Introduction
Supraventricular tachycardia (SVT) is frequently manifested in emergency pediatric care units and is difficult to solve because the hemodynamic state of the patient may be involved.
The frequency of this disorder ranges from 1 in 250 to 1000 children.1 Forty percent of children who has SVT suffer from factors that predispose them to this condition, such as congenital heart disease, Wolff-Parkinson-White (WPW) syndrome, infections, fever or adverse reactions due to exposure to various different drugs. SVT is characterized by episodes of rapid cardiac rhythm caused by abnormal electrical circuits in the heart. The electrocardiogram (EKG) shows short PR interval, delta wave and complex prolongation of QRS. Propafenone is beta-blocking drug recommended for the treatment of SVT. It acts as a potent sodium channel blocker and is a weak b-adrenergic and calcium antagonist. Its main electro-physiological effect is to slow down conduction in tissues of quick response. Due its main electrophysiological effects, propafenone has a wide spectrum of activity and efficacy for the treatment against SVT including those of the WPW syndrome and recurrent atrial fibrillation. It has also been used safely in the treatment o various tachyarrhythmias among children.2 The efficacy and safety of propafenone for the treatment of infantile arrhythmia has been reported, with an average dose of 300mg/m2/day (ranging from 250-400 ng/mL) every 8h.3 Children with SVT were successfully treated with this drug without adverse or proarrhythmic effects. Due to its pharmacokinetic characteristics, propafenone has variable bioavailability when taken orally.4 The therapeutic plasmatic concentrations are between 0.2 and 1.5µg/ mL, following a dose of 150-200 mg/m2/day, with a dose increase of up to 600 mg/m2/day.5 Its elimination is of 11 mL/min/kg. Though uncommon, especially in cases of very high doses, adverse effects include lack of appetite, bloating sensation, feeling of nausea, bitter taste, and in some cases blurred vision and dizziness.6
Clinical case
The case of a 2-year-old boy, born and resident in Xochimilco, Mexico City, who had been given cardiological health care at the National Pediatric Institute from May 2005 (since the age of four months) is described. The boy was diagnosed with secondary SVT of the WPW syndrome, and treated with a magistral suspension of propafenone, starting May 2005. The drug was prepared according to an approved formula tested previously in laboratory studies.7 The mother is an apparently healthy 29-year-old housewife; the father, aged 31 years, has a degree in hotel administration, is apparently healthy and smokes 3/24, whilst denying alcoholism or drug addiction. The parents mentioned genetic inheritance of arterial hypertension on the paternal side and the patient has an apparently healthy 4- year-old brother.
This child is the outcome of a second pregnancy, which evolved normally with prenatal control from the first term of gestation, and the mother reported consuming iron and folic acid. It is reported that during the 37th week of gestation, an abdominal ultrasound was carried out which indicated extreme fetal suffering, due to nuchal cord, which required an urgent cesarean section to be carried out on February 16, 2005, producing a single outcome, with an Apgar score of 7/9, at 37 weeks of gestation, weight of 2.500 kg, size 49 cm, without any complications at birth, the child and mother left the hospital 72 hours later. He was breast fed until the end of the first year, taking solids at 6 months, and is currently incorporated into the family diet, which includes; vegetable consumption 3-4/7, fruit 7/7, red meat 4/7 and green vegetables 3/7, egg 1/7, milk and derivatives 3/7, pulses 1/7. He does not eat lentils or beans, only in soup. Psycho-motor development was as follows: social smile at four months, sitting up at 6/2, crawling 8/2, with walking now in process. His vaccines are complete.
His current illness began on May 11, 2005, suffering from tachycardia, sweating and indications of poor perfusion, suffering from sweating with a cardiac frequency of 310 per minute and general signs of poor perfusion; together resulting in a diagnosis of SVT.
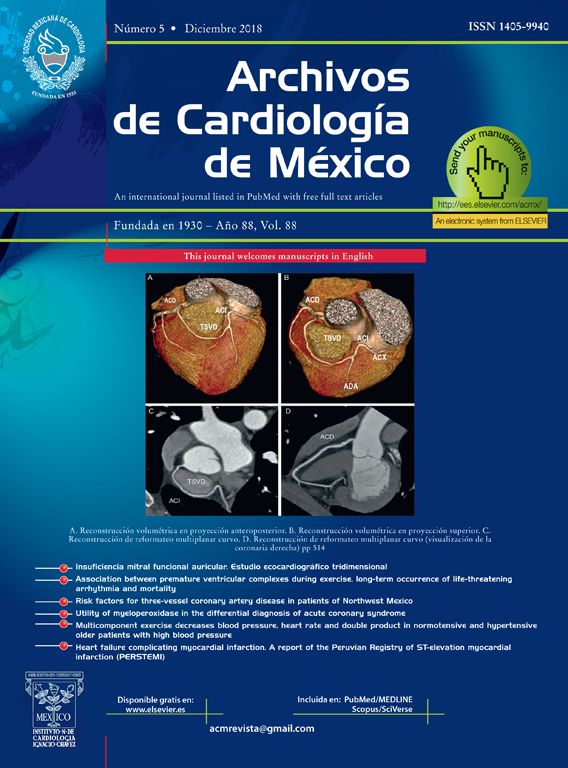
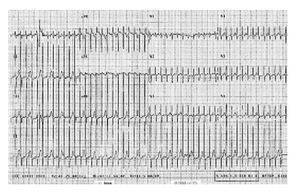
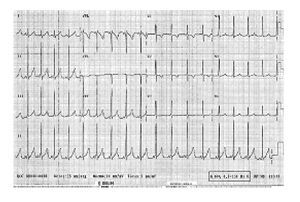
After non effective vagal stimulation maneuvers, propafenone was administered intravenously, however, due to unsuccessful therapeutic actions, patient underwent one attempt of transthoracic electrical cardioversion to restore normal sinus rhythm (Figure 1).
Figure 1 Electrocardiogram with supraventricular tachycardia taken before the propafenone treatment
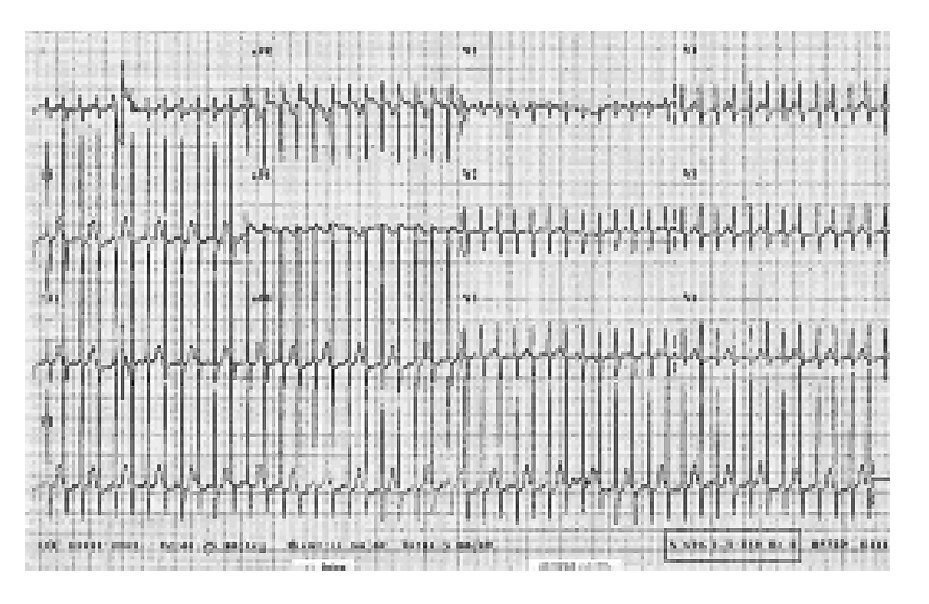
The child was placed in the Intensive Therapy Unit to monitor and stabilize hemodynamic activity and was discharged on May 15, 2005. From the time he was placed in hospital, he received treatment with a magistral suspension of propafenone as previously was mentioned, he received at the beginning 3 mg/8h for 4 months, followed by 6 mg/8h, and finally 11 mg/8h. He has been without symptoms for the last year. Figure 1 shows an EKG with SVT that was taken before propafenone treatment. Figure 2 shows the current EKG with SVT of the patient after one year of treatment with propafenone suspension.
Figure 2 Current electrocardiogram of the patient with supraventricular tachycardia after one year of treatment with propafenone suspension
Discussion
Research undertaken in the pharmacological laboratory of the National Pediatric Institute includes the development and evaluation of drugs at adequate doses for pediatric use, which are currently not available in our country. On this occasion, a magistral suspension was prepared using commercial tablets. The suspension was prepared by grinding 1 tablet of 150 mg of hydrochloride propafenone (Norfenon, Abbott Lab, Mexico) in a mortar. The finely ground tablets were then powdered and forced to pass through a mesh (size 100) in order to homogenize the particle size. Pomegranate syrup (La Madrileña, Mexico), which was used as diluent, was added to obtain a final concentration of 1.5 mg/mL of propafenone.
The obtained suspension was subjected to stability tests over a 90 day period, stored at room temperature (15 ± 5 °C) and refrigeration (3-5 °C), and turned out to be stable; neither physicochemical changes nor microbiological growth were observed. High performance liquid chromatography was used to validate dosage and to determinate the plasmatic levels of the drug.8
Previous to this study we carried out an analysis of the bioavailability of the suspension utilized for the present study compared with commercial tablets.7 The results of that study showed acceptable value of bioavailability as previously reported.9 Results demonstrate the reliability to administrate the drug. Plasma levels observed in children with SVT reached concentrations after they took the suspension. There are not reports in children that mention therapeutics range of propafenone, in this study we found that plasma concentration of drug was correlated with the clinical improvement of patients. The EKG done simultaneously during monitoring of propafenone showed an improvement respect initial exam reported during the diagnostic process.
To monitor propafenone levels in the patient, concentration of drug was measured at 3.5 h post administration, obtaining 11.7 ng/mL (0.0117 µg/mL) of propafenone in plasma. This result coincided with the dose administered; however, it differed from results reported in the literature, even though the clinical response of the patient was favorable with a dose of 2.5 mg/kg/day. The patient´s health improved under the constant medical care by the personnel of the Cardiological Health Care Unit, and monitoring through EKG, reveling a favorable evolution of the patient.
Limitations of case
During clinical surveillance, the patient was maintained in control of the disease that was enough for physician to decide not to ask for therapeutic monitoring of propafenone levels, but if this would be indicated, a strong relationship between levels and therapeutic response.
We concluded that chronic use of propafenone is indicated for patients with SVT secondary to WPW syndrome.
As a temporal solution the use of magistral formulations in pediatric population has to be evaluated in terms of safety and efficacy but in terms of relationship between risk-benefit during prolonged use of propafenone, overall when such relationship will be compared with a spontaneous favorable evolution in the group of patients younger than one year of age diagnosed of WPW syndrome.
This study was supported partially by Abbott Laboratories, specifically for its presentation in the 35th Annual Meeting of American College of Clinical Pharmacology, held in Cambridge (MA), 2006 September 16-19.
There is not conflict of interest.
*Corresponding author: Carmen Flores Pérez.
Avenida Imán No.1 3er piso, Colonia Cuicuilco, 04530 México, D.F. México.
Telephone & Fax: (55) 5255-1084-3883.
Correo electrónico: cfp3575@yahool.com.mx
Received: July 4, 2007;
accepted: December 15, 2008.