Background and rationale for the study. To investigate thyroid function in patients with acute-on-chronic liver failure (ACLF) caused by hepatitis B virus infection and to determine whether thyroid hormone levels can be used as prognostic markers for assessing severity and prognosis of ACLF patients. We enrolled 75 patients with ACLF and70 patients with chronic hepatitis B (CHB).Continual serum samples were collected during hospitalization from the ACLF patients. The serum thyroid hormone levels (triiodothyronine [T3], thyroxine [T4], free (F)-T3, FT4, and thyroid stimulation hormone [TSH]) were measured by chemiluminescence. The Model for End-stage Liver Disease (MELD) score was used to assess severity.
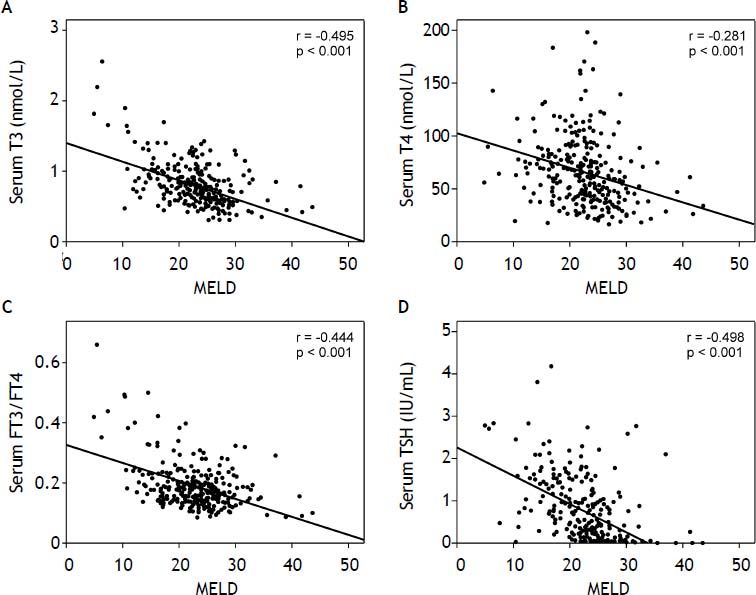
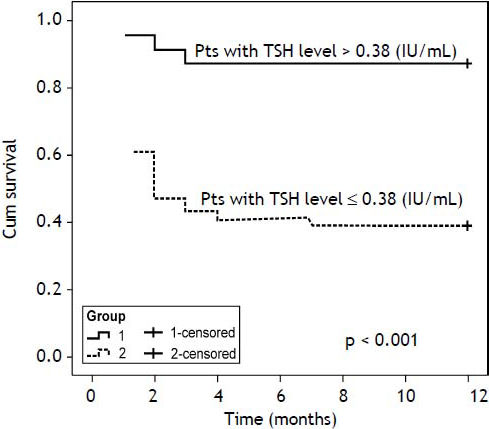
Results. ACLF patients showed significantly (p < 0.001) lower values of serum T3, T4, FT3/FT4 and TSH than CHB patients. The T3, T4, and TSH levels in ACLF patients were negatively correlated with the MELD score (T3: r = -0.495, p < 0.001; T4: r = -0.281, p < 0.001; TSH: r = -0.498, p < 0.001), suggesting that serum thyroid hormone levels reflect disease severity. At 1 year, 31 patients died. The T3 (p = 0.016), T4 (p = 0.008), and TSH (p = 0.003) levels in non-survivors were significantly lower than in survivors. The serum TSH level was a significant factor for predicting mortality in ACLF patients (optimal cutoff value = 0.38 IU/mL). The cumulative survival rate was decreased significantly when the serum TSH level was < 0.38 IU/mL (39.2%, p < 0.001).
Conclusion. Serum TSH level may be a useful indicator for assessing severity and prognosis in ACLF patients.
The liver plays an important role in thyroid hormone metabolism, specifically in its conjugation, excretion, and mono-de-iodination. Liver disease can affect thyroid hormone metabolism. Altered thyroid hormone metabolism resulting in a low serum triiodothyronine (T3), normal to low thyroxine (T4), a high reverse T3 (rT3) with an inappropriately normal thyroid stimulation hormone (TSH) in the absence of clinical hypothyroidism is well documented in chronic and cirrhotic liver diseases of various etiologies, such as hepatitis virus infection,1–4 alcoholic,3,4 autoimmunization,4,5 non-alcoholic fatty liver disease,5,6 haemochromatosis,3 and cryptogenic.3,4 These specific thyroid alterations cause euthyroid sick syndrome.
Acute-on-chronic liver failure (ACLF) affects patients with previously well-compensated liver disease in whom an acute decompensation of liver function occurs due to a precipitating event.7 In China, hepatitis B virus (HBV)-infected ACLF patients account for > 80% of ACLF patients, because of the high incidence of chronic HBV infection.8,9 The progressive nature of ACLF affects many organ systems. However, data on thyroid hormone changes in patients with HBV-related ACLF are lacking.
The Model for End-stage Liver Disease (MELD) score is commonly used to classify the severity of liver impairment and to predict the mortality of liver diseases. Previous studies addressed changes in thyroid hormone levels in relation to the Child-Pugh classification scheme in patients with cirrhotic liver diseases,10–11 but few studies have addressed the relationship with the MELD score.
Furthermore, thyroid hormones are potent mediators of multiple physiological processes. The liver is a typical target organ of thyroid hormones; therefore, the alterations of thyroid hormones can affect liver function. Previous studies reported that serum T3 concentrations may be a useful indicator for assessing the severity and prognosis of patients with liver diseases.12–15 However, the usefulness of serum thyroid hormones as a prognostic index remains to be established in ACLF patients.
The purposes of this study were to investigate whether serum thyroid hormone levels (T3, T4, free (F)-T3, FT4, and thyroid stimulation hormone [TSH]) were changed in ACLF patients and to determine whether thyroid hormone levels can be used as prognostic markers for assessing severity and prognosis in ACLF patients.
Material and MethodsThe protocol was approved by the Ethical Committee of Beijing 302 Hospital. All procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all included study patients.
SubjectsWe enrolled 75consecutive patients with HBV-related ACLF and 70 patients with chronic hepatitis B (CHB) at the Beijing 302 Hospitalfrom January 2012 to June 2012. ACLF is defined as acute liver decompensating on the basis of chronic liver disease with mandatory jaundice (serum total bilirubin > 171.0 μmol/L or a rapid rise > 17.1 μmol/L/day), coagulopathy (plasma prothrombin activity < 40%), and the recent development of complications.16 Patients with CHB met the diagnostic criteria of CHB according to the 2000 Xi’an viral hepatitis management scheme.17
The exclusion criteria were cardiac dysfunction, respiratory dysfunction, renal dysfunction, cancer, primary thyroid diseases, smoking, alcoholism, and those being treated with thyroid hormones or anabolic drugs.
SamplesSerum samples of all patients were collected at admission. ACLF patients were followed for 1 year, and serum thyroid hormones were measured during hospitalization for these patients. A total of 358blood samples were collected.
Biochemical assayThe biochemical parameters were routinely performed in the Central Clinical Laboratory of Beijing 302 Hospital. The T3, T4, FT3,FT4, and TSH levels were determined by chemiluminescence (VITROS reference nos. 1322528, 8744468, 1315589, 1387000, and 1912997; VITROS 3600 System; Ortho-Clinical Diagnostics, Rochester, NY,USA). Patient severity was assessed using the MELD score, which was calculated from the following formula: MELD Score = 9.6 × ln (creatinine [mg/dL]) + 3.8 × ln (bilirubin [mg/dL]) + 11.2× ln (international normalized ratio [INR]) + 6.4.18
Statistical analysisAll statistical analyses were performed using the Statistical Package for Social Science (SPSS), version 16.0 (SPSS, Inc., Chicago, IL, USA). Data were expressed as mean±standard deviation (SD). Comparisons between the two groups were made using the Student’s t-test for continuous variables. Categorical data were compared using the χ2 test. Correlations between two parameters were determined by using Pearson’s correlation coefficient(r). Cox’s regression analyses were performed to identify which of the following factors were predictive of mortality inACLF patients: age, sex, T3, T4, and TSH. Variables that reached statistical significance (p < 0.1) by univariate analyses were subjected to multivariate analysis inorder to identify predictors of the prognosis. We also determined the sensitivity and specificity of theTSH level for predicting the prognosis of ACLF patients by using receiver operating characteristic (ROC) curves. The Kaplan-Meier method and log rank test were adopted to test the survival differences. P < 0.05 was considered as significant.
ResultsComparison of clinical parameters between ACLF and CHB patients
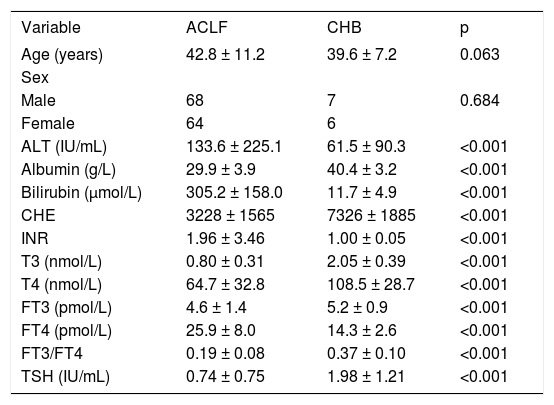
We enrolled 68 men and 7 women with ACLF due to hepatitis B infection (mean age: 42.8 ± 11.2 years) and 64 men and 6 women with CHB (mean age: 39.6 ± 7.2 years). HBV-infected ACLF patients showed lower values of serum T3 (0.80 ± 0.31 nmol/L, p < 0.001), T4 (64.7 ± 32.8 nmol/L, p < 0.001), FT3/FT4 (0.19 ± 0.08, p < 0.001), and TSH (0.74 ± 0.75 IU/mL, p < 0.001) levels than the CHB patients (Table 1).
Levels of thyroid hormones in patients with acute-on-chronic liver failure (ACLF) and chronic hepatitis B (CHB).
| Variable | ACLF | CHB | p |
|---|---|---|---|
| Age (years) | 42.8 ± 11.2 | 39.6 ± 7.2 | 0.063 |
| Sex | |||
| Male | 68 | 7 | 0.684 |
| Female | 64 | 6 | |
| ALT (IU/mL) | 133.6 ± 225.1 | 61.5 ± 90.3 | <0.001 |
| Albumin (g/L) | 29.9 ± 3.9 | 40.4 ± 3.2 | <0.001 |
| Bilirubin (μmol/L) | 305.2 ± 158.0 | 11.7 ± 4.9 | <0.001 |
| CHE | 3228 ± 1565 | 7326 ± 1885 | <0.001 |
| INR | 1.96 ± 3.46 | 1.00 ± 0.05 | <0.001 |
| T3 (nmol/L) | 0.80 ± 0.31 | 2.05 ± 0.39 | <0.001 |
| T4 (nmol/L) | 64.7 ± 32.8 | 108.5 ± 28.7 | <0.001 |
| FT3 (pmol/L) | 4.6 ± 1.4 | 5.2 ± 0.9 | <0.001 |
| FT4 (pmol/L) | 25.9 ± 8.0 | 14.3 ± 2.6 | <0.001 |
| FT3/FT4 | 0.19 ± 0.08 | 0.37 ± 0.10 | <0.001 |
| TSH (IU/mL) | 0.74 ± 0.75 | 1.98 ± 1.21 | <0.001 |
ALT: alanine aminotransferase. CHE: Cholinesterase. INR: international normalized ratio’s, thyroid stimulation hormone.
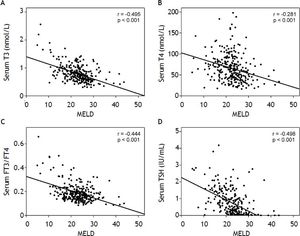
The MELD score was significantly correlated with the T3, T4, FT3, FT4, FT3/FT4, and TSH levels. There was a reverse correlation between the MELD score and the T3 (r = -0.495, p < 0.001), T4 (r = -0.281, p < 0.001), FT3 (r = -0.219, p = 0.012), FT3/FT4 (r = -0.444, p < 0.001), and TSH (r = -0.498, p < 0.001) levels, whereas there was a positive correlation between the MELD score and the FT4 (r = 0.286, p < 0.001) level (Table 2, Figure 1).
Correlation coefficients between the Model for End-stage Liver Disease (MELD) score and thyroid hormones.
| MELD score | T3 | T4 | FT3 | FT4 | FT3/FT4 | TSH |
|---|---|---|---|---|---|---|
| r | -0.495 | -0.281 | -0.219 | 0.286 | -0.444 | -0.498 |
| p | <0.001 | <0.001 | 0.012 | <0.001 | <0.001 | <0.001 |
TSH: thyroid stimulation hormone. The correlations between two parameters were determined by Pearson’s correlation coefficient.
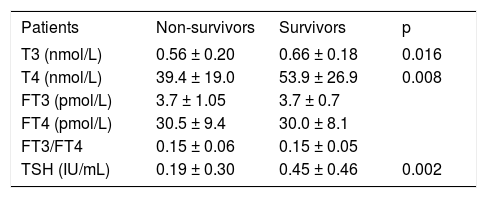
ACLF patients were followed for 1 year, and 31 patients died during that period. The serum levels of T3 (0.56 ± 0.20 nmol/L, p = 0.016), T4 (39.4 ± 19.0 nmol/L, p = 0.008), and TSH (0.19 ± 0.30 IU/mL, p = 0.002) in non-survivors were significantly lower than those in survivors (Table 3).
Comparison of thyroid hormones levels in non-survivors and survivors.
| Patients | Non-survivors | Survivors | p |
|---|---|---|---|
| T3 (nmol/L) | 0.56 ± 0.20 | 0.66 ± 0.18 | 0.016 |
| T4 (nmol/L) | 39.4 ± 19.0 | 53.9 ± 26.9 | 0.008 |
| FT3 (pmol/L) | 3.7 ± 1.05 | 3.7 ± 0.7 | |
| FT4 (pmol/L) | 30.5 ± 9.4 | 30.0 ± 8.1 | |
| FT3/FT4 | 0.15 ± 0.06 | 0.15 ± 0.05 | |
| TSH (IU/mL) | 0.19 ± 0.30 | 0.45 ± 0.46 | 0.002 |
TSH: thyroid stimulation hormone.
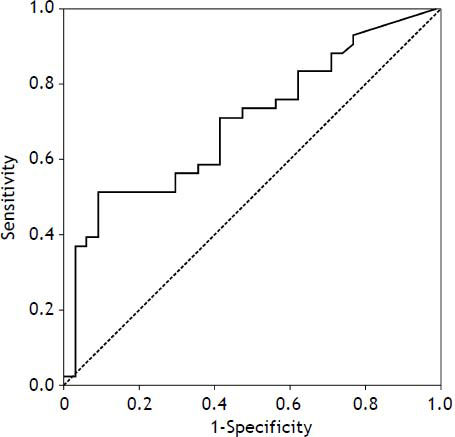
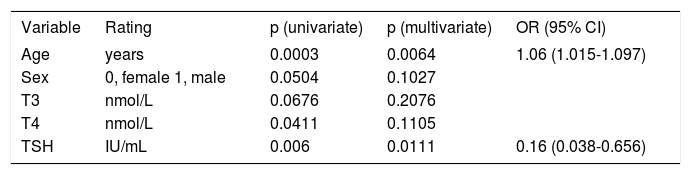
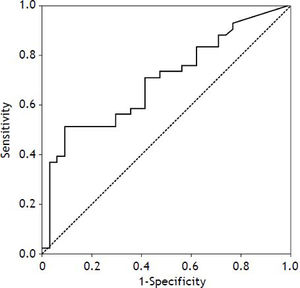
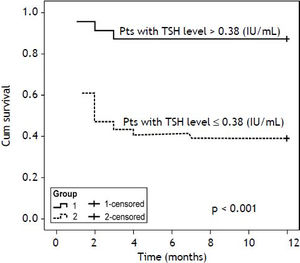
Age, T3, T4, and TSH levels were significant factors for predicting mortality according to the univariate Cox’s regression analysis, and serum TSH level (odds ratio [OR], 0.16; 95% confidence interval [CI], 0.038-0.656; p = 0.0111) was a significant factor for predicting mortality according to multivariate regression analysis(Table 4). Receiver operating characteristic curve (ROC) analysis was performed (Figure 2), and the area under the curve (AUCs)+ for the TSH was 0.698 (95% CI: 0.581-0.799; p = 0.0011). With an optimal cutoff value of 0.38 IU/mL, the sensitivity and specificity for predicting mortality were 91.2 and 51.2%, respectively. The cumulative survival rate of the ACLF patients differed significantly when all enrolled patients were classified according to the cutoff value (p < 0.001).The survival rate was 39.2% when serum TSH levels were < 0.38 IU/mL, and the survival rate was 87.5% when serum TSH levels were >0.38 IU/mL (Figure 3).Our results suggested that the serum TSH concentration may be used as a parameter for prognosis, because the low level reflected an extremely poor prognosis.
Cox’s regression analyses for mortality in patients with acute-on-chronic liver failure.
| Variable | Rating | p (univariate) | p (multivariate) | OR (95% CI) |
|---|---|---|---|---|
| Age | years | 0.0003 | 0.0064 | 1.06 (1.015-1.097) |
| Sex | 0, female 1, male | 0.0504 | 0.1027 | |
| T3 | nmol/L | 0.0676 | 0.2076 | |
| T4 | nmol/L | 0.0411 | 0.1105 | |
| TSH | IU/mL | 0.006 | 0.0111 | 0.16 (0.038-0.656) |
OR: odds ratio. CI: confidence interval. TSH: thyroid stimulation hormone.
It has been increasingly evident that several conditions other than thyroid disease change the concentrations of thyroid hormones. Many studies have demonstrated that severe systemic nonthyroid disease, including chronic and cirrhotic liver diseases, induce several abnormalities in thyroid function tests, which is probably a result of low serum T3, normal to low T4 with normal TSH (i.e., euthyroid sick syndrome).1–6
In our studies, serum T3, T4, FT3, FT3/FT4, and TSH concentration was significantly decreased in HBV-infected ACLF patients, suggesting that euthyroid sick syndrome may have occurred in these patients. Overall, the serum thyroid hormone concentrations appeared to parallel the severity of liver dysfunction, which is similar to the results of previous studies on cirrhotic liver diseases.1,2 These, observed alterations in the thyroid hormones are generally believed to be part of the acute adaptive mechanisms of the individual. The liver plays an important role in thyroid hormone metabolism, and liver disease can affect thyroid hormone metabolism. ACLF is associated with massive cell death, which may be responsible for the altered metabolism of T4 and its metabolic products. Greater than 80% of serum T3 is produced by peripheral deiodination. Parenchymal liver damage in ACLF patients may decrease T3 generation due to the impaired conversion of T4 to T3 in the liver.19
Earlier studies have indicated the interrelationship of hypothalamic dysfunction and the degree of liver impairment. It has been demonstrated that an increased impairment of the hypothalamic-pituitary-adrenal axis existed according to the severity of cirrhosis. While serum TSH concentration was normal, the function of the hypothalamic-pituitary-thyroid axis was not altered markedly.20 In our study, the serum T3 and T4 concentration decreased in ACLF patients, while the serum TSH concentration decreased significantly. These findings indicated that hypothalamic-pituitary-thyroid axis dysfunction may exist in ACLF patients. Although the underlying mechanisms of the suppression of the hypothalamic-pituitary-thyroid axis in ACLF are not yet known, an increase in levels of cytokines such as interleukin-1, interleukin-6, and tumor necrosis factor, as well as alterations in hepatic steroid metabolism may play a role.21–25
Previous studies have reported that serum T3 concentrations can indicate the severity and prognosis of patients with cirrhotic liver diseases.12–15 In our study, the TSH was regarded as a more sensitive marker than T3, T4, FT3, FT4, and FT3/FT4, suggesting that it is a useful indicator for assessing severity, and it may play an important role in elucidating the prognosis of ACLF patients.
Thyroid hormones are potent mediators of multiple physiological processes, including embryonic development, cellular differentiation, metabolism, and cell growth.13–14 In addition to their crucial roles in maintaining cellular homeostasis, abnormal thyroid hormones can cause multiple disorders, including cardiovascular disease,26,27 diabetes mellitus,28,29 and liver disease.1–6 The liver is a typical target organ of thyroid hormones. Equal amounts of the thyroid hormone receptors α1 and β1 antagonists are expressed in human hepatocytes.30 Previous studies reported that treatment with T3 analogs prevents hepatic statuses and hepatitis.15,31–37The multipotent functions of the thyroid hormones and thyroid hormone receptors are variable, but they are essential for normal growth and proliferation of the liver. In our study, the serum T3, T4, FT3, and TSHlevels were significantly decreased in ACLF patients, and they paralleled the severity of liver dysfunction. The serum T3, T4, and TSH levels in non-survivors were significantly lower than those in survivors. Thus, further investigation is required on whether the treatment of thyroid hormones analogs is needed in ACLF patients.
ConclusionOur study suggested that serum levels of thyroid hormones were altered in ACLF patients and were related to the severity of the diseases. The serum TSH levels may be a useful indicator for assessing severity and prognosis in ACLF.
Abbreviations- •
ACLF: acute-on-chronic liver failure.
- •
AUCs: area under the curve.
- •
CHB: chronic hepatitis B.
- •
CI: confidence interval.
- •
HBV: hepatitis B virus.
- •
MELD: Model for End-stage Liver Disease.
- •
OR: odds ratio.
- •
ROC: receiver operating characteristic curve.
- •
SD: standard deviation.
- •
SPSS: Statistical Package for Social Science.
- •
T3: triiodothyronine.
- •
T4: thyroxine.
- •
TSH: thyroid-stimulation hormone.
This study was supported by the 12th Five-Year National Science and Technology Major Project for Infectious Diseases (No: 2012ZX10002004-005) and the 12th Five-Year Grand Project of PLA (No: BWS11J075).
Conflicts of InterestNone.