPSSstatistical programme for social sciences
ALTalanine transaminase
AZAazathioprine
IQRinterquartil range
Financial supportThe authors have financial support to disclose.
Conflict of interestThe authors have no conflicts of interest to declare.