Since 2012, in the autonomous region of Andalusia, Spain, the “Review of Patients with Potential Prescription Problems” program has been developed annually. Among the patients targeted by this program, there are type 2 diabetic patients, whose treatment complexity favours the appearance of Potential Safety Problems (PSP).
This retrospective study conducted from June to September 2019 was carried out by an integrated care pharmacy service, with an assigned population of 406,701 patients. The objective of this study was to evaluate the magnitude effect of a multidisciplinary intervention on Non-insulin Antidiabetics (NIA) PSP.
NIA-related PSP were included, with three types of PSP being selected for review: prescriptions that exceed the summary of product characteristics recommended doses, prescriptions combining more than three NIA and monotherapy prescriptions with acarbose, miglitol or guar gum.
The study was conducted in three phases:
Phase I: A cross-sectional analysis was performed which detected patients with PSP in April 2019, pharmacists then recommended a dose modification or drug deprescription for each PSP. This information was made available to physicians through the web application “Portal de Farmacia”, which allows communication between clinicians and pharmacists and serves as a database.
Phase II: Intervention period by primary care physicians.
Phase III: Retrospective analysis of prescription modifications.
PSP were classified according to the intervention carried out by physicians as:
- -
Modified: PSP was resolved by deprescription or dose reduction.
- -
Justified: the prescription was not modified, and this action was justified by physicians through “Portal de Farmacia”.
- -
Not revised: the prescription was not modified or justified.
Physician's acceptance of pharmacist recommendations was measured according to whether the prescription was modified thus resolving the PSP.
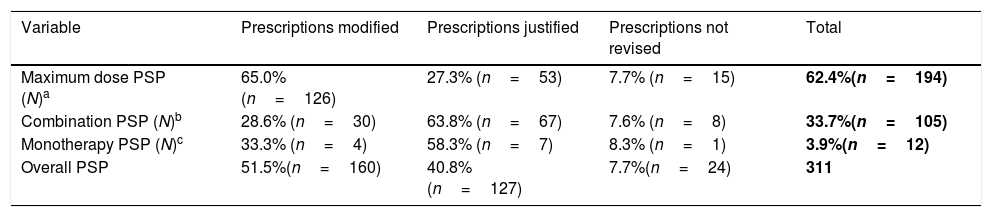
The analysis carried out identified 24,438 patients on treatment with at least one NIA, in this population 311 PSP were detected in 1.2% (n=296) of the patients, the median age was 65 (30–97) years and 45.6% (n=135) of patients were polymedicated. Table 1 shows the measures carried out by physicians after PSP review.
Measures carried out by primary care physicians after PSP review.
| Variable | Prescriptions modified | Prescriptions justified | Prescriptions not revised | Total |
|---|---|---|---|---|
| Maximum dose PSP (N)a | 65.0% (n=126) | 27.3% (n=53) | 7.7% (n=15) | 62.4%(n=194) |
| Combination PSP (N)b | 28.6% (n=30) | 63.8% (n=67) | 7.6% (n=8) | 33.7%(n=105) |
| Monotherapy PSP (N)c | 33.3% (n=4) | 58.3% (n=7) | 8.3% (n=1) | 3.9%(n=12) |
| Overall PSP | 51.5%(n=160) | 40.8%(n=127) | 7.7%(n=24) | 311 |
| Variable | Prescriptions modified | Prescriptions not modifiedd | Pearson χ2P value |
|---|---|---|---|
| Suggestion of dose modification | 126 | 68 | <0.0001 |
| Suggestion of drug discontinuation | 34 | 83 |
Overdosing was the most frequent PSP, with high rates of prescription modifications being observed for biguanides (86.1%, n=31), sulfonylureas (74.5%, n=35) and iDDP-4 (71.0%, n=44), however, a low modification rate was observed for aGLP-1 (28.6%, n=12).
Inadequate combination therapy PSP were caused by the combination of four NIA in 93.8% (n=98) of cases and five NIA in the remaining 6.3% (n=7). Commonest pharmacological groups prescribed were sulfonylureas, metformin, sodium-glucose cotransporter-2 inhibitors and iDDP-4, this four-drug combination was also the most common, accounting for 56.3% (n=59) of cases.
Regarding the demographic characteristics of the population studied, the median age was over 60 years and about half of the patients were classified as polymedicated; both had been described as risk factors for hypoglycaemia,1 therefore, interventions aimed at reducing the number of NIA prescribed could contribute to a decrease in polypharmacy, drug interactions and adverse events.
An alternative to NIA in patients who do not achieve adequate glycaemic control is early insulinization therapy, which can allow long-term control and an improvement in life-quality.2 However, patients frequently avoid insulinization due to the discomfort of subcutaneous administration and the perception of an increased risk of hypoglycaemia.3
In conclusion, the number of PSP identified was low (1.2%), indicating a very adequate prescription of NIA. The acceptance rate of pharmacist recommendations was moderate, PSP detected were reduced by half after two months of interventions, with similar studies describing comparable results.4,5 Interestingly, suggestions of dose modifications to resolve a PSP were more frequently accepted by physicians than drug discontinuation suggestions.
Funding sourceThe present investigation has not received specific aid from agencies from the public sector, commercial sector or non-profit entities.
Conflict of interestsNo potential conflict of interest was reported by the authors.