Introduction
Cardiac resynchronization therapy (CRT) creates simultaneous or near simultaneous biventricular stimulation aimed at correcting the lack of ventricular synchrony found in some patients with congestive heart failure and, most commonly, those with left bundle branch block. CRT is among the most important contemporary advances in the treatment of heart failure.1 Several randomized clinical trials have demonstrated benefits in survival, hospitalizations for heart failure, functional capacity and improvement in left ventricular function and architecture among patients with advanced systolic dysfunction (ejection fraction equal to or less than 35% and New York Heart Association functional class III) and QRS duration equal or greater to 120 milliseconds. The benefit is additional to that of optimal medical therapy.2-11 Two recent clinical studies have suggested similar benefits, although more modest, in patients with low ejection fraction (35% -40%) and wide QRS and less severe heart failure, class II (NYHA).12-15 Although there is still no consensus on the routine use of CRT in this population, it is very possible that in the near future these indications expand to include less symptomatic patients as a therapeutic or prophylactic measure to avoid the progression of the disease. Based on the large volume of existent clinical evidence, CRT has acquired a solid position in the treatment of heart failure. However, clinical trials have also shown that only 60% - 80% of patients receiving these devices experience clinical or electrocardiographic improvement.3,16,17
At the present time, a QRS complex with duration greater than 120 milliseconds, regardless of the underlying conduction defect, is considered sufficient electrocardiographic criteria for patients with an ejection fraction of less than 35% and symptoms consistent with class III (NYHA) heart failure to be candidates for the implantation of a resynchronization cardiac device.18-21 These criteria are based on the clinical trials mentioned above. The scientific community has explored persistently newer techniques for the assessment of ventricular dyssynchrony to optimize the selection of patients for this therapy. Taking into account the diversity among imaging modalities that are currently available and the existing resources for their process and analysis, it is expected that imaging methods would make possible the most accurate diagnosis of ventricular dyssynchrony. The reliable identification of dyssynchrony should allow us to predict whether a patient will respond positively to CRT, regardless of the QRS duration, in order to offer the procedure to those patients who are more likely to benefit and likewise prevent unnecessary implantation in patients with low likelihood of clinical improvement. Mechanical dyssynchrony has been demonstrated in patients with normal QRS duration and can be absent in up to 20% of patients with left bundle branch block, as shown recently by magnetic resonance imaging.22-27 With all its benefits, CRT is not exempt of risks. The implantation of CRT devices include the risks inherent to the implantation process such as pneumothorax, cardiac perforation, dissection of the coronary sinus, contrast induced nephropathy, bleeding and infection of the device, among others. Additionally, there is subsequent risk of paradoxical worsening of heart failure if the biventricular stimulation exacerbates the degree of dyssynchrony, causes diaphragmatic stimulation, or complicated by lead dislocation.28-31
This manuscript aims to review available techniques for the study of ventricular dyssynchrony for patient selection and the results of its application in clinical trials.
Dyssynchrony
Electromechanical ventricular dyssynchrony is the pathophysiologic abnormality targeted by CRT. In the case of interventricular dyssynchrony the focus is the temporal difference in activation between both ventricles, as compared to intraventricular dyssynchrony, which refers to the relative delay of activation among different segments of the left ventricle. Complete electrical activation occurs in less than 80 milliseconds in the normal heart, by fast conduction through the His-Purkinje system.32 Delay in the activation of a large enough segment of the left ventricle (electrical dyssynchrony) may result in an asymmetrical and inefficient systolic contraction (mechanical dyssynchrony) that eventually compromises cardiac pump function, with subsequent adverse remodeling of the left ventricle and development or progression of heart failure. This phenomenon is critical in patients with low myocardial contractile reserve as in ischemic and dilated cardiomyopathies.
The best model of ventricular dyssynchrony is due to left bundle branch block, in which the left ventricle is activated later and slower than normal following activation of the right ventricle. Once the right ventricle has been activated through the right bundle, depolarization spreads through the interventricular septum resulting in delayed activation of the posterior and lateral walls of the left ventricle.33 The sequence of depolarization determines the sequence of mechanical contraction. When the right ventricle and the septum are activated before the left ventricle, the septum is pulled away from the lateral wall of the left ventricle, which ends up contracting late, at a time when the septum is shifted to the opposite direction. At first glance, this phenomenon is responsible for the characteristic paradoxical septal motion observed in the echocardiogram of patients with left bundle branch block (apical four-chamber view). It has been demonstrated that the sequence of conduction and ventricular activation can shown individual variations among different patients with similar degree of left bundle branch block.34,35
The time elapsed between the time of maximal impulse in the septum and the lateral wall of the left ventricle is one of the most common measures of ventricular dys-synchrony and the normal value varies depending on the method used and its validation. It is understood that left intraventricular dyssynchrony is more important in the pathophysiology of heart failure than interventricular dyssynchrony. Most clinical studies have focused on the activation and mechanical function of the left ventricle during the evaluation of dyssynchrony in patients with heart failure.
Left bundle branch block is present in about 15-20% of patients with heart failure due to systolic dysfunction and confers a negative prognosis, determined by decreased survival and accelerated progression of the disease.36-40 Even in patients that lack systolic dysfunction but have increase risk of cardiovascular disease, presence of left bundle branch block represents an increased risk for heart failure and mortality.41-43 Similarly, the mechanical dys-synchrony induced by iatrogenic left bundle branch block, which is characteristic of artificial stimulation of the right ventricle in the case of traditional pacemakers, has been associated with increased risk of impaired left ventricular function in patients with or without heart failure, and favorable response to biventricular stimulation with CRT.44-48 It has been accepted that dyssynchrony associated with left bundle branch block is a factor that contributes negatively to the pathophysiology of heart failure. Excessive prolongation of the QRS duration in patients with advanced heart failure may also be a passive marker of the severity of the disease and not only an active factor in the process. Rosenmbaum, Elizari and Lazzari described the variants of bundle branch blocks.49 These include block at the level of the branches "bundle branch block"; blockade in the major subdivisions known as "segmental block"; blockade in the Purkinje network called "reticular" or "Purkinje block" and "parietal blocks" that occurs due to delayed conduction in the myocardium itself. This classification although frequently ignored, is essential to understand the variety of possible patho-physiological scenarios involved in the pattern and duration of the QRS and the complexity of the clinical problem.
The QRS duration as a measure of electrical dyssynchrony and the pattern of the left bundle branch block, suggest the presence of mechanical dyssynchrony and are often associated with it. However, the delay in conduction, as might be inferred, does not necessarily correspond consistently with the measures of mechanical dys-synchrony obtained with imaging modalities. As discussed above, some patients presenting with left bundle branch block and advanced heart failure do not show meaningful evidence of mechanical dyssynchrony by echocardiography. Likewise, evidence of ventricular dyssynchrony has been found in patients with conduction defects other than left bundle branch block or in patients with normal QRS duration. Based on these electro-mechanical inconsistencies, some researchers have reported that mechanical resynchronization and its benefits can be achieved with CRT regardless of QRS duration.50 This finding, adds to the arguments against the exclusive use of QRS duration as a measure of mechanical dyssynchrony and strict criteria in patient selection for CRT.
Since not every patient with prolonged QRS duration have mechanical dyssynchrony demonstrable with imaging techniques, it is understandable that with the current selection criteria for CRT, there will be a percentage of patients that do not benefit from the treatment. Similarly it is conceivable that some patients with "normal" QRS duration and currently considered not candidates for CRT, will exhibit mechanical dyssynchrony with advanced imaging modalities, enough to justify a CRT device and show clinical improvement. These observations have turned the study of ventricular dyssynchrony into a rapidly expanding field in medical research.
Diagnosis of dyssynchrony by echocardiography
The need to identify an accurate method for the selection of the ideal patient, the one with the highest probability to respond to CRT, has fueled the development of imaging techniques to study ventricular dyssynchrony. Most of these techniques are based in echocardiography because of its convenience, availability and relatively low cost. Clinical studies mostly from single centers, with relatively small samples, initially yielded promising results.51-64
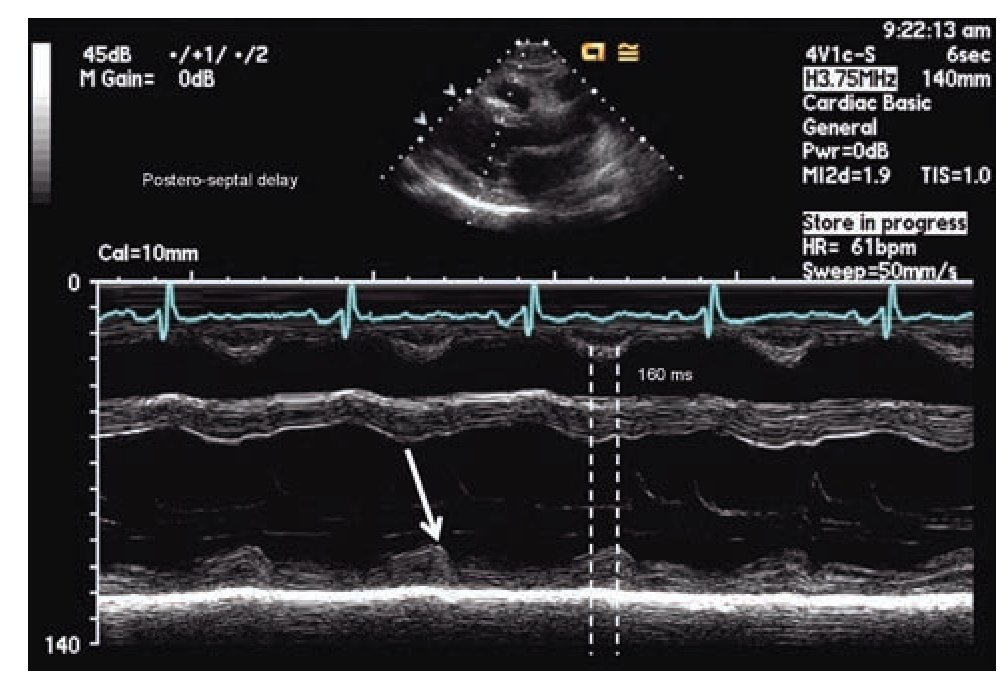
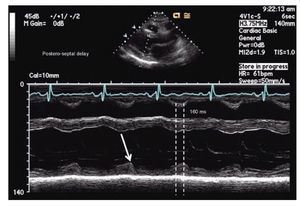
The simplest echocardiographic technique for the study of ventricular dyssynchrony is the analysis of M mode ultrasound that registers the delay between the left ventricular septum and the posterior wall. In the parasternal window, longitudinal or transverse axis, the M-mode ultrasound cursor is positioned at the level of the mid ventricle where papillary muscles may be observed and with the record speed set at 50-100 mm/s, the time delay between the point of maximum displacement of the septum and the posterior wall is measured (Figure 1). More than 130 milliseconds of difference is considered the cutoff value for the diagnosis of dyssynchrony.52,53 The addition of tissue color Doppler to M-mode echocardiography may help to more precisely identify the time of maximum displacement of the ventricular walls. Although this is a very practical technique, the variability between successive measurements and the lack of predictability in the response to CRT in more recent studies suggest that it cannot be used as a single reliable measure in the selection of patients for CRT.65,66
Figure 1. M-mode echocardiography in para septal long axis demonstrating a delay of 160 milliseconds between the point of maximum contraction in the antero-septum and the posterior wall of the left ventricle, consistent with mechanical dyssynchrony (≥ 130 milliseconds).
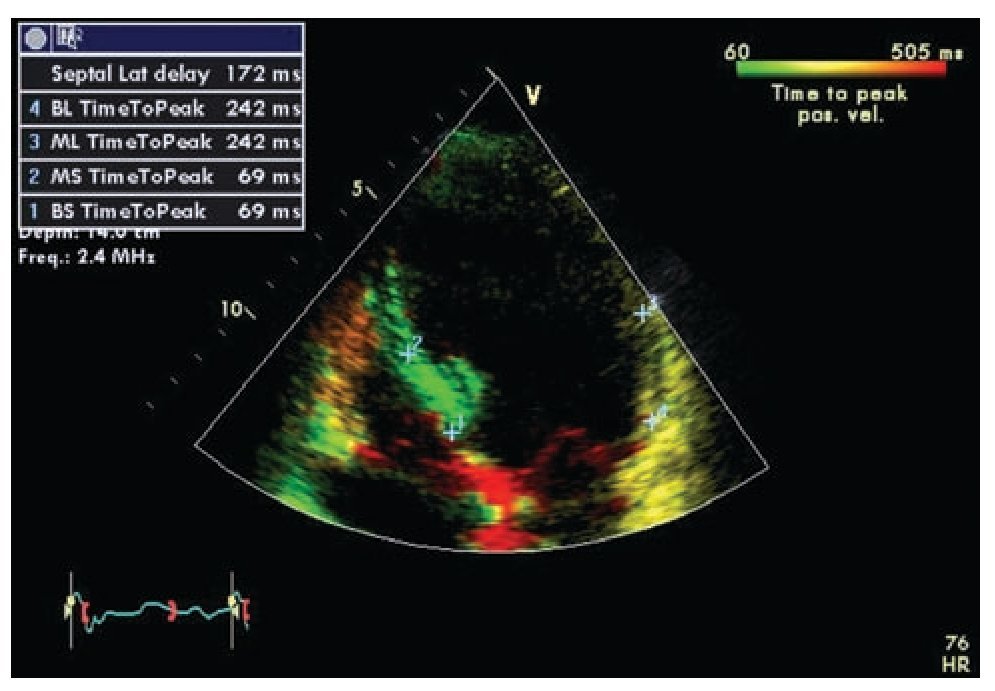
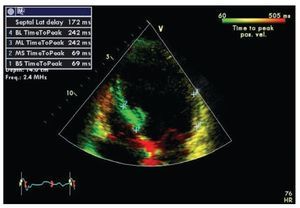
Tissue Doppler is the technique most frequently used in the study of ventricular dyssynchrony and is currently by consensus the recommended method.67 Longitudinal and radial shortening velocities can be recorded with tissue Doppler in different segments from different windows to assess the time of contraction of each segment and quantify the difference among individual segments. Time intervals are measured in reference to the electrocardiogram using the beginning of the QRS complex and the peak systolic velocity recorded in selected regions of interest on basal and mid segments of opposing walls wherever the maximal systolic velocity is found to be stable. The good quality measurements from different beats for a segment on the same plane are usually averaged.67 Several authors have evaluated the data obtained by this method using different combinations of measures including the use of the septal and lateral basal segments in the apical four-chamber view (dyssynchrony present when the delay in activation of opposite walls is more than 65 msec) or the use of a dyssynchrony index that incorporates measurements in 12 segments in all three standard planes of the apical window (basal anterior, inferior, septal and lateral in the two and four chamber and longitudinal views).54,58,61,68 The index corresponds to the standard deviation of the times of maximum velocity of the 12 segments, with a normal cutoff value 33 ms.69 A feature known as tissue synchronization imaging allows some computers display color-coded images of automated speed times superimposed on 2D images, providing a quick visual guide to identify segments of interest.70,71 (Figure 2)
Figure 2. Tissue synchronization imaging of the left ventricle from the apical four-chamber view showing color-coded tissue Doppler consistent with maximal delay at the base and mid lateral wall (yellow). The top left frame shows records of maximum systolic velocity for basal ("BL" and "BS") and mid ("ML" and "MS") segments of the lateral wall and septum. A TSI (tissue synchronization imaging) index can be calculated based on these measurements.
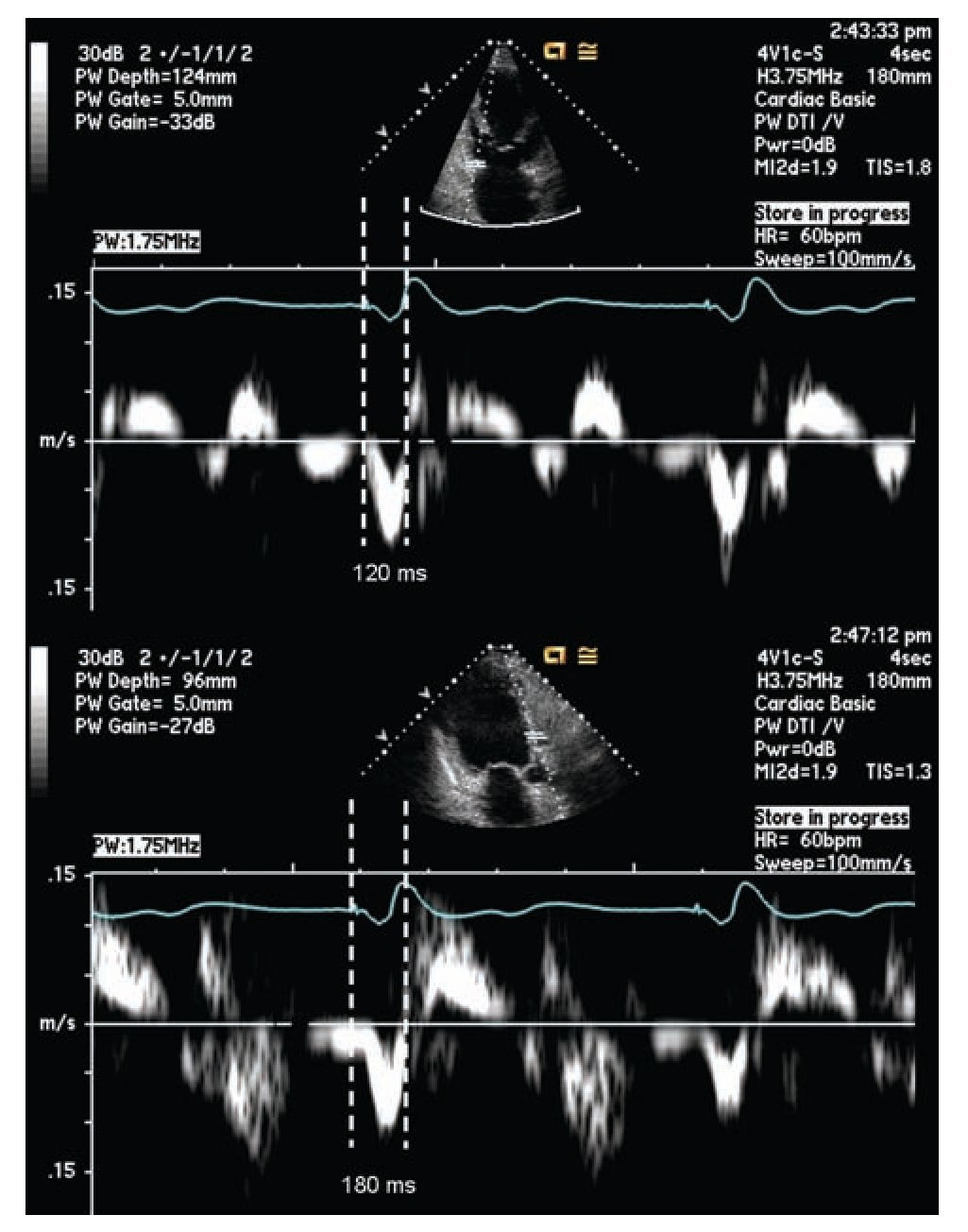
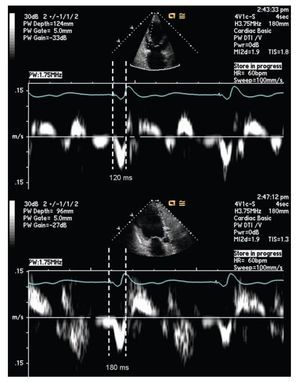
Pulsed tissue Doppler imaging without color can also be used in the evaluation of dyssynchrony. The cursor should be located in the areas where the signal is reproducible. The measurement is done from the beginning or peak of the QRS and the onset of the systolic velocity signal corresponding to the segment of interest. A difference of 60 msec or more on opposite walls is considered diagnostic for dyssynchrony.56,67,72 (Figure 3)
Figure 3. Pulsed tissue Doppler imaging demonstrating mechanical delay between the base of the left anterior and inferior walls, from the apical long axis view. The measurement is taken between the pacing artifact and the beginning of displacement of the evaluated segment determined by tissue Doppler. This measurement is dependent on technology, good signal quality is essential for a reliable measurement. The example shows a delay of 60 milliseconds in the lateral wall consistent with mechanical dyssynchrony between these segments (≥ 60 milliseconds).
Another approach has been developed around the concept of myocardial deformation. Instead of using the Doppler registered tissue velocity, the myocardial deformation is calculated as a proportionate measure of the sampled tissue shortening along the Doppler cursor line. The proposed advantage of this technique is the accuracy to register myocardial contraction independently of passive motion. Unfortunately this method has technical limitations because it is dependent on the Doppler's angle of incidence; the acquisition of images is complicated by an increased incidence of noise and low reproducibility. Initial studies suggested that deformation measures corresponded better with mechanical dyssynchrony than myocardial velocity.73 However, it showed little correlation with the echocardiographic response to CRT.58,64,74 Some of the limitations of this technique may have improved with the addition of speckle tracking. Speckle tracking is an additional resource that eliminates the influence of the Doppler's angle of incidence, improving the diagnostic value of the deformation parameters, in both short and long axis.75-78 The presence of dyssynchrony in the two modes of longitudinal and radial deformation has shown to correspond with a greater likelihood of response to CRT.79,80 Ventricular displacement can also be obtained from tissue Doppler and its association with response to CRT has been demonstrated.26,81-82 However the advantages and diagnostic superiority shown with speckle tracking,74 has popularized this technique; further perfection and inclusion in larger clinical trials would probably be necessary for its adoption as a preferred technique in the diagnosis of dyssynchrony.
Other Imaging Modalities
Three-dimensional echocardiography with various imaging processing techniques, stress echocardiography, magnetic resonance and nuclear myocardial perfusion imaging are other diagnostic modalities used in the assessment of ventricular dyssynchrony and resynchronization.
The initial application of three-dimensional echo-cardiography in the study of CRT was to evaluate the effects of biventricular pacing in volume and ventricular function.83,84 Using tissue Doppler, the systolic dyssynchrony index may be calculated which has shown good correlation with the response to CRT with a sensitivity of 88-96% and specificity of 85-88%, according to different authors using slightly different cutoff values (5.6% -10%).85-89 Although there is evidence that the measurement of the systolic dyssynchrony index with this technique is reproducible,88,90 when compared with the measurement of dyssynchrony using 2D echocardiography tissue Doppler, the correlation and agreement between them is poor.89,91 Preliminary studies suggest that identifying the more delayed segment of the left ventricle using three-dimensional echocardiography, allows determining the ideal segment for the placement of the left ventricular lead.92-94 At present, the use of three-dimensional echocardiography is limited by its availability, technical complexity, and lower temporal-spatial resolution.95
Several studies with small sample sizes (42-71 patients) and different designs have explored the value of exercise or dobutamine pharmacological stress echocardiography. Overall, they show that the presence of contractile reserve or myocardial viability is an independent predictor of clinical improvement and increased ejection fraction in patients with CRT with sensitivity between 70% and 100% and specificity between 43.8% and 88%.96-100 The correlation is kept regardless of the presence of demonstrable dyssynchrony at rest suggesting an additional prognostic value conferred by the presence of contrac-tile reserve.97 The demonstration of dyssynchrony during exercise has also shown to have superior predictive value than the assessment made at rest with 89% of positive predictive value compared to 70% in a study involving 64 patients.98 The presence of contractile reserve in the segment corresponding to the location of the left ventricular lead has shown to be of particular importance, and could be useful especially in the planning of the procedure to increase the likelihood of clinical response.99-100 The results of a prospective multicenter clinical trial designed to compare the predictive value of pharmacological stress echocardiography with low doses of dobutamine in patients with conventional indications for CRT have not been published. In this study follow-up was planned for one year with clinical response parameters and evaluation of left ventricular reverse remodeling, it will also include a comparison of dyssynchrony measurements using conventional echocardiographic methods.101
Single photon emission computed tomography (SPECT) of myocardial perfusion, as well as magnetic resonance imaging is useful in defining areas of scar and myocar-dial viability. Patients with perfusion defects or extensive areas of scar are less likely to experience clinical improvement, reduction of left ventricular dimension or increased ejection fraction in response to CRT102-109. Analysis protocols for gated perfusion "SPECT " have been developed for the assessment of ventricular dyssynchrony and validated with tissue Doppler.110,111 Subsequent studies have confirmed its predictive value for clinical response in patients receiving CRT devices.112-115 A percentage of total scar less than 15% has been associated with an increased likelihood of clinical improvement.116,117 Demonstration of mechanical dyssynchrony by different magnetic resonance techniques may also help predict the response to CRT independently of the presence of electrical dyssynchrony or scar.106,108,109,116-118 More studies and larger samples are needed to determine the role of this technology in clinical practice. Cost, technical complexity, equipment availability, time to complete the studies and its relative incompatibility with pacemakers and defibrillators, besides referral bias are some of the advantages of the echocardiographic methods over MRI.
Clinical Trials and dyssynchrony measurements
The use of echocardiographic parameters in identifying suitable patients for CRT was studied in a large multicenter clinical trial, the study of predictors of response to CRT (PROSPECT).66 The study involved 53 centers in the United States, Europe and China. A total of 498 patients with standard indications for CRT were studied with twelve echocardiographic parameters and were followed for six months to evaluate clinical response and ventricular endsystolic volume. The ability of echocardiographic parameters to predict clinical, anatomical or both responses was the study objective. The authors found wide variability in the analysis of the echocardiographic parameters regardless of the training provided to operators at each center. The parameters studied in PROSPECT included two M-mode echocardiography measurements, one combined with pulsed Doppler; three parameters based on pulsed Doppler and seven using tissue Doppler including confirmation by longitudinal deformation rate in one of them. Clinical improvement, determined with a standard instrument, occurred in 69% of patients and reduction of end-systolic volume of over 15% was registered in 56% of the patients in whom this data could be analyzed. The sensitivity and specificity from the 12 echocardiographic parameters evaluated to predict response to CRT significantly varied between 6-77% and 31-93% respectively. The authors concluded that none of the parameters studied could be recommended to improve patient selection for CRT. A subsequent study analyzed the factors associated with clinical improvement and decrease left ventricular end systolic volume in the patient population of PROSPECT. Patients with higher percentage of volumetric improvement (reduction greater than or equal to 30%) were female with nonischemic cardiomyopathy, wider QRS complex and with more evidence of dyssynchrony. Advanced Class IV heart failure in the NYHA classification and history of ventricular tachycardia were associated with a negative response characterized by increased left ventricular systolic volume.119
In another recent clinical trial including 239 patients undergoing CRT, the predictive value of the time to peak systolic velocity and peak systolic deformation using tissue Doppler in the four basal segments, was evaluated. Median follow up was 20 months and mortality was 33%. The maximum delay in reaching the peak systolic velocity was found to be an independent predictor value of lower cardiac mortality after CRT with a relative risk of 0.46.120 PROMISE-CRT was a prospective multicenter study of nine centers in Minnesota (United States) in which 71 patients were studied with tissue Doppler and speckle tracking prior to CRT. Improvement in mechanical dyssynchrony was not associated with clinical response but changes in radial dyssynchrony determined with speckle tracking were associated with reduction in ventricular volume, suggesting reverse remodeling.121
The RethinQ study the role of CRT in patients with narrow QRS (defined in the study as less than 130 milliseconds) and evidence of mechanical dyssynchrony. The technique included M-mode echocardiography and delay in opposite walls determined with pulsed tissue Doppler. The results were disappointing. The improvement in peak oxygen consumption during cardiopulmonary exercise testing, mortality, quality of life and 6 min walk were evaluated showing no difference between patients receiving CRT and controls. While the idea of resynchronizing a heart that shows no electrical dyssynchrony could be considered unreasonable, measurements of mechanical dyssynchrony and its previous validation, even among patients with heart failure and narrow QRS has maintained interest on this population.122
Discussion
While there is continuous search for novel techniques 0f assessing ventricular dyssynchrony, the complexity of the patho-physiological process of heart failure, have made the practical and clinical application of these techniques a big puzzle to be solved. Judging the clinical response to CRT has been nothing less than controversial. While it is true that a considerable proportion of patients do not show significant clinical improvement to CRT, they still might be receiving a less obvious benefit. The definition of favorable clinical response is particularly difficult to assess in heart failure. Clinical evaluation systems that take into account the patient's responses to standarized quality of life questionnaires, 6 minute walk, NYHA functional class, recurrent hospital admissions, ejection fraction, measures of left ventricular geometry and survival are some of the frequently used criteria to assess response to treatment in heart failure.122 Slowing or stopping the progression of heart failure, measurements of systemic inflammation, neurohumoral activity and changes at the genetic and histological levels among others, are also potential benefits that have been suggested as a result from CRT but more difficult to quantify with precision and reproducibility in a large-scale clinical trial.123-126 These more subtle effects may lead to a decrease or delay in long term morbidity and mortality without the patient necessarily experiencing subjective clinical improvement in the short term. So far there is no clear consensus on the best way to measure the patient's response to a therapeutic modality and what parameters have definitive prognostic value. Patients who are considered treatment failures by the criteria of some clinical studies could still received benefits from the treatment that goes unnoticed due to the design of the study.
Multiple comorbidities and the dynamic nature of its progression, often present in patients with advanced systolic failure may confuse the interpretation of the clinical response to CRT. These include cardiac arrhythmias, anemia, lung disease, pulmonary hypertension, valvular heart disease, right ventricular failure and renal insufficiency, among others. Many of these conditions have been identified as factors that reduce the clinical response to CRT.127-134 At the present time we do not have adequate tools to accurately predict the progression of the disease in a particular patient. The point in the progression of the disease at which the resynchronization device is implanted and the rate of the disease progression can be decisive in the patient's clinical "response".
Technical difficulties implanting the coronary sinus lead is a major limiting factor in the clinical evaluation of CRT devices. The anatomy of the coronary sinus is often a determinant factor in the position of the lead. Although the operator could accurately identify the ideal segment to stimulate, the anatomy of the coronary sinus may not necessarily provide an appropriate branch for a stable lead in that area. Furthermore, there is risk of phrenic nerve stimulation, which runs in close proximity to the lateral free wall of the left ventricle, where quite often a suitable ideal branch for the lead placement is found.135-138
Right bundle branch block has been recognized as another negative prognostic factor for response to CRT,139-142 however at least two retrospective studies with limited number of patients found that patients with right bundle branch block and left anterior fascicular block are more likely to receive a benefit from these devices.143,144 Just like not all patterns of left bundle branch block reflect the same conduction system defect,49 right bundle branch block may also be present in hearts with different degrees of dyssynchrony; in some cases enough to benefit from resynchronization. While the widening of the QRS complex is usually the result of a conduction defect, in an individual patient it may correspond to myocardial disease per se, and thus the assessment of myocardial reserve and viability appear important in predicting the response to CRT.
All the techniques discussed in this manuscript have shown in a variety of studies their value and ability to predict clinical response and prognosis in patients who are considered candidates for CRT. We have intentionally not included in this work AV or interventricular optimization, a subject which in itself is a vast field of controversial research and clinical practice that could be considered another factor in CRT response.145-153 The task would be to create a combination of techniques and parameters with the best reproducibility, lowest cost and highest prognostic value. An ideal method should identify and characterize the presence and quality of electrical and mechanical dyssynchrony and their correspondence; at the same time be able to identify the presence, extent and distribution of myocardial viability and scar tissue, yielding the highest positive predictive value for the reverse remodeling process, clinical response and survival in a prospective multicenter clinical trial including a large patient population. Additional benefits of this method would include its ability to identify the ideal position for the electrode catheter, and the availability of target veins.
Conclusions
While it is true that a proportion of patients receiving CRT devices do not appear to respond clinically to this mode of treatment and even a smaller proportion could worsen, the clinical results obtained applying the current selection criteria are impressive considering the relatively crude technique of implantation and the overwhelming volume of limitations and adverse clinical situations. Despite tremendous progress in the technology available for the assessment and diagnosis of ventricular dyssynchrony, the ideal method has not been identified. At present, the duration of the QRS complex in the surface ECG remains the most reliable criteria for selecting patients who may respond to CRT. Inclusion or exclusion criteria based on images of dyssynchrony will have to go through the slow and costly process of prospective multicenter clinical trials before being widely accepted and recommended as clinically useful. Meanwhile, the use of these technologies in clinical practice should be framed in the process of gathering information in the form of databases or clinical trials in order to generate knowledge or as an additional tool for clinical judgment in particularly difficult cases looking for very specific answers; otherwise its routine use at present would be no more than an exercise of good will with good intentions and uncertain results.
Corresponding author: Mario D. Gonzalez.
Professor of Medicine. Director, Clinical Electrophysiology 500 University Drive, P.O. Box 850. Hershey,
PA 17033-0850.FAX: (717) 531-4077.
E-mail: mgonzalez@hmc.psu.edu
Received on August 30, 2010;
accepted on October 6, 2010.