Background & Aim. Despite treatment with glucocorticoids, mortality remains high in patients with severe alcoholic hepatitis. Oxidative stress and depletion of mitochondrial glutathione are implicated factors in liver injury. The aim of this study was to evaluate the impact of the addition of metadoxine, a drug which possesses a multifactorial mechanism of action, including antioxidant properties, to standard treatment with glucocorticoids in patients with severe alcoholic hepatitis.
Material and methods. This randomized open label clinical trial was performed in Mexico’s General Hospital (Registry Key DIC/10/107/03/043). We randomized 70 patients with severe alcoholic hepatitis. The first group received prednisone (40 mg/day), and the second group received prednisone (40 mg/day) plus metadoxine tablets (500 mg three times daily). The duration of treatment in both groups was 30 days. Survival at 30 and 90 days, development of complications, adverse events and response to treatment (Lille model) were assessed.
Results. In the group receiving metadoxine, significant improvements were observed, as follows: survival at 30 days (74.3 vs. 45.7%, P = 0.02); survival at 90 days (68.6 vs. 20.0%, P = 0.0001). There was less development or progression of encephalopathy (28.6 vs. 60.0%, P = 0.008) and hepatorenal syndrome (31.4 vs. 54.3%, P = 0.05), and the response to treatment (Lille model) was higher in the metadoxine group (0.38 vs. 0.63, P = 0.001; 95% CI 0.11 to 0.40). There were no differences between groups regarding the development or progression of variceal hemorrhage or infection. The incidence of adverse events, mainly gastrointestinal, was similar in both groups.
Conclusions. Addition of metadoxine to glucocorticoid treatment improves the short-term survival of patients with severe alcoholic hepatitis and diminishes the development or progression of encephalopathy and hepatorenal syndrome.
Heavy drinkers may develop episodes of alcoholic hepatitis. Severe alcoholic hepatitis (SAH) is characterized by a Maddrey’s discriminant function (MDF) over 32, and implies a two-month mortality near to 50% if untreated.1–3 The presence of acute renal failure (ARF) has a poor prognosis.4 Treatment with steroids has been demonstrated to diminish mortality to 35% within the following six months.5–7 However, through detection of an early change in the bilirubin level (ECBL), defined as a serum bilirubin level at 7 days lower than the bilirubin level on the first day of treatment, it has been reported that 27 to 40% of patients with SAH fail to respond to steroid treatment.2
Recently, Di Mambro, et al. studied in-vitro steroid resistance measured in peripheral blood mononuclear cells (PBMCs). They found that in vitro steroid resistance correlates with the clinical outcome; the response failure to steroid treatment contributes to a greater mortality rate.8
The Lille model is a useful tool to predict the response to steroid treatment. A Lille score > 0.45 predicts a response failure to steroid treatment and a mortality rate up to nearly 75%.9 A short publication regarding a historic cohort of Mexican patients reported that 90% of the patients with a Lille score > 0.45 failed to respond to steroid treatment.10 A multicenter study performed in Mexican patients demonstrated that the mortality rate was high; the overall in-hospital and 90-day mortality rate were 36% and 51%, respectively. The main causes of death at 90 days were sepsis (20%), liver failure (24%) and multi-organ failure (46%).11
The pathophysiology of SAH is complex. Kupffer cell (KC) activation, production of pro-inflammatory cytokines and reactive oxygen species (ROS) are implied factors in liver damage.12,13 Moreover, there is severe depletion of mitochondrial glutathione (mGSH), which is the principal antioxidant agent in hepatocytes.14,15
Many investigations have demonstrated that treatment with metadoxine is effective in patients with acute alcohol intoxication and chronic alcoholic liver disease and may be effective in patients with non-alcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH).16–19 Furthermore, studies have revealed the multifactorial mechanism of action of metadoxine, which protects hepatocytes by increasing ATP, reduces free fatty acid accumulation and inhibits TNF-α.20–22
Study objectiveThe aim of this study was to evaluate the impact of adding metadoxine to the current standard treatment with prednisone in patients with SAH.
Material and MethodsStudy designThe present investigation was a randomized, open-label clinical study performed at the Hospital General de México, Dr. Eduardo Liceaga from April 2010 to March 2012. The protocol was reviewed and approved by the Institutional Investigation and Ethical Committee (Register key DIC/10/107/03/043).
Inclusion criteriaPatients between 18 and 65 years old and with clinical and biochemical criteria for SAH23,24 characterized by a history of chronic and heavy alcohol intake (> 80 g per day within the previous 5 years), rapid onset of jaundice in the absence of biliary tract obstruction by ultrasound, painful hepatomegaly and ascites, transaminases increased more than two times above the normal value, an aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio greater than 2 times normal, leukocytosis with a predominance of neutrophils, total bilirubin > 5 mg/dL, and MDF > 32 (calculated with the formula [4.6 x (patient prothrombin time (PT)-control PT, in seconds) + total bilirubin in mg/dL]) were included in the study.
Exclusion criteriaPatients with the following concomitant diseases or conditions were excluded: acquired immunodeficiency syndrome; neoplasms; autoimmune diseases; psychiatric disorders different from alcoholism, such as depression and anxiety according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DMS-IV), criteria; history of atopy or asthma; diabetes; obesity; pregnancy; hepatitis B virus (patients with positivity for any of the following: HBsAg, anti-HBc, IgM anti-HBc) and/or hepatitis C virus infection; and tuberculosis. In addition, patients with intake of illicit drugs, herbal products, antioxidant supplements (multivitamins, S-adenosyl-L-methionine, metadoxine, silymarin), or previous treatment with steroids or pentoxifylline within the previous two years were excluded. Patients without family support or without access to telephone communication were also excluded from the study.
Screening phaseAll patients provided written informed consent before enrollment (for patients with hepatic encephalopathy, informed consent was obtained from the next of kin). On the admission day (baseline), we performed a clinical history and collected peripheral blood samples to determine glucose, urea, creatinine, total bilirubin, alkaline phosphatase (AP), gamma glutamyl transpeptidase (GGT), alanine transaminase (ALT), aspartate aminotransferase (AST), sodium, potassium, albumin, leucocytes, neutrophils, hemoglobin, platelets, PT and the international normalized ratio (INR). Patients were also tested for hepatitis B surface antigen (HBsAg), hepatitis B surface antibody (anti-HBs), total hepatitis B core antibody (anti-HBc), IgM antibody to hepatitis B core antigen (IgM anti-HBc), serology for hepatitis C virus (HCV) and human immunodeficiency virus (HIV). Screening for bacterial infections included urine, blood and ascites cultures, as well as chest radiography and the neutrophils count in ascites. Liver ultrasound and endoscopy to determine the presence of varices was performed in all patients.
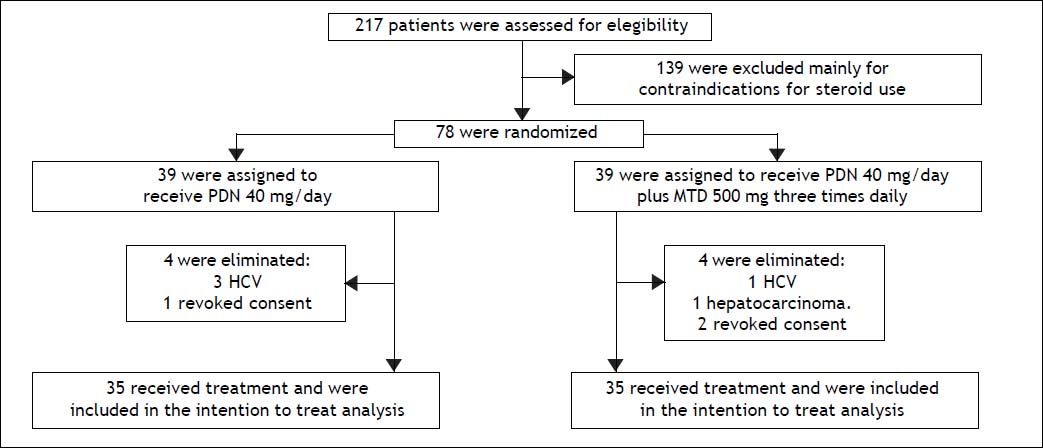
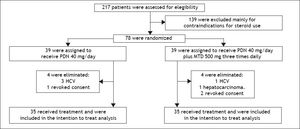
RandomizationIn total, 217 patients were evaluated, of whom 70 patients diagnosed with clinical and biochemical criteria for SAH met the inclusion criteria. These patients were randomized into two treatment groups of 35 patients each. The first group received prednisone, 40 mg once daily. The second group received prednisone (40 mg daily) plus oral metadoxine (500 mg three times daily). The treatment duration in both groups was 30 days or until death if death occurred before day 30 (Figure 1).
Study phase and outcomesThe primary outcome was survival at 30 and 90 days, respectively. The secondary outcomes were development or progression of acute renal failure (ARF), variceal bleeding (VB), hepatic encephalopathy (HE), bacterial or fungal infections, adverse effects and response to treatment according to the Lille model.
Patients were monitored weekly during the first month and then two times per month until the third month (day 90). Each visit included clinical examinations and peripheral blood samples to determine glucose, urea, creatinine, total bilirubin, AP, GGT, AST, ALT, sodium, potassium, albumin, hemoglobin, platelets, leucocytes, neutrophils, PT and INR. These tests were performed more frequently, if necessary, based upon the individual patient’s medical condition. During the first two weeks, all patients were hospitalized; subsequently, each investigator determined the duration of hospitalization depending on the individual patient’s medical condition. For hospitalized patients, the medications were administered under the supervision of physicians and nurses. For outpatients, the adherence to treatment was monitored by a family member who was dedicated to take care of the patient, administered the treatment drugs and reported in a control diary. To increase control of the medication intake, patients were required to return the empty blisters at each visit.
In patients who were suspected of developing an infection during the study, cultures and chest radiography were performed if necessary. In patients who were suspected of developing odynophagia or dysphagia, an endoscopic study was performed to identify esophageal candidiasis; when suspicious lesions were seen, brushing and mycological examinations were performed. When patients developed complications, such as ARF, VB, HE or infection, additional laboratory and radiography tests were performed.
Management of complications- •
ARF. This condition was defined, according to the actual criteria from the Acute Kidney Injury Network, as the abrupt reduction (48 h) of renal function, characterized by an increase of 0.3 mg/dL in the serum creatinine compared with the baseline value. Patients who had a baseline value of serum creatinine greater than 1.5 mg/dL at the time of admission were considered ARF cases.25,26 The initial therapy consisted of intravascular volume expansion with an albumin infusion at 1 g/kg for 48 h. Patients without a response were evaluated for hepatorenal syndrome (HRS) according to Ascites International Club criteria; patients who met the criteria received a vasopressor (terlipressin or norepinephrine) and intra-vascular volume expansion with albumin.27,28
- •
HE. This condition was defined clinically by neuropsychiatric alterations and neuromuscular signs according to the West-Haven criteria.29 Patients having HE grade I or II were treated with L-ornithine–L-aspartate orally. Patients with HE grade III or IV were treated with L-ornithine–L-aspartate intravenously. In patients for whom L-ornithine-L-aspartate was contraindicated, oral lactulose for HE grade I or II and lactulose enemas for HE grade III or IV were prescribed.
- •
VB. This condition was defined by the presence of melena or hematemesis associated with gastroesophageal varices as determined by endoscopy. These patients were treated with terlipressin or octreotide. Fresh frozen plasma and blood were collected as necessary. When acute VB was diagnosed, endoscopic band ligation for esophageal varices or cyanoacrylate injection for gastric varices was performed. Antibiotic prophylaxis was prescribed. Subsequently, the patients received secondary prophylactic therapy with propranolol and an endoscopic examination.30
- •
Spontaneous bacterial peritonitis (SBP). Patients with an ascites neutrophil count greater than 250/mm3 were diagnosed with SBP and were given empirical antibiotic therapy with cefotaxime (2 g intravenously, three times daily). The patients were re-evaluated after 72 h with a clinical examination and diagnostic paracentesis to determine the neutrophil count. In case of improvement, empiric antibiotic therapy was continued up to 6 days. If there was no improvement, antibiotic therapy was adjusted according to the ascites culture results. Next, the secondary prophylaxis therapy with nor-floxacin was continued.28
- •
Other infections. Urinary tract infection was diagnosed in patients having urinary symptoms associated with abnormal urinary examinations and urinary cultures, including a bacterial count greater than 100,000 CFU. Antibiotic therapy was prescribed based on the urinary culture results.
Pneumonia was diagnosed in patients who developed cough with expectoration and consolidation in the chest X-ray. Treatment was initiated with ceftriaxone (1 g) and clarithromycin (600 mg three times daily), after which the antibiotic therapy was adjusted depending on the sputum culture results.
When patients developed odynophagia or dysphagia, esophageal candidiasis was considered. The diagnosis was confirmed through oral cavity examinations and endoscopy with the presence of compatible lesions; brushing for mycological examination was performed in all cases, and treatment with fluconazole (100 mg twice daily) was prescribed.
Patients with diarrhea were evaluated by microscopic fresh stool examinations and stool cultures. These patients were treated empirically with ciprofloxacin and/or metronidazole.
Statistical analysisThe distribution of variables was analyzed; in case of quantitative variables with a non-normal distribution base, a 10-logarithmic transformation was performed to normalize their distribution. Descriptive statistics were used, quantitative variables were expressed as the mean and standard deviation (SD), and qualitative variables were expressed as proportions and percentages. Analysis with intention to treat (ITT) was conducted. To compare primary and secondary outcomes between groups, the chi-square test, Fisher’s exact test or Student’s t-test was used, based on the variable type. Survival analysis was performed using Kaplan-Meier curves for up to 30 and 90 days, respectively, and compared with the log-rank test.
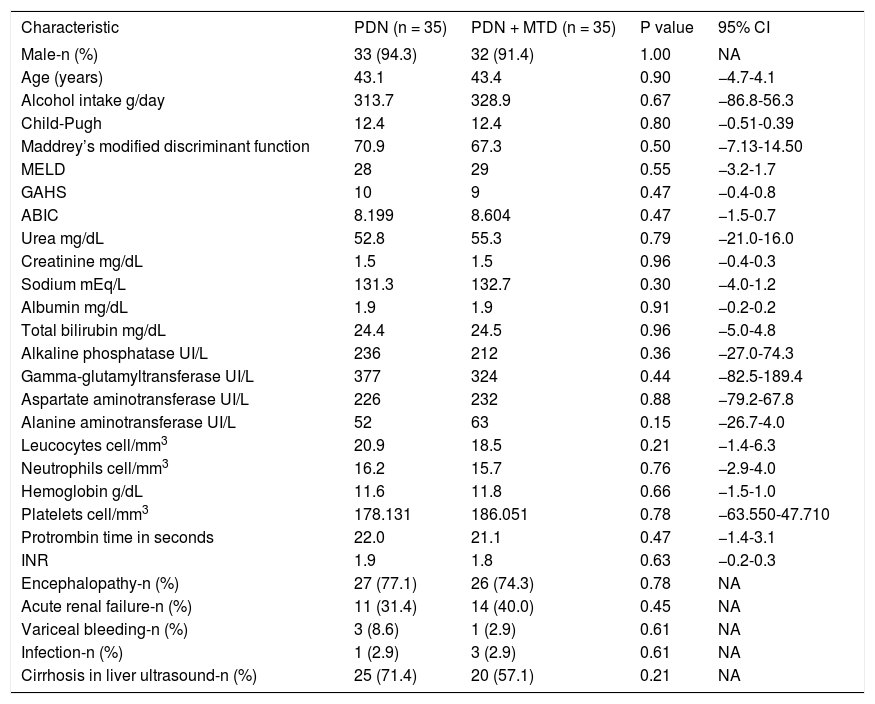
ResultsThe baseline characteristics of patients are listed in table 1.
Basal characteristics of patients.
| Characteristic | PDN (n = 35) | PDN + MTD (n = 35) | P value | 95% CI |
|---|---|---|---|---|
| Male-n (%) | 33 (94.3) | 32 (91.4) | 1.00 | NA |
| Age (years) | 43.1 | 43.4 | 0.90 | −4.7-4.1 |
| Alcohol intake g/day | 313.7 | 328.9 | 0.67 | −86.8-56.3 |
| Child-Pugh | 12.4 | 12.4 | 0.80 | −0.51-0.39 |
| Maddrey’s modified discriminant function | 70.9 | 67.3 | 0.50 | −7.13-14.50 |
| MELD | 28 | 29 | 0.55 | −3.2-1.7 |
| GAHS | 10 | 9 | 0.47 | −0.4-0.8 |
| ABIC | 8.199 | 8.604 | 0.47 | −1.5-0.7 |
| Urea mg/dL | 52.8 | 55.3 | 0.79 | −21.0-16.0 |
| Creatinine mg/dL | 1.5 | 1.5 | 0.96 | −0.4-0.3 |
| Sodium mEq/L | 131.3 | 132.7 | 0.30 | −4.0-1.2 |
| Albumin mg/dL | 1.9 | 1.9 | 0.91 | −0.2-0.2 |
| Total bilirubin mg/dL | 24.4 | 24.5 | 0.96 | −5.0-4.8 |
| Alkaline phosphatase UI/L | 236 | 212 | 0.36 | −27.0-74.3 |
| Gamma-glutamyltransferase UI/L | 377 | 324 | 0.44 | −82.5-189.4 |
| Aspartate aminotransferase UI/L | 226 | 232 | 0.88 | −79.2-67.8 |
| Alanine aminotransferase UI/L | 52 | 63 | 0.15 | −26.7-4.0 |
| Leucocytes cell/mm3 | 20.9 | 18.5 | 0.21 | −1.4-6.3 |
| Neutrophils cell/mm3 | 16.2 | 15.7 | 0.76 | −2.9-4.0 |
| Hemoglobin g/dL | 11.6 | 11.8 | 0.66 | −1.5-1.0 |
| Platelets cell/mm3 | 178.131 | 186.051 | 0.78 | −63.550-47.710 |
| Protrombin time in seconds | 22.0 | 21.1 | 0.47 | −1.4-3.1 |
| INR | 1.9 | 1.8 | 0.63 | −0.2-0.3 |
| Encephalopathy-n (%) | 27 (77.1) | 26 (74.3) | 0.78 | NA |
| Acute renal failure-n (%) | 11 (31.4) | 14 (40.0) | 0.45 | NA |
| Variceal bleeding-n (%) | 3 (8.6) | 1 (2.9) | 0.61 | NA |
| Infection-n (%) | 1 (2.9) | 3 (2.9) | 0.61 | NA |
| Cirrhosis in liver ultrasound-n (%) | 25 (71.4) | 20 (57.1) | 0.21 | NA |
ABIC: age-bilirubin-international normalized ratio-creatinine. INR: international normalized ratio. GAHS: Glasgow alcoholic hepatitis scale. MELD: Model for End Stage Liver Disease. PDN: prednisone. PDN + MTD: prednisone + metadoxina.
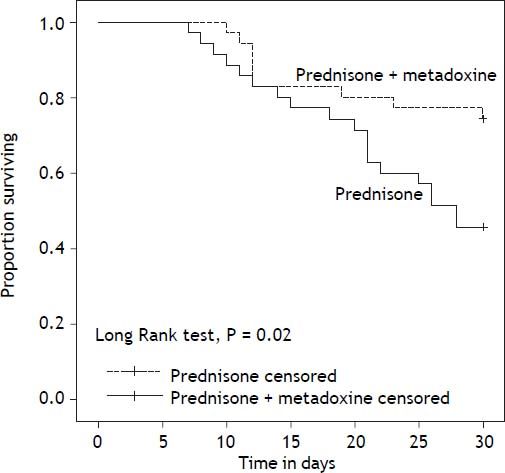
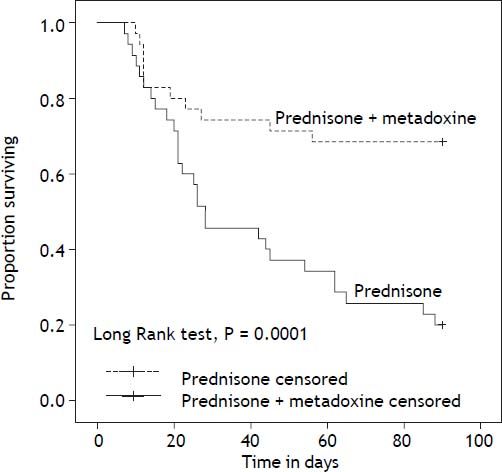
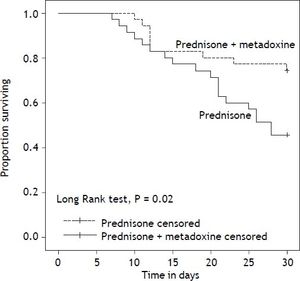
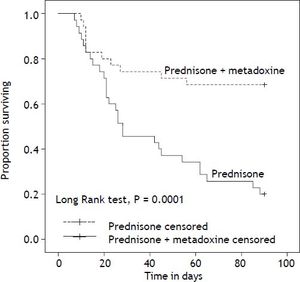
With regard to the primary outcome, the survival rate at 30 days in the group of patients who received concomitant treatment with prednisone and metadoxine was 74.3% (26 of 35), compared with 45.7% (16 of 35) in the group that received prednisone alone P = 0.02 (Figure 2). At 90 days, the survival rate in the group of patients who received concomitant treatment with prednisone and metadoxine was 68.6% (24 of 35), compared with 20.0% (7 of 35) in the group that received prednisone alone P = 0.0001 (Figure 3).
Kaplan Meier curves displaying the 30-day survival according to the treatment group. PDN censored: on the plot, the small vertical tick-marks indicate the primary outcome of the 30-day survival rate in the patients treated with prednisone. PDN + MTD censored: on the plot, the small vertical tick-marks indicate the primary outcome of the 30-day survival rate in the patients treated with prednisone and metadoxine. x-axis: days. y-axis: proportion surviving. PDN: Prednisone. MTD: metadoxine.
Kaplan Meier curves displaying the 90-day survival according to the treatment group. PDN censored: on the plot, the small vertical tick-marks indicate the primary outcome of the 90-day survival rate in the patients treated with prednisone. PDN + MTD censored: on the plot, the small vertical tick-marks indicate the primary outcome of the 90-day survival rate in the patients treated with prednisone and metadoxine. x-axis: days. y-axis: proportion surviving. PDN: Prednisone. MTD: metadoxine.
The response to treatment according to the Lille model was greater in the group receiving concomitant treatment than in the group receiving prednisone alone (0.38 vs. 0.63 P = 0.001; 95% CI 0.11 to 0.40).
Regarding the development or progression of complications, 10 patients developed or exhibited a progression of the HE grade in the group receiving concomitant treatment with prednisone and metadoxine, compared with 21 patients in the group receiving prednisone alone (28.6 vs. 60.0%, P = 0.008); 11 patients developed HRS in the group receiving concomitant treatment, versus 19 patients in the group receiving prednisone alone (31.4 vs. 54.3%, P = 0.05).
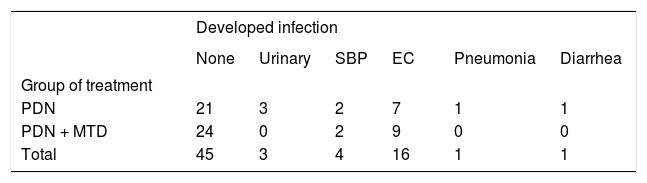
There were no significant differences between groups with regard to the development or progression of VB, which was present in 10 patients in the group receiving prednisone and metadoxine, compared with 13 patients in the group receiving prednisone alone (28.6 vs. 37.1%, P = 0.44). No significant differences were observed between groups with regard to the development of infections, which was present in 11 patients in the prednisone and metadoxine group, compared with 14 patients in the group receiving prednisone alone (31.4 vs. 40%, P = 0.45). The frequency of documented infections is presented in table 2.
Frequency of developed infections in patients with severe alcoholic hepatitis.
| Developed infection | ||||||
|---|---|---|---|---|---|---|
| None | Urinary | SBP | EC | Pneumonia | Diarrhea | |
| Group of treatment | ||||||
| PDN | 21 | 3 | 2 | 7 | 1 | 1 |
| PDN + MTD | 24 | 0 | 2 | 9 | 0 | 0 |
| Total | 45 | 3 | 4 | 16 | 1 | 1 |
PDN: prednisone. PDN + MTD: prednisone + metadoxine. SBP: spontaneous bacterial peritonitis. EC: esophageal candidiasis. SBP: patients with positive ascites culture and/or neutrophils count in ascites greater than 250/mm3 were diagnosed with SBP and given empirical antibiotic therapy with cefotaxime 2 g intravenously three times daily. Patients were re-evaluated after 72 h with clinical examination and diagnostic paracentesis to determine neutrophils count. In case of improvement, empiric antibiotic therapy was continued up to 6 days. If there was no improvement, antibiotic therapy was adjusted according to the ascites culture results. Then, secondary prophylaxis therapy with norfloxacine was continued. Urinary tract infection: was diagnosed in patients having urinary symptoms associated with abnormal urinary examination and urinary culture, having a bacterial count greater than 100,000 CFU. Antibiotic therapy was prescribed according to urinary culture results. Pneumonia: was diagnosed in patients who developed cough with expectoration and consolidation in the chest X-ray. Treatment was initiated with ceftriaxone 1 g and clarithromycin 600 mg three times daily, after which antibiotic therapy was adjusted depending upon culture results of the expectoration. Esophageal candidiasis: Diagnosis was confirmed through oral cavity examination and endoscopy with the presence of compatible lesions, brushing for mycological examination was obtained in all cases and treatment with fluconazole 100 mg twice daily was prescribed. Diarrhea: patients were evaluated with microscopic fresh stool examination and stool cultures. These patients were treated empirically with ciprofloxacin and/or metronidazole.
The occurrence of adverse effects was similar in both groups, principally epigastric burning, nausea and vomiting, due to which 4 patients in the prednisone and metadoxine group, as well as 3 patients in the group that received prednisone alone, decided by themselves to suspend treatment between weeks 14 and 21. These patients were included in the intention to treat analysis because we verified that they take at least 80% of the treatment. Serious adverse effects were not reported in either group.
DiscussionUp to 40% of patients with severe alcoholic hepatitis die within 6 months after the onset of the clinical syndrome.24 Several studies have demonstrated that steroid treatment improves the survival rate. Carithers, et al.5 found that patients with SAH treated with methylprednisolone (32 mg/day for 28 days) had a lower mortality rate compared with patients who received a placebo (6 vs. 35%, P = 0.006). Ramond, et al.31 also found that the survival rate at 2 months was better in patients with SAH treated with prednisolone (40 mg/day for one month) than in patients who received a placebo.
In 2010, Mathurin, et al.32 performed a metaanalysis and found that patients treated with corticosteroids have a better survival rate at 28 days compared with patients who do not receive corticosteroids (79.97 ± 2.8% vs. 65.7 ± 3.4%, P = 0.0005). In this meta-analysis, patients are classified according to their response to treatment with steroids (based on the Lille model) as complete responders (Lille < 0.16), partial responders (Lille 0.16 to 0.56) and null responders (> 0.56). The survival rate at 28 days was strongly associated with these subgroups (91.1 ± 2.7% vs. 79.4 ± 3.8% vs. 53.3% ± 5.1%, P < 0.0001).
Corticosteroids had a significant effect on the survival at 28 days in complete responders (HR 0.18, P = 0.006) and in partial responders (HR 0.38, P = 0.04), but not in null responders. In our study, the group of patients who received prednisone alone had a mortality rate of 54.3% at 30 days and 80% at 90 days, which was similar or even greater than that reported with the placebo. In our patients, treatment with steroids alone did not yield significant clinical benefits. According to this meta-analysis, our patients are classified as null responders when the Lille model is applied, with a mean value of 0.63 in patients who received prednisone alone. A possible explanation for this fact is steroid resistance.
In a study by Di Mambro, et al.,8 the in vitro steroid resistance in PBMCs from patients with SAH correlated with clinical steroid resistance defined by a decrease of less than 25% in the level of serum total bilirubin within 6 days of starting treatment, or mortality in the next 6 months. Furthermore, interleukin-2 (IL-2) may contribute to this steroid resistance because in vitro blocking of the IL-2 receptor with basiliximab improved the in vitro sensitivity to steroids.
In our study, the mortality in patients treated with PDN alone was as high as 80%. The mortality is also high in other studies conducted in Mexican patients with SAH. Garrido-García, et al. reported a mortality rate of 59.9% at 28 days in their patients despite treatment with PDN.33 We do not attribute this high mortality to the duration of treatment with PDN because if we consider the studies by Carithers, et al.5 or Ramond, et al.,31 their patients treated for 28 days and for one month with a steroid had a lower mortality than the placebo group did.
Moreover, Dominguez, et al.34 and Altamirano, et al.11 demonstrated that despite treatment with steroid or pentoxifylline, the mortality rate is mainly related to the ABIC class, being 0 to 13% in class A, 30 to 50% in class B, and 75 to 81% in class C at 90 days of follow-up.
Stewart, et al.35 evaluated the effect of antioxidants in patients with SAH, the patients were stratified by sex and steroid use. The treatment group received a starting dose of N-acetylcysteine (150 mg/ kg), followed by 100 mg/day for one week, and vitamin supplements daily for 6 months. The control group received a placebo. The survival rate at 6 months was similar between the groups (52.8 vs. 55.8%, P = 0.699), and there were no differences between the subgroups by sex or steroid use.
A recent study by Nguyen-Khac, et al.36 evaluated the possible synergistic effect of N-acetylcysteine with prednisolone vs. prednisolone alone. The mortality rate at 30 days was lower in the group that received N-acetylcysteine (8 vs. 24%, P = 0.006).
However, the mortality rates at 90 days and at 6 months were similar in both groups. Infections were less common in the group that received N-acetylcysteine (P = 0.001). In our study, we did not find differences with regard to the development of infection between the group taking metadoxine, which has been demonstrated to have an antioxidant effect, and the group that received prednisone alone.
Our study demonstrated that concomitant treatment of metadoxine with prednisone significantly improves the short-term (30- and 90-day) survival rate of SAH patients. Furthermore, the treatment response (based on the Lille model) was superior in the group that received metadoxine, with a Lille mean score of 0.38, indicating partial responders. In addition, serious complications, such as HE and HRS were less frequent in the group receiving metadoxine. It is noteworthy that a previous study conducted in our hospital demonstrated that ARF and HE are risk factors that increase the mortality rate at 30 days, with a relative risk (RR) of 6.7 (P = 0.02) and 8.9 (P = 0.001), respectively.37
Caballería, et al. conducted a multicenter, randomized, double-blind clinical study including patients who had active chronic alcoholism and were diagnosed with alcoholic fatty liver disease; treatment with metadoxine (500 mg three times daily) for three months accelerated the normalization of the liver function tests and yielded ultrasonographic improvement, even in patients with persistently active alcoholism, thereby recommending metadoxine as a therapeutic alternative during the early stages of alcoholic liver disease.20
We decided to administer 500 mg metadoxine three times daily based on two main concerns: first, because this dose was the maximum therapeutic dose used in a clinical trial in humans, and second, because the drug’s scavenger and antioxidant capacity is dose-related.38
Recently, Leggio, et al. conducted a retrospective study that evaluated patients who had been treated with metadoxine for acute alcohol intoxication, in whom markers of liver injury and markers of alcohol consumption, such as AST, ALT, GGT and mean cell volume (MCV) have been determined. Notably, metadoxine reduced the alcohol consumption and improved the adherence to abstinence; furthermore, an improved AST/ALT ratio was reported.21
Despite the many studies that have revealed the clinical benefits of metadoxine treatment for various pathologies related to the acute and chronic consumption of alcohol, as well as improvements in certain nonspecific serum markers of liver damage, and reduction of steatosis in fatty liver disease, so far no study has been performed to evaluate the efficacy of metadoxine for treating a serious condition such as severe alcoholic hepatitis. Therefore, our investigation is important because it demonstrates, using evidence-based clinical data, that the addition of metadoxine to the 30-day standard treatment with a glucocorticosteroid is a recommended alternative for patients classified as non-responders to glucocorticosteroid treatment according to the Lille model. Our study indicates that combined treatment with metadoxine and a glucocorticosteroid has a life-saving impact on the therapy of patients with severe alcoholic hepatitis, significantly improving the survival rate of these patients at 30 and 90 days.
Abbreviations- •
ALT: alanine aminotransferase.
- •
AP: alkaline phosphatase.
- •
ARF: acute renal failure.
- •
AST: aspartate aminotransferase.
- •
DSM-IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition.
- •
ECBL: early change in the bilirubin level.
- •
GGT: gamma glutamyl transpeptidase.
- •
HBV: hepatitis B virus.
- •
HCV: hepatitis C virus.
- •
HE: hepatic encephalopathy.
- •
HIV: human immunodeficiency virus.
- •
HRS: hepatorenal syndrome.
- •
INR: International Normalized Ratio.
- •
ITT: intention to treat.
- •
KC: Kupffer cell.
- •
MCV: mean cell volume.
- •
MDF: Maddrey’s modified discriminant function.
- •
mGSH: mitochondrial glutathione.
- •
MTD: metadoxine.
- •
NAFLD: non-alcoholic fatty liver disease.
- •
NASH: non-alcoholic steatohepatitis.
- •
PBMC: peripheral blood mononuclear cell.
- •
PDN: prednisone.
- •
PT: prothrombin time.
- •
ROS: reactive oxygen species.
- •
RR: relative risk.
- •
SAH: severe alcoholic hepatitis.
- •
SBP: spontaneous bacterial peritonitis.
- •
SD: standard deviation.
- •
US: United States.
- •
VB: variceal bleeding.
The authors declare no conflicts of interest.
Eurodrug Laboratories de Mexico S.A. de C.V. donated metadoxine 500 mg tablets (ABRIXONE®) to Mexico’s General Hospital for this study.
This work was awarded First Prize “Rolando Figueroa Barrios” in 2012, awarded by Asociación Latinoamericana para el Estudio de Hígado (ALEH) (The Latin American Association for the Study of the Liver).
Financial SupportThis work was partially supported by funds granted to Higuera de la Tijera MF through the Stimulus “Angeles Espinosa Yglesias 2010” granting FUNSALUD AC, AMPARO Foundation and FUNDHEPA A.C., Mexico.
Eurodrug Laboratories donated metadoxine (ABRIXONE®) to Mexico’s General Hospital for this study.
This work was awarded First Prize “Rolando Figueroa Barrios” in 2012, contributed by the Asociación Latinoamericana para el Estudio de Hígado (ALEH).