Case history. A 62-year-old woman was evaluated at the outpatient clinic for shortness of breath and mild substernal heaviness, which increased in severity with moderate exertion. The patient had a history of hypertension and coronary artery disease (CAD), and had suffered an acute inferior myocardial infarction six years earlier, for which she underwent bare-metal stenting in the distal right coronary artery (RCA). Her physical examination was normal and the ECG revealed normal sinus rhythm at 75 beats per minute, with Q waves and T-wave inversion in leads II, III and aVF. Suspicion for in-stent restenosis was raised and she was scheduled for a coronary computed tomography angiogram (coronary CT angiogram) after consultation with a cardiologist.
The patient underwent a cardiac CT with a dual-source CT system (DSCT: two X-ray sources and 64 detectors with temporal resolution of 83 ms; SOMATOM Definition, Siemens Medical Solutions, Forchheim, Germany) in dual-energy mode for performing coronary CT angiogram , and myocardial perfusion evaluation, as recently described.1-2 From the dual-energy scan, 3 different image reconstructions were obtained using the automated dual-energy image reconstruction software of Syngo MultiModality Workplace (Siemens) and compared to previous reports.1 The DSCT system delivers the lowest possible radiation dose in cardiac CT because DSCT images the heart two times faster; furthermore, adaptative ECG-pulsing delivers the necessary radiation amount for cardiac imaging in less than half the time required by the most dose-efficient single source CT scanners. The protocol described is completed under 10 mSv, which is lower than the dose of a single day SPECT scan.2 No pharmacological heart rate control was used.
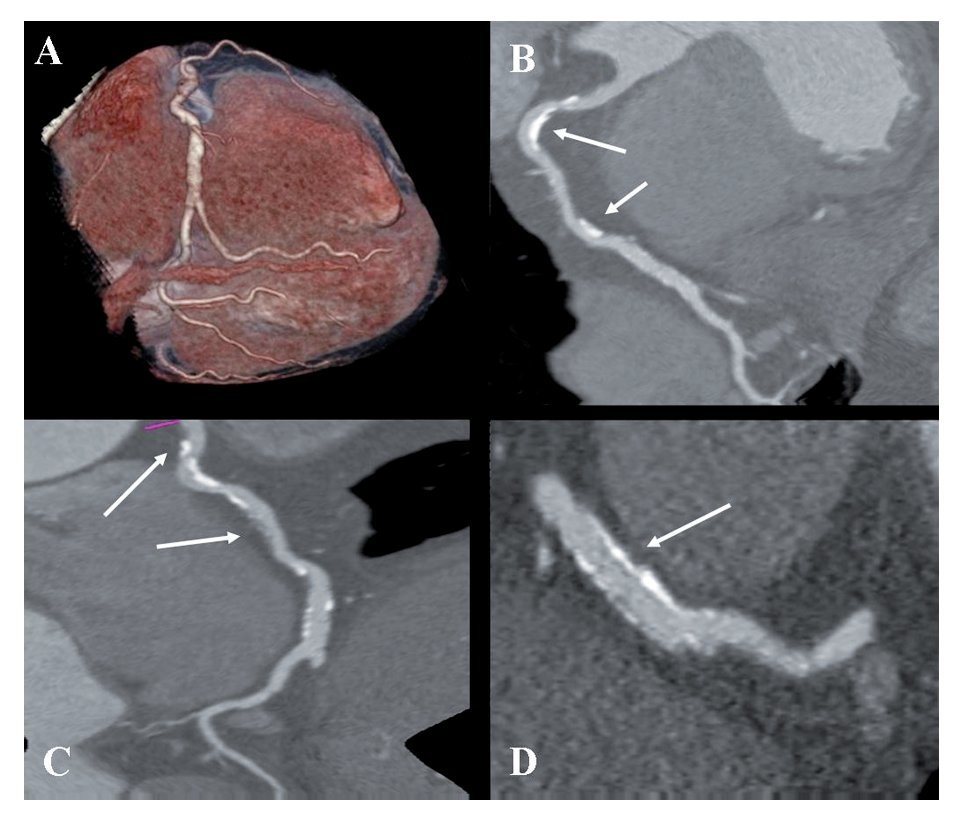
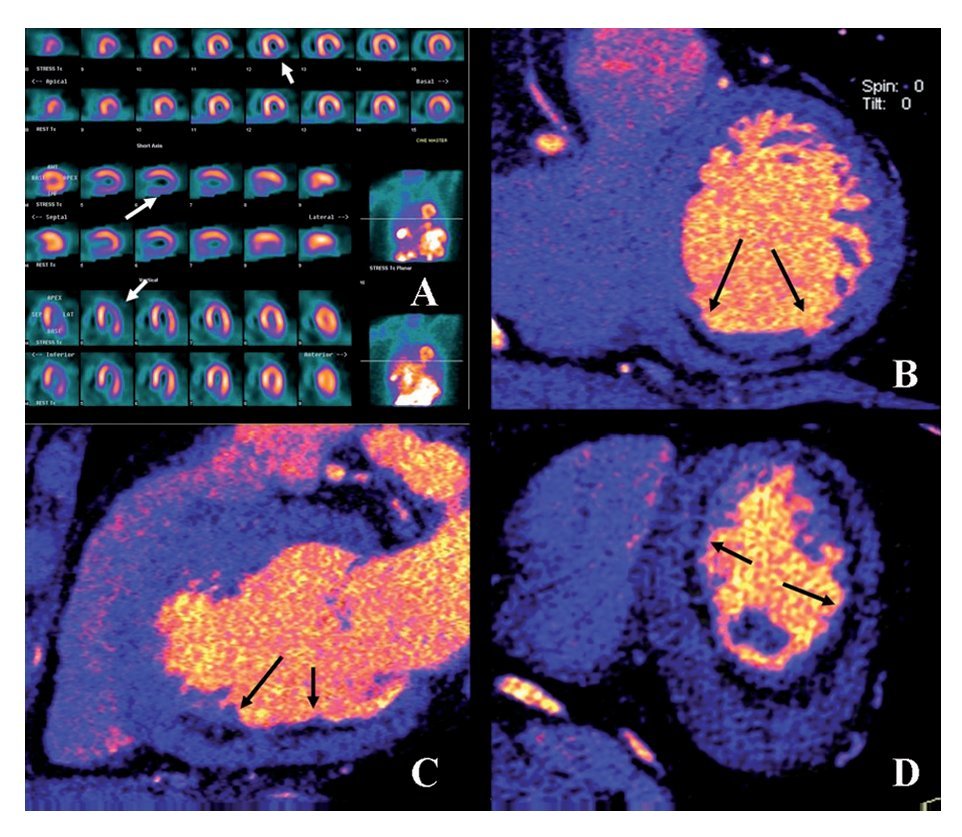
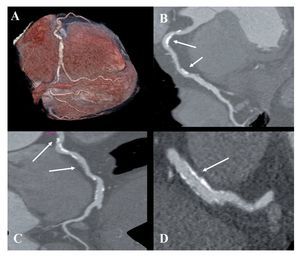
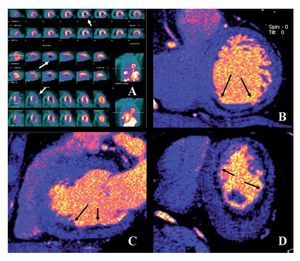
Figure 1 coronary CT angiogram images revealed right dominant circulation with normal left main coronary artery and non-significant stenosis in the left anterior descending and circumflex arteries territory. Analysis of the RCA showed two high-density calcified plaques with an intermediate stenosis in the middle segment of the RCA (Figures 1B and 1C). Multiplanar images with a special post-processing B46f reconstruction filter showed a patent stent with a very mild neointimal hyperplasia (thin dark line inside the stent lumen) (Figure 1D). Figure 2 coronary CT angiogram images demonstrated a subendocardial perfusion defect in the right coronary territory, within the inferior and inferolateral wall of the left ventricle (area that appears as a black thick line at the subendocardial region) (Figures 2B, 2C and 2D).
Figure 1.Retrospective ECG-gated dual-energy CT scan reconstruction; merging 70% of the 140-kV spectrum and 30% of the 100-kV spectrum shown as volume rendering of the right coronary artery (RCA) (A); curved multiplanar reconstruction revealing the presence of two-highly calcified plaques in the RCA (arrows in B and C), and a longitudinal multiplanar image at the stent level in the RCA, processed with a s edge-enhancing kernel (B46f) to optimize the visualization of the stent lumen which demonstrated a mild neointimal hyperplasia (arrows in D).
Figure 2.Exercise stress TC-99m sestamibi SPECT perfusion images demonstrated an inferior and inferolateral transmural infarct with mild ischemia extending from base to apex (Arrows in A). Retrospective ECG-gated dual-scan CT multiplanar reformations in short-axis (B), vertical-axis (C) and horizontal-axis (D) views of dual-energy CT-based images showing a subendocardial perfusion defect (arrows) in the inferior wall of the left ventricle.
Due to clinical history and the findings of coronary CT angiogram (RCA with two intermediate stenoses), the patient was scheduled for SPECT myocardial perfusion imaging (SPECT MPI). Exercise-stress Tc-99m sestamibi SPECT MPI demonstrated an inferior and inferolateral transmural infarct extended from base to apex with mild ischemia (Figure 2A). Gated SPECT images showed inferior and inferolateral akinesia with normal left ventricular ejection fraction. The patient was managed medically without symptoms during follow-up evaluations.
Discussion
Cardiac imaging has become the cornerstone in the workup of patients with suspected or known CAD. The integration of multimodality imaging represents a natural extension of current imaging paradigms for diagnosing CAD, assessing risk and guiding therapeutic decision-making. The latest generation of multidetector CT scanners and the software configuration of 64-section CT scanners provide an appealing alternative for noninvasive luminal assessment in patients with chest pain.3,4 However, a major limitation of MSCT is that only anatomic information is obtained, whereas no information on the hemodynamic significance of CAD is evaluated.5,6 As a result, a wide discrepancy may be present between anatomic and functional testing results.
Recently, it has been published that single dual-energy CT provides morphological information on coronary artery luminal integrity and, using the different imaging spectra contained within the same scan, allows the reproducible differentiation of iodine distribution within the myocardium to delineate myocardial perfusion defects, in good correlation with standard techniques.1 In our case, DS-MSCT demonstrated a very high diagnostic performance to exclude in-stent restenosis when using a dual-energy protocol. Furthermore, due to the higher spatial resolution of DS-MSCT, compared to SPECT, dual-energy CT images clearly demonstrated subendocardial distribution of the myocardial perfusion defect, in contrast to the transmural defect seen within the SPECT images. As a result, the integration of a dual-energy CT protocol imaging to evaluate myocardial blood pool during the assessment of coronary anatomy in this patient, may redefine the diagnostic power of DS-MSCT.
However, myocardial blood deficits demonstrated with DS-MSCT cannot differentiate between reversible ischemia and scar with current imaging protocols, because coronary CT angiogram is acquired only at rest; this is true for any relative or absolute evaluation of myocardial perfusion reserve (MPR). Recently, it has been described an experimental protocol using DS-MSCT to assess both stress and rest myocardial perfusion, in order to identify areas of infarcted or ischemic myocardium.7 However, disadvantages of CT perfusion imaging protocols include: a) radiation exposure, b) administration of a large volume of iodinated contrast, c) poor contrast resolution, d) artifacts that can result in false positive images, e) unable to quantify myocardial blood flow, and f) they are highly dependent on appropriate bolus timing. Therefore, to further define the sequelae of coronary atherosclerosis, functional testing for assessment of MPR is ideally performed by nuclear perfusion imaging. Advantages of cardiac SPECT imaging over other cardiovascular imaging modalities (cardiovascular magnetic resonance, contrast echocardiography and computed tomography) include an extensive literature supporting efficacy and prognostic value, standardized protocols for performing studies, published user guidelines and appropriated criteria, as well as a proven cost effectiveness for diagnosis, management and risk assessment.8
Thus, rather than being competitive, MSCT and SPECT imaging should be considered to be complementary for both diagnostic and a prognostic perspective,9 as it has been shown in this case. The use of the latest generation of multidetector CT scanners and developing novel imaging protocols, combined SPECT and MSCT cardiac imaging, will play a prominent role to detect, quantify, and characterize both clinical and subclinical atherosclerosis, with potential reduction of radiation burden.
Corresponding author:
Enrique Vallejo.
Nuclear Cardiology and Cardiac CT, Hospital Ángeles del Pedregal. Camino a Santa Teresa 1055-C No. 645, Col. Héroes de Padierna, C.P. 10700, México, D.F., México.
Phone and Fax number: 5255 5135 2984.
E mail: vallejo. enrique@gmail.com
Received: March 20, 2009;
accepted: August 20, 2009