Background. Visfatin is a novel adipocytokine predominantly expressed and secreted by visceral adipose tissue. It is realized for its multiple functions of central importance in NAD biosynthesis, innate immunity and inflammation. Its phosphoribosyl transferase activity regulates cellular energetics and NAD dependent enzymes such as SIRTUINS. Although its expression in various tissues and circulating levels are documented, visceral visfatin levels in Nonalcoholic fatty liver disease (NAFLD) patients have not been reported.
Objective. The aim of the present study was to assess visceral adipose tissue visfatin levels in NAFLD.
Materials and methods. A total of 115 patients undergoing diagnostic laparoscopy were recruited in the study and categorized into two groups based on standard criteria for NAFLD. Visceral adipose tissue TNF-α, IL-6 and visfatin levels were measured by ELISA. Blood glucose, lipids, liver enzymes and non esterified fatty acids (NEFA) were estimated using standard procedures. Formalin fixed, Hematoxylene Eosin stained liver biopsy specimens were examined for the presence of steatosis and the degree of steatosis was ascertained as per Brunt’s classification.
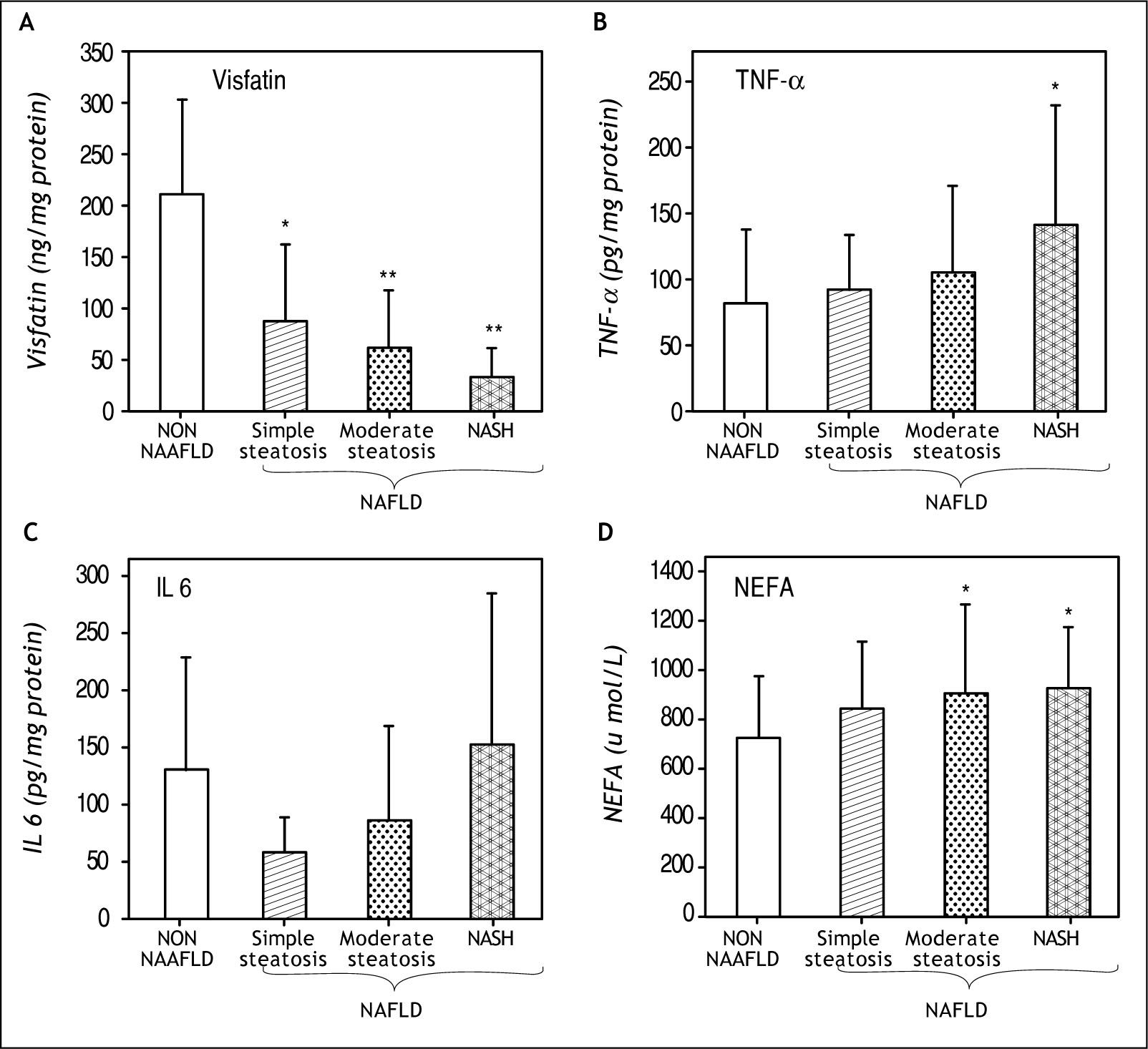
Results. The visceral visfatin level declined significantly (P < 0.001) in all groups of NAFLD as compared to non NAFLD group, while plasma NEFA level increased with progressive steatosis (P < 0.02). Significant increase in TNF a was observed in all groups of NAFLD, while IL-6 increased in NASH only.
Conclusion. A significant decline in visceral adipose tissue visfatin level was found to be associated with degree of steatosis in NAFLD patients.
Visfatin (PBEF/NAMPT/Visfatin), a novel adipo-kine predominantly expressed in visceral adipose tissue, has gained increasing importance not only in metabolism but also in inflammatory1 and immunological processes.2 It was initially identified in lymphocytes as pre β-cell colony enhancing factor (PBEF) which, along with IL-7, acts synergistically to promote the differentiation of β cell precur-sors.3 Its phosphoribosyl transferase activity (NAMPT) is the rate limiting step in NAD biosynthesis.4 Thus, it regulates cellular energetics and NAD dependent enzymes such as silent information regulator proteins (SIRTUINS).5 Many investigators have demonstrated the role of adipose tissue derived adipokines in the pathogenesis and in the progression of NAFLD to NASH (Non alcoholic steatohepatitis).6,7 Despite large number of reports on the role of proinflammatory cytokines including adiponectin in NAFLD studies on visceral adipose tissue visfatin in NAFLD are scanty. Visfatin was shown to be expressed in various tissues including liver8 and upregulated in visceral adipose tissue (VAT) of mice and humans9–11 under the conditions of obesity. VAT has been proposed to be a major contributor of steatosis in NAFLD.12 Recently decrease in plasma visfatin was shown to be associated with NASH,13 while the incidence of portal inflammation increased with increase in circulating visfatin level.14 Considering the importance of the portal delivery of visceral adipose tissue factors on liver, role of visceral visfatin in NAFLD needs to be elucidated.15 In view of this, the present study was conducted to assess the level of visceral adipose tissue visfatin in NAFLD.
Materials and MethodsSubjectsA total of 115 patients (non diabetic) referred to Asian Institute of Gastroenterology, Hyderabad, India for diagnostic laparoscopy were recruited in the study. Those with a history of diabetes mellitus (treated/untreated), chronic liver diseases, excessive consumption of alcohol (< 20 gm/day) and those on medication that influence glucose metabolism were excluded from the study. Patients who were radiologically diagnosed to have fatty liver and devoid of any history of alcohol consumption were considered as NAFLD group and patients without fatty liver were considered separately as non NAFLD group. The number of obese patients were fourteen in NAFLD and six in non NAFLD groups. Informed consent was obtained from all the patients and the study protocol was approved by the institutional ethical and scientific review committee.
MethodsSamplesVisceral adipose tissue from greater omentum was obtained at the time of laparoscopy in Dulbecco’s minimum essential medium (DMEM) containing 1000 mg/L glucose and 27 BSA for estimation of pro inflammatory cytokines and visfatin. Formalin preserved liver biopsy specimens taken from the patients were subjected to histopathologi-cal examinations. Fasting blood samples (5 ml) were collected in heparin for the estimation of glucose, lipids, liver enzymes and non esterified fatty acids (NEFA).
Measurment of proinflammatory cytokines and visfatinThe visceral adipose tissues were washed with PBS (pH 7.4) and extracted immediately using cell extraction buffer (Biosource, Camarillo, CA, USA) containing cocktail of protease inhibitors. The extracts were stored at-80° C until analysis. TNF-α (Tumor necrosis factor-α) and IL-6 (Interleukin-6) levels were estimated using ELISA kits (Sanquin reagents, AD Amsterdam, Netherlands) as per manufacturers’ instructions. Visfatin level was estimated by ELISA (Adipogen, Seoul, Korea) employing microplate wells pre-coated with monoclonal antibody specific for human visfatin. The bound visfatin in the adipose tissue extract was captured by HRP conjugated rabbit anti human visfatin. The extent of color developed with the addition of substrate was measured at 450nm using an ELISA plate reader (Bio-Rad, Tokyo, Japan). Lipid profiles, glucose and liver enzymes were estimated on autoanalyser (Daytona, Randox laboratories, Co. Antrim, UK) and non esterified fatty acids (NEFA) were measured on spectrophotometer (Bec-kman Coulter, Fullerton, CA, USA) using commercial kits (Randox).
Histopathological examination of liver biopsyFormalin fixed liver biopsy specimens were routinely processed, sectioned and stained with haema-toxylin-eosin. Biopsies were evaluated by two independent pathologists. The degree of steatosis was graded as per Brunt’s classification.16 Simple hepatocellular fat accumulation was considered as indicative of simple steatosis, moderate steatosis was diagnosed when predominant ballooning degeneration was observed while NASH was diagnosed by the presence of lobular inflammation and pericellu-lar fibrosis.
Statistical AnalysisThe data was analysed by ANOVA analysis of variance, Pearson’s correlation, and Kendell’s correlation as and when applicable using SPSS software, version 13.0 (SPSS, IL USA). A P value of < 0.05 was considered significant throughout the study.
ResultsDemographic and clinical details of the patients are given in table 1. Grouping of the patients (n = 115; male: female ratio = 63:52) on the basis of histopathological grading resulted in two groups, NAFLD (n = 77), non NAFLD (n = 38).
Demographic and clinical details of the patients.
| Non NAFLD (n=38) | Simple Steatosis | NAFLD (n=77) Moderate Steatosis | NASH | |
|---|---|---|---|---|
| Age (yrs) | 46.5 ± 16.48 | 48.94 ± 13.12 | 46.2 ± 12.22 | 49 ± 13.19 |
| RBS (mg/mL) | 128.08 ± 60 | 150.6 ± 81.13 | 129.29 ± 53.58 | 136.12 ± 40 |
| Cholesterol (mg/dL) | 184.71 ± 70.24 | 214.37 ± 72.39 | 210.6± 54.93 | 222.8 ± 59 |
| TG (mg/dL) | 135.63 ± 77.73 | 175.14 ± 96.83 | 173.66 ± 69.47 | 184 ± 82.9 |
| ALT (U/L) | 22.75 ± 14.8 | 28.05 ± 21.69 | 66.89 ± 47.79 | 52.27 ± 33.8 |
| Insulin (mlU) | 75.6± 103 | 98.9 ± 147 | 89.8 ± 172 | 108.4 ± 130 |
| Visfatin (ng /mg pro) | 210.38 ± 93.16 | 87.25 ± 75.22* | 61.36 ± 56.46** | 31.83 ± 29.43** |
| TNF a (pg/mg pro) | 81.3 ± 56.23 | 92.1 ± 41.9 | 105.7 ± 65.9 | 141.2 ± 91.2* |
| IL-6 (pg/mg pro) | 131.12 ± 96.91 | 58.39 ± 30.28 | 86.7 ± 82.1 | 152.23 ± 134.7 |
| NEFA (μmol/L) | 726.06 ± 248.75 | 838.23 ± 277.19 | 910 ± 359.3* | 929.09 ± 251.03* |
| HPE of liver biopsy | No fat accumulation | Up to 66% hepatocytes showed fat accumulation | Steatosis with ballooning degeneration | Above 66°% hepatocytes showed fat accumulation ballooning degeneration, lobular inflammation and peri cellular fibrosis |
*
P < 0.05. **P < 0.001. HPE: Histopathological examination. RBS: Random blood sugar. TG:Tri glycerides.
Histopathological examination of liver biopsies revealed varying degrees of steatosis in NAFLD group ranging from simple steatosis (457, n = 35), moderate steatosis (407, n = 30) to NASH (157, n = 12). Subjects from Non NAFLD group were devoid of any histological signs of steatosis (Table 1).
Visceral adipose tissue visfatinVisceral adipose tissue visfatin levels were high in non NAFLD group (210 ± 93.16 ng /mg protein). Significant decline in visceral visfatin level was evident in all the groups of NAFLD which was more pronounced in simple steatosis (87.25 ± 75.22 ng/mg protein) as compared to non NAFLD group. This decline maintained the same waning trend in patients having moderate steatosis (61.36 ± 56.46 ng/mg protein) and reduced drastically in those diagnosed for NASH (31.83 ± 29.43 ng/mg protein) as depicted in figure 1A. The decrease in simple steato-sis showed an inverse correlation between the level of visceral visfatin and occurrence of simple steato-sis in NAFLD (P < 0.001; r – 0.576).
Visceral adipose tissue proinflammatory cytokinesAs shown in figure 1B, TNF α levels in visceral adipose tissue was higher in all the groups of NA-FLD including patients with NASH, showing an increase of 13.27, 307 and 737 in simple steatosis, moderate steatosis and NASH patients respectively. Visceral adipose tissue IL 6 levels (Figure 1C) showed a considerable decrease in simple steatosis and an increase in NASH group of NAFLD as compared to non NAFLD group. Although there was an increasing trend in IL 6 levels in NASH, statistical significance could not be attained for such observed increase.
Circulating nonesterified fatty acids (NEFA)In comparison to non NAFLD group, circulating NEFA levels increased in NAFLD group as evidenced by an increase of 15.47, 25.27 and 27.97 in simple steatosis, moderate steatosis and NASH respectively. The elevated levels of NEFA in moderate steatosis and in NASH patients were found to be statistically significant (P < 0.02) (Figure 1D).
DiscussionThe present study was conducted to estimate visceral adipose tissue visfatin levels in NAFLD. This study demonstrated a significant decline in visceral visfatin levels i.e. 607 decline in simple steatosis 707 in moderate steatosis and 857 in NASH. This result corroborates with recent reports in which circulating visfatin was shown to be decreased in
NASH patients13 and with worsening of the clinical stage of liver disease in cirrhosis17 as well as aetherogenesity of lipid profile.18 The observed decline in visceral visfatin levels in the present study was found to be independent of BMI as measured by standard methods (data not shown). Although association of insulin resistance measured by HOMA model with NASH was demonstrated,18 our study did not find any association of visceral visfatin with IR in NASH patients. Non association of visceral visfatin was also true with circulating glucose, cholesterol and triglycerides. Alagasham and Barakat also did not find any correlation between visfatin and BMI, insulin, HOMA IR (Homeostasis Model Assessment-Insulin Resistance).19
Our study reports increased levels of adipose tissue TNF-α in all the groups of NAFLD as compared to non NAFLD group emphasizing its role in the pathogenesis. Earlier studies also reported a potential role for TNF-α in NAFLD and NASH.20,21 Since visceral adipose tissue is considered as an independent risk factor for NAFLD,12 increased visceral adipose TNF-α levels even in simple steatosis implicate its significant role in steatogenesis. While TNF-α levels increased, visceral visfatin levels decreased in all the groups of NAFLD in this study. These results corroborate with similar recent observations which showed that TNF-α down regulated the expression of adipose triglyceride lipase, adiponectin, visfatin and PPAR (Peroxisome proliferator activator receptor) gamma. 22 Decrease in IL-6 levels observed in simple steatosis in this study may indicate a hepato-protective role of IL-6 in steatotic livers.13 Contrary to this finding, IL-6 levels increased in moderate steatosis to NASH, which is in agreement with a previous study which showed increase in both IL-6 and IL-6R levels in NASH patients. 23 Despite these reports, the role of IL-6 in pathogenesis of NAFLD is not clear.
A significant increase in circulating NEFA level was observed in moderate steatosis and in NASH patients (P < 0.02) as compared to non NAFLD group in this study. Hepatic steatosis in NAFLD is attributed either to increased delivery of FFA (NEFA) to the liver from visceral adipose tissue through portal delivery or decreased FA degradation or impaired TG release from the liver. Although pro-inflammatory nature of visfatin has been demonstrated, its phosphoribosyl transferase activity in visceral adipose tissue may have an important central role in fatty acid metabolism and triacyl glycerol release from the visceral adipose tissue. Significant decrease of tissue visfatin level in NAFLD as
observed in the present study might be indicative of a potential link between the NAD generating properties of visfatin and adipose tissue fatty acid metabolism.
Limitation of our study is the small number of obese patients in both NAFLD and non NAFLD groups. In spite of the limitation, decline in visceral visfatin was evident in NAFLD group. Measurement of NEFA levels in the portal vein might throw more light on the autocrine effect of visfain on visceral adipose tissue lipid metabolism which need further study. To our knowledge, this is the first report involving visceral adipose tissue visfatin in NAFLD. In agreement with recent reports which indicate a protective role of visfatin in steatosis of liver,24 our data shows that there is strong association between increased TNF-α and decreased levels of adipose tissue visfatin in NAFLD patients.
AcknowledgementsThe authors wish to acknowledge Asian Health care Foundation for funding the project. Authors express their gratitude to Dr. B. Sadasivudu for the intellectual inputs and critical advise on the manuscript.