(r = 0,72) y a las 2 h de la sobrecarga oral de glucosa (r = 0,43). El 30% de los pacientes con glucemia basal entre 110 y 125 mg/dl presentaó valores de A 1c superiores a los límites de referencia. Una técnica combinada de diagnóstico basada en una glucemia basal > 125 mg/dl o de 110-125 mg/dl con una A 1c >= 3 DE (5,94%) demostró una sensibilidad del 92% y una especificidad del 95%. Conclusiones. En sujetos con una determinación de glucemia basal no concluyente (110-125 mg/dl), los valores de A 1c por encima de la media +3 DE (> 5,94%) son útiles para orientar el diagnóstico de diabetes e identificar a los que requieren tratamiento.
Introduction
The diagnostic criteria for type 2 diabetes mellitus (DM2) as a clinical entity are based on a number of population-based studies that documented a bimodal distribution of plasma glucose concentrations (a continuous laboratory variable), with a higher prevalence of complications typical of DM in the population subgroup with higher values.1 Statistical procedures were used to artificially dichotomize plasma glucose levels, and a cutoff value was thus set for the diagnosis of DM2. The diagnostic criteria for diabetes were developed by the National Diabetes Data Group (NDDG)2 and the World Health Organization (WHO).3 Later, the American Diabetes Association (ADA),4 in response to the inconvenience, variability and nonphysiological nature of the oral glucose tolerance test (OGTT), recommended abandoning its routine use in favor of lowering the diagnostic cutoff value for fasting plasma glucose (FPG) to >=126 mg/dL, which seemed to better predict the risk of developing macrovascular complications.5,6 In 1999 the WHO included this recommendation in its new diagnostic criteria.7
After the discovery that one fraction of hemoglobin in red blood cells (glycosylated hemoglobin, or HbA1c) is elevated in the presence of hyperglycemia lasting for
4-8 weeks, the A1c fraction became the main tool for following metabolic control in persons with diabetes. The importance of this parameter has been reinforced by studies such as the DCCT8 and the UKPDS,9 which showed that better control of plasma glucose levels reduced the risk of developing long-term complications.
If diabetes is diagnosed on the basis of plasma glucose values, and if HbA1c accurately reflects plasma glucose levels during the preceding weeks, why not use HbA1c to diagnose DM2? This option has its attractions, because it would obviate the inconvenience and poor reproducibility of the OGTT; moreover, HbA1c correlates well with the likelihood of developing chronic complications. Since this idea was first proposed, different studies have yielded dissimilar results.6,10 Important drawbacks are the lack of standardization of different methods used to quantify HbA1c, the high cost of this test (placing it beyond the reach of developing countries) and the lack of studies that document the clinical validity of a given diagnostic cutoff point. (It has traditionally been claimed that the diagnostic sensitivity of HbA1c is poor.11-15)
A group of 18 metaanalyses16 concluded that although HbA1c cannot be used alone to diagnose diabetes, it is more useful than the OGTT for therapeutic decision-making. One of these articles17 proposed that diabetes should not be diagnosed in subjects with a fasting plasma glucose concentration lower than 140 mg/dL unless they also had a clearly abnormal level of HbA1c. Patients with moderately elevated FPG (110-139 mg/dL) but without an excessively elevated HbA1c value should be diagnosed as having impaired fasting glucose (IFG) and should be treated with appropriate dietary measures and physical exercise.
The aim of our study was to evaluate the usefulness of HbA1c measurements to diagnose DM2 in a population seen in a primary care center with risk factors for developing DM2.
Material and Methods
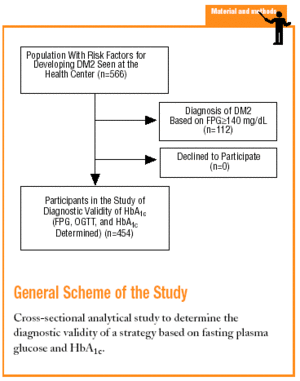
The study was done at the Raval Sud Primary Care Center in Barcelona. The population served by this center is characterized by its low economic level, high rate of immigration (20%), and high rate of morbidity and mortality for certain diseases and disorders. In addition, 29% of the population is older than 65 years. The recorded prevalence of diabetes is 6.6%, one of the highest rates in Spain.18
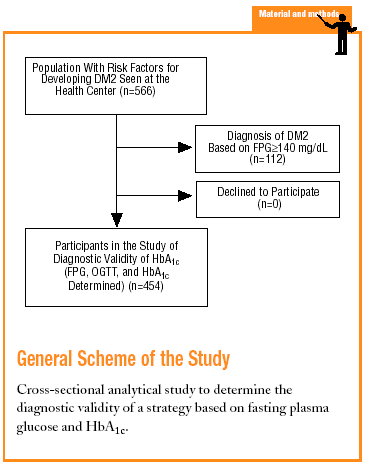
The Raval Sud study was begun in 1992. This project comprises a number of dynamic cohort studies aimed at documenting the epidemiologic features of glucose metabolism disorders and the rate of appearance of chronic, specific complications. For the purposes of the present study, we used data recorded from the time the patient was included in the Raval Sud program. Consequently, the study used a cross-sectional analytical design to validate a diagnostic test.
Subjects eligible for inclusion in the present analysis had at least one of the risk factors for developing DM2 described in the ADA 1997 guidelines (family history of DM2, personal history of carbohydrate intolerance or gestational diabetes, prolonged use of a drug able to raise glucose levels, obesity with a body mass index [BMI] >30, hypertension, HDL-cholesterol levels <35 mg/dL, or triglyceride levels >250 mg/dL).4 We excluded persons who did not wish to take part in the study.
The variables we recorded for each participant were sociodemographic characteristics (age and sex), clinical characteristics (BMI in kg/m2 and blood pressure in mm Hg, in accordance with the guidelines of the Joint National Committee on Prevention Detection Evaluation and Treatment of High Blood Pressure (JNC VI),28 and laboratory values including FPG in a venous blood sample, OGTT after a 75-g glucose overload (if FPG was lower than 140 mg/dL) in blood samples drawn after 30, 60 and 120 minutes (G2h), and HbA1c measured by high pressure liquid chromatography (HPLC) with a Menarini HA8141 autoanalyzer (normal range =3.8%-5.5%; m=4.65%; SD=0.43%). To standardize the results we recalculated the absolute values of HbA1c in terms of the number of standard deviations above the mean. For example, HbA1c=7.23% was considered equivalent to the mean value +6 SD).
On the basis of FPG and OGTT values we classified the participants according to the WHO 1999 criteria as having normal glucose levels (FPG<110 mg/dL and G2h<140 mg/dL), IFG (FPG, 110-125 mg/dL), impaired glucose tolerance (IGT) (FPG<126 mg/dL and G2h, 140-199 mg/dL), or DM2 (FPG>125 mg/dL or G2h>199 mg/dL).
The sample consisted of 454 subjects. For this sample size and our study conditions (P=.066 and *=.05), the absolute percentage error rate (e) was 2.3%.
The descriptive parameters used for the statistical analysis were the mean, standard deviation (SD) and 95% confidence intervals. Normality of the numerical variables was checked with the Kolmogoroff-Smirnov test. Bivariate analysis was based on chi-squared tests, ANOVA, Pearson's correlation coefficient (r) and their nonparametric counterparts.
Diagnostic value was analyzed by calculating sensitivity, specificity, positive predictive value, negative predictive value, and global value or efficacy (GV) of each cutoff point. "Efficacy" was defined as the proportion of subjects correctly classified with reference to the total number of cases. Predictive values were calculated for a prevalence of 6.6%
Results
The study included 454 individuals (235 men and 219 women). Mean age was 64.6 (13.2) years, and 20% of the patients were older than 75 years. Mean BMI was 29.63 (4.97) kg/m2, and 3% of the patients were classified as having morbid obesity (BMI>40 kg/m2).
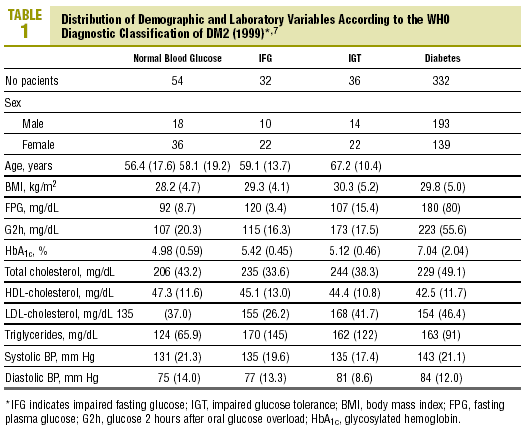
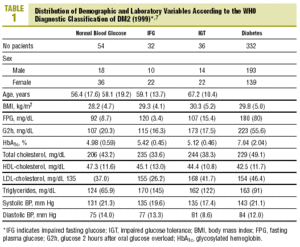
The distribution of demographic characteristics and laboratory findings according to the diagnostic classification are shown in Table 1. In the present descriptive analysis we found that plasma glucose values were significantly lower in normal subjects than in subjects with abnormal glucose levels (IFG or IGT), and were in turn lower in the latter group than in patients with diabetes (P<.001). Mean HbA1c was significantly higher in patients with diabetes than in all other categories of participants: 7.04% versus 4.98% (normal glucose level), 5.42% (IFG), and 5.12% (IGT) (P<.001). Patients with IFG had slightly higher HbA1c values than those with IGT, although the difference did not reach statistical significance.
Levels of HbA1c correlated significantly with FPG (r=0.72; P<.001) and glucose measured 30 min (r=0.27; P=.01), 60 min (r=0.39; P<.001) and 2 h (r=0.43; P<.001) after an oral glucose overload.
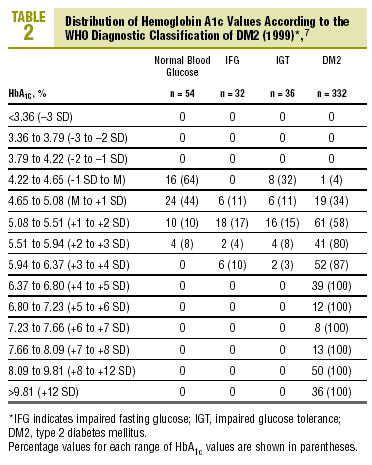
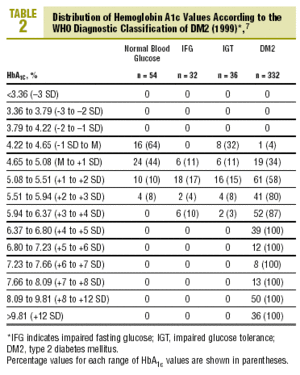
Table 2 shows the distribution of HbA1c values according to the diagnostic classification. All groups with HbA1c values above 6.37% (x+4 SD) had DM2, whereas none of the patients with HbA1c below 4.22% (x1 SD) had diabetes. Patients with IFG had HbA1c levels that ranged from 4.65% to 6.37% (between the mean value and +4 SD). In the group with IGT, levels of HbA1c were between 4.22% and 6.37% (between 1 SD y +4 SD), and interestingly, HbA1c levels were lower than the mean (between 4.22% and 4.65%) in 8 of the 36 patients with IGT (22%).
The diagnosis of DM2 in patients with FPG<110 mg/dL (but with an abnormal OGTT) was rare, accounting for only 1.2% of all cases of DM2. Of the 80 patients with FPG between 110 and 125 mg/dL, 30% had HbA1c values above the normal range (HbA1c>5.5%), 15% had HbA1c levels >5.94% (+3 SD), and none of them had HbA1c levels >6.8% (+5 SD)
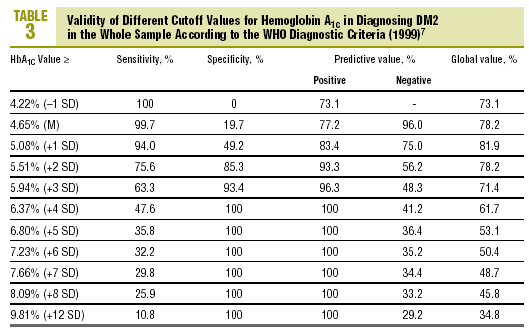
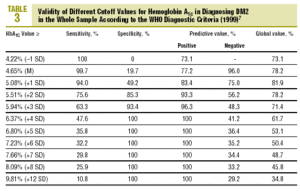
Table 3 shows the validity for the entire sample of the different cutoff values of HbA1c in establishing a diagnosis of DM2.
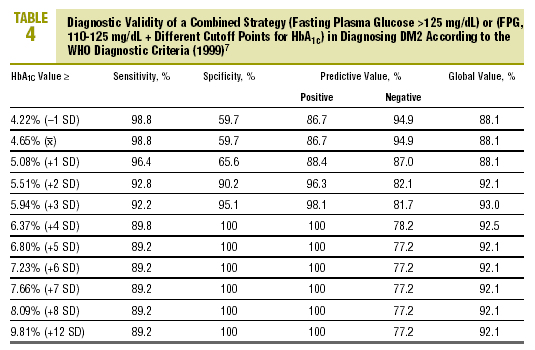
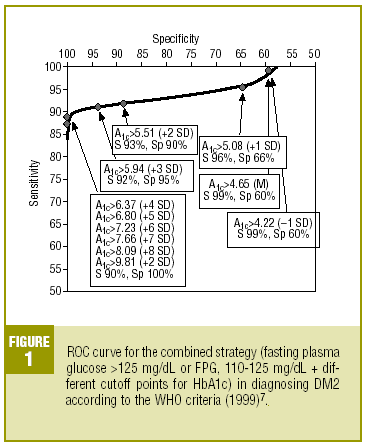
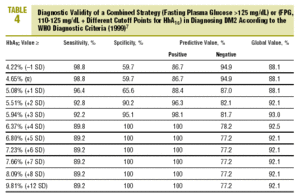
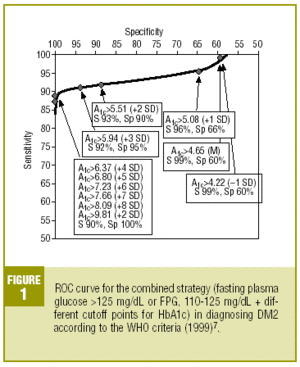
Table 4 shows the diagnostic validity of a combined strategy based on FPG and HbA1c values: patients were considered to have diabetes when FPG>125 mg/dL, or when FPG>=110 mg/dL and HbA1c was abnormally high (>= the cutoff point). We found that the validity of the HbA1c cutoff points increased markedly as the percentage value increased. Maximal efficacy (93% GV) was found for HbA1c>=5.94% (x+3 SD), with a sensitivity of 92.2% and a specificity of 95.1%. The Figure illustrates the ROC curve for different HbA1c values.
Discussion
The present study was done at a primary care center located in the Raval district, a depressed area in downtown Barcelona with particular demographic characteristics. The very low sociocultural level of the population may have led to underdiagnosis of DM2. In addition, mean age of the sample we studied was close to 65 years (64.6 years, with 20% of the sample older than 75 years) and the rate of obesity was high (mean BMI, 29.6 kg/m2). Standard diagnosis based on FPG and OGTT was used for a selected population at high risk for developing diabetes. For these reasons the prevalence of DM2 in our sample was much higher than in the general population, but this was expected for a sample of the characteristics described above. We believe this may have made overestimation of the positive and negative predictive values more likely, but probably did not affect the sensitivity or specificity of the different cutoff points in the diagnostic test.
A number of prospective studies have confirmed the relationship between circulating glucose values and the onset of chronic complications.19-21 It is thus logical for the diagnosis of DM2 to be based on glucose values. The main problem, however, lies in the attempt to establish a cutoff point for this continuous quantitative variable.
A second important problem is the choice of method for measuring plasma glucose that best diagnoses the disease, i.e., that predicts the presence of chronic complications: FPG, postprandial glucose level, OGTT, or 24-hour glucose profile (among other possible tests). Some approaches such as the OGTT are poorly reproducible and highly variable,22 while others show poor predictive capacity (e.g., a single glucose measurement).23,24 If hyperglycemia is toxic,25 it would be logical to use a measure that quantifies the intensity and duration of hyperglycemia--i.e., glycosylated hemoglobin.
The relationship between HbA1c and measures of glucose levels has been widely documented.5,6,17 In the present study, HbA1c values correlated most clearly with FPG (r=0.72). We also found that FPG and HbA1c appeared to be most useful in detecting diabetes, whereas G2h appeared to be most useful in identifying normal subjects (who have significantly lower values).
As the first step in our test of the usefulness of HbA1c in diagnosing DM2, we analyzed different cutoff points for this laboratory value for the whole sample of patients at risk for DM2. When an HbA1c value of >=5.51% (x+2 SD) was used to classify persons as having diabetes, the attendant sensitivity (76%) and specificity (85%) were acceptable. When a higher cutoff point was used, specificity increased, but this was logically at the expense of reduced sensitivity. Differences in the reference populations notwithstanding, our values were similar to those from other studies. For example, Engelgau et al6 reported that an HbA1c of >=6.7% had a sensitivity of 68% and a specificity of 99%. The metaanalysis published by Peters et al16 noted sensitivities of 66% and 36%, with specificities of 98% and 100% for cutoff points of 6.3% (x+2 SD) and 7.3% (x+4 SD), respectively. Both of these studies were done in groups consisting exclusively of persons whose diabetes had been diagnosed with OGTT. We opted to use as our reference population all cases of diabetes in the sample (diabetes diagnosed with OGTT or with FPG according to WHO 1999 criteria), as this was a better reflection of the situation encountered in daily practice. A study of a US population (Rohlfing et al)26 obtained a sensitivity of 63.2% and a specificity of 97.4% with an HbA1c cutoff value of >=6.1 (+2 SD). Other authors27 reported similar results. The Japanese Diabetes Society28 recommended using an HbA1c value of >=6.1% (+2 SD) as the criterion for diagnosing DM2.
However, one of our aims was to identify a simple, reliable strategy for diagnosis. The OGTT is neither simple nor reliable. In our setting it is rarely requested and almost never used as the basis for therapeutic decisions. Moreover, DM2 is diagnosed only exceptionally when FPG in repeat analyses is below 110 mg/dL (1.2% of all cases of DM2 in the present study).
The study by Ko et al29 investigated the importance of the OGTT in confirming the diagnosis of DM2. Their results suggested that an OGTT was necessary only for persons with FPG values between 110 and 140 mg/dL and HbA1c values >=5.5% (with 84% sensitivity and specificity).
Many studies have shown that to diagnose diabetes, measuring HbA1c or FPG is at least as useful as measuring plasma glucose 2 h after an oral glucose overload.5,16,17,22,23 Tsuji et al27 reported that simultaneously measuring FPG and HbA1c increased the number of
diagnoses in a statistically significant manner in comparison to the use of either of these tests alone.
On the basis of these findings we designed a logical strategy for diagnosis based on current recommendations for FPG values (FPG>125 mg/dL) and the validity of HbA1c, which makes it possible to forgo OGTT when FPG is nondiagnostic (between 110 and 125 mg/dL). In this situation, if HbA1c is >=5.94% (mean, +3 SD), the diagnosis of DM2 is reliable and accurate in 93% of the cases.
Conclussions
Despite the limitations of this study, we would like to offer some practical considerations that are consistent with the findings of other authors.5,10.16,17,22,26-29 We believe the combination of FPG and HbA1c values is extremely useful in establishing the diagnosis of diabetes under the following conditions:
If repeated FPG measurements are above 125 mg/dL, a diagnosis of DM2 should be established, and HbA1c measurement will be needed to evaluate metabolic status and decide on appropriate treatment.
If repeated FPG measurements are below 110 mg/dL, the individual should probably be considered normal.
If FPG is between 110 and 125 mg/dL, carbohydrate tolerance may be impaired and HbA1c should be determined. If the result is below the mean +3 SD (<5.94% in the present study), periodic follow-up is advisable with particular attention to risk factors for the appearance of DM2 (obesity, sedentary lifestyle, etc.). If HbA1c is above this value, the patient should be considered diabetic in practical terms. More aggressive health and dietary measures should be started, and short-term pharmacological treatment should probably be considered.
We hope that the not too distant future sees standardization of the laboratory techniques for measuring HbA1c. This would make it possible to readily identify individuals who require intervention to prevent the appearance and progression of diabetes and its complications.