(93 varones y 111 mujeres). Mediciones principales. Antecedentes familiares/personales, actividad física, fecha de menarquia, peso, talla e índice de masa corporal relativo, hemoglobina (Hb), volumen corpuscular medio (VCM), índice de saturación de transferrina (IST) y ferritina (FS) a uno de cada 4 adolescentes en el examen de salud correspondiente a su edad. Eran motivo de exclusión una PCR positiva y/o VSG elevada. Se consideró depleción de los depósitos férricos si FS < 12 ng/dl, ferropenia si además IST < 14% y/o VCM < 75 fl, y anemia ferropénica si además Hb < 12 g/dl. Resultados. Un 8,6% de varones y un 12,6% de mujeres adolescentes presentaban una carencia de hierro en alguno de sus diferentes estadios, bien como depleción de los depósitos férricos (varones, 2,2%; mujeres, 6,3%), ferropenia (varones, 3,2%; mujeres: 3,6%), o anemia (varones, 3,2%, mujeres, 2,7%), sin que existieran diferencias significativas entre ambos sexos. Conclusiones. En los adolescentes de 10-14 años de nuestro entorno asistencial la carencia de hierro es relativamente importante, y su prevención, detección y control deberían contemplarse específicamente en los Programas de Atención a la Población Infantil y Adolescente.
Introduction
Iron deficiency is one of the most frequent nutritional problems in the world, and iron-deficiency anemia continues to be the most prevalent hematological alteration. Although the prevalence is higher in developing countries, nutritional iron deficiency is also common in industrialized countries.1-5
Adolescents are a population group at risk for iron deficiency, as together with a considerable increase in nutritional requirements (especially for iron) with a number of physiological factors (growth spurt, changes in body composition, menstrual losses), the dietary supply is often deficient owing to psychosocial factors such as participation in sports, preoccupation with body image, fear of obesity, and fast food.6-10
The development of iron-deficiency anemia is a dynamic process that begins with the depletion of iron stores (latent iron deficiency) and progresses to iron-deficient erythropoiesis (iron deficiency), culminating in iron-deficiency anemia. Biochemical and hematological tests make it possible to detect the different stages of iron deficiency. However, the diagnostic sensitivity of laboratory techniques for each of these phases differs. Consequently there is no predetermined diagnostic algorithm; instead, a combination of two or three tests is recommended to diagnose iron deficiency and iron-deficiency anemia (for example). On the other hand, a very specific marker--serum ferritin--is available for earlier stages of deficiency. Lower-than-normal values are diagnostic of depeleted iron stores.11-15
The aim of the present study was to investigate the prevalence of depletion of organic iron stores, iron deficiency and iron-deficiency anemia in adolescents (10-14 years old) in our health district, and to analyze different factors (such as growth, nutrition, participation in sports, and menarche) that may be related with nutritional iron deficiency.
Material and methods
The Estella (Navarra province) Basic Health Zone comprises a total population of 14 037 inhabitants, of whom 811 (5.8%) are adolescents aged between 10 and 14 years. This population group represented the sample population (811 adolescents, 410 boys and young men, 401 girls and young women). To calculate sample size we used as a reference value an expected prevalence of 20%, a 95% confidence interval and a level of precision of 0.05; optimum sample size was 189 cases.
The participants were recruited by random, systematic sampling. During the 1999-2000 school year, as part of the Child and Adolescent Population Care Program,16 one of every 4 adolescents who were seen for a regular check-up was asked to provide information on family and personal antecedents, usual physical activity and (in young women) date of menarche. We also recorded weight and height, relative body mass index (%BMI), growth rate and hematological and biochemical indicators related with nutritional iron status, eg, hemoglobin (Hb), hematocrit (Ht), mean corpuscular volume (MCV), transferrin saturation index (TSI) and serum ferritin (SF).
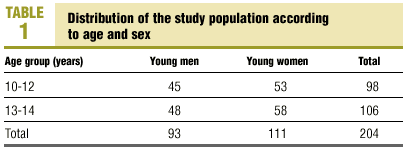
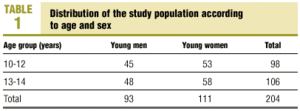
Table 1 shows the numbers of adolescents of each sex in each of the age groups. A total of 204 young people (93 males, 111 females) were divided into 2 groups according to age. The first group consisted of 45 young men and 53 young women (total 98) aged 10.0 to 12.9 years (10-12 group); the second consisted of 48 young men and 58 young women (total 106) aged 13.0 to 14.9 years (13-14 group). We exclude all patients with any acute or chronic disease (chronic respiratory disease, hematological disease, cancer, others) that might condition the hematological or biochemical results. Positive C-reactive protein (CRP) or elevated erythrocyte sedimentation rate (ESR) were also criteria for exclusion.
The different stages of iron deficiency were defined on the basis of established criteria.4,13-15,17 Depletion of iron stores (latent iron deficiency) was defined as SF<12 ng/dL, iron deficiency was said to exist when, in addition, TSI was <14% or MCV was <75 fL. Iron-deficiency anemia was said to exist when, in addition to the aforementioned alterations, Hb was <12 g/dL.
The results were expressed as the mean and as a percentage, with 95% confidence intervals (95% CI). Statistical analyses (Student´s t test, comparison of the proportions and linear regression) were done with the SIGMA-PLUS program (Hardware, 97).
Results
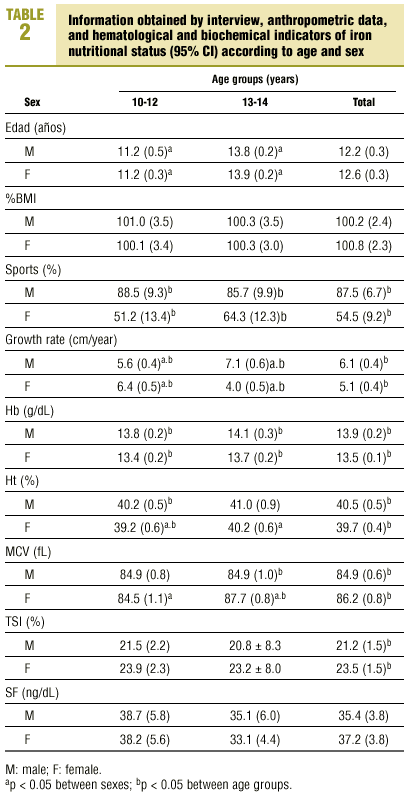
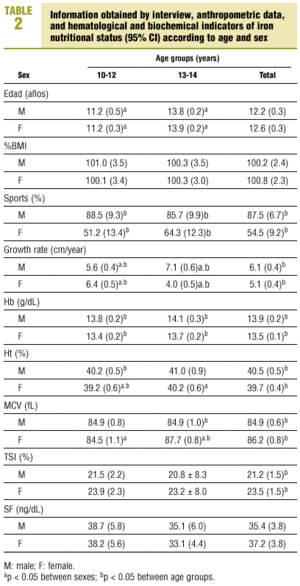
Table 2 shows the mean values obtained from the interview and mean anthropometric values, along with hematological and biochemical indicators of iron nutritional status in different sexes and age groups. When we compared the results for young men and young women, we found that the following were significantly higher in young men: participation in sports (87.5% versus 54.5%; P<0.05), growth rate (6.1±1.8 versus 5.1±2.3 cm/year; P<0.05), Hb (13.9±0.9 versus 13.5±0.7 g/dL; P<0.05) and Ht (40.5±2.3% versus 39.7±2.2%; P<0.05). Significantly higher values were found in young women for MCV (84.9±3.1 versus 86.2±4.1 fL; P<0.05) and TSI (21.2±7.7% versus 23.5±8.2%; P<0.05). There were no significant differences for %BMI (100.2±12.1 versus 100.8±12.2; P<0.05) or SF (34.4±19.0 versus 37.2±20.4 ng/dL; P<0.05). When we compared mean values for the two age groups, we found that growth rate was significantly higher in young men in the older group (5.6±1.4 versus 7.1±2.1 cm/year; P<0.05), and in young women in the younger group (6.4±1.8 versus 4.0±2.1 cm/year; P<0.05). Values for Ht (39.2±2.2% versus 40.2±2.0%; P<0.05) and MCV (84.5±4.2 versus 87.7±3.3 fL; P<0.05) were significantly higher in young women in the older group.
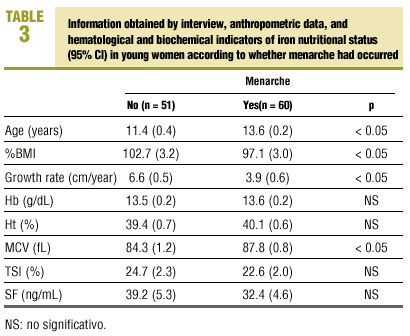
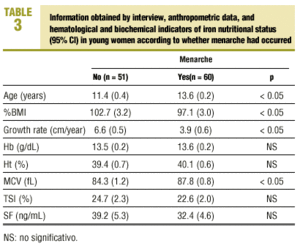
Of all adolescent women included in the study (n=111), 54.1% (n=60) had had their first menstrual period, during a period that ranged from 2.4 to 36 months previously (mean, 15.3±10.1 months). Table 3 shows the mean values for anthropometric variables and for hematological and biochemical analyses of iron nutritional status in relation to the occurrence of menarche. Mean age was significantly higher in the subgroup of young women who had reached menarche (11.4±1.4 years versus 13.6±0.8 years; P<0.05), whereas %BMI (102.7±11.7 versus 97.1±12.0; P<0.05) and growth rate (6.6±2.0 versus 3.9±2.3 cm/year; P<0.05) was significantly higher in the subgroup of adolescent women who had not reached menarche. There were no significant differences between hematological or biochemical markers of iron nutritional status except in relation with MCV, which was significantly higher in the post-menarche subgroup (84.3±4.2 versus 87.8±3.3 fL; P<0.05).
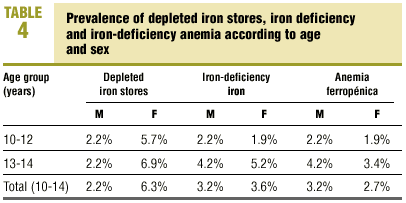
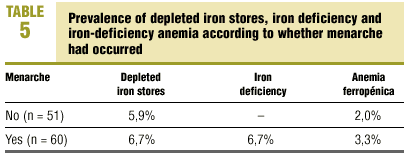
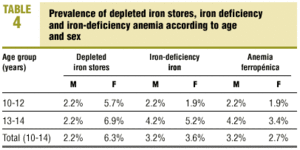
Table 4 summarizes the prevalence of depleted iron stores, iron deficiency and iron-deficiency anemia according to sex and age group. Iron stores were depleted in 2.2% of the young men and 6.3% of the young women (6.9% in the 13-14 group); iron deficiency was found in 3.2% of the young men (4.2% in the 13-14 group) and 3.6% of the young women (5.2% in the 13-14 group); and iron-deficiency anemia was seen in 3.2% of the young men (4.2% in the 13-14 group) and 2.7% of the young women (3.4% in the 13-14 group). There were no significant differences between the sexes or between the two age groups. In other words, 8.6% of the young men (6.6% in the 10-12 group and 10.4% in the 13-14 group) and 12.6% of the young women (9.5% in the 10-12 group and 15.5% in the 13-14 group) had SF values below 12 ng/dL, some stage of iron deficiency, or both. Table 5 shows the prevalence of depleted iron stores, iron deficiency and iron-deficiency anemia in relation with the occurrence of menarche. Of all young women who had reached menarche, 6.7% had depleted iron stores, 6.7% had iron deficiency, and 3.3% had iron-deficiency anemia. There were no significant differences in comparison to young women who had not yet reached menarche. In other words, 16.7% of the post-menarche and 7.9% of the pre-menarche young women had SF values below 12 ng/dL, some stage of iron deficiency, or both. We found no significant correlations between age, %BMI, growth rate, menarche (months since menarche), participation in sports, and values for Hb, Ht, MCV, TSI and SF.
Discussion
The diagnosis, and hence calculation of the prevalence of iron deficiency and iron-deficiency anemia, are not always straightforward, as no single diagnostic test can evaluate with absolute precision how far the problem has progressed along the sequence of deficiency. Serum ferritin is directly related with the status of organic stores of iron, and is the only marker that does not yield false positive results. Thus values below 12 ng/dL provide a reliable diagnosis of depleted organic iron reserves. However, SF behaves as an acute phase reactant, and its values may be increased, even in situations in which iron deficiency actually exits, in the presence of infectious or inflammatory processes, just as ESR and CRP are increased under these circumstances. For this reason we excluded from the present study all patients with elevated ESR or CRP values. Sideremia is an unreliable indicator to diagnose iron deficiency; TSI should always be requested, as regardless of the results for other serum markers including SF, a decrease in TSI can be considered diagnostic of iron deficiency. The diagnostic sensitivity of free erythrocyte protoporphyrin (FEP) varies, as in depletion of organic stores and in the initial stages of iron-deficient erythropoiesis, FEP values remains normal. Although FEP is a low-cost technique, it is rarely used in conventional laboratories, as we found in the present study.
Microcytosis is highly indicative of iron deficiency or iron-deficiency anemia, but frequently no alteration is seen in MCV in early stages of iron deficiency. Another candidate is hypochromia, which is evaluated as a decrease in mean corpuscular hemoglobin (MCH) and specifically by counting hypochromic red blood cells. However, not all cell counters are designed to provide this value, which can be altered despite normal MCV and MCH values.
In its most characteristic form, iron-deficiency anemia first appears as a decrease in hemoglobin together with alterations in hematometric indicators (hypochromia and microcytosis) and a decrease in transferrin saturation and SF. However, this is not always the case. It can nonetheless be said that in persons with anemia, the finding of decreased SF is practically diagnostic of iron-deficiency anemia; moreover, in the absence of hypoferritinemia, iron-deficient erythropoiesis can be revealed as a decrease in TSI. The diagnostic capacity of a given laboratory is markedly enhanced by the additional information provided by FEP or hypochromic red blood cell count. In uncertain cases the determination of soluble transferritin receptor may provide the answer, and as a last resort, bone marrow aspirate can be used.11-14,18-20 The criteria used in the present study allowed us to evaluate the prevalence of iron deficiency at different stages of its evolution. Organic iron stores were measured as SF concentration, iron deficiency was studied by measuring two or more biochemical markers (SF and TSI or MCV), and iron-deficiency anemia was identified by considering Hb in the light of other markers necessary to evaluate iron deficiency (SF and TSI or MCV).
The prevalence of depleted organic iron stores, iron deficiency and iron-deficiency anemia that we found in the population of adolescents in our health care area agree, both for young men (2.2%, 3.2% and 3.2%) and young women (6.3%, 3.6% and 2.7%), with those in recent studies published in Spain17,21-23 and in other Western countries.4,9,24,25 Although there have been some improvements in industrialized countries1,3-5, these figures remain high. In fact, in our health care setting about 8.6% of all adolescent men (6.6% in the 10-12 group and 10.4% in the 12-13 group) and 12.6% of all adolescent women (9.5% in the younger group and 15.5% in the older group) had SF values below 12 ng/dL, some stage of nutritional iron deficiency, or both. This shows that adolescents, as a population group, are highly susceptible to nutritional iron deficiency.
Several factors that are associated with pathophysiological mechanisms of iron deficiency coincide in adolescents to give rise to increased nutritional requirements, eg, the adolescent growth spurt, physical activity, and (in young women) menstrual losses. The increased requirement is not always accompanied by an adequate dietary supply.6,7,10,26-30 In the population of adolescents we studied, the prevalence of different stages of iron deficiency was higher in the older subgroup in both sexes. Several factors might contribute to the higher risk or otherwise explain this higher prevalence. For example, young men aged 13.0 to 15.0 years were in the middle of their growth spurt (7.1±2.1 cm/year), consequently a series of changes in body composition, including increased lean mass, blood volume and erythrocyte mass, together with increased physical activity (85.7% regularly participated in some sport), might have led to an increase in urinary losses of iron or mechanical hemolysis. Of the young women aged 13.0 to 15.0 years, although they had passed the apex of the growth spurt and their physical activity was lower, most had reached menarche, hence menstrual losses might have led to iron deficiency. In fact, 16.7% of the young women in this study who had started menstruating had SF values below 12 ng/dL, in comparison to 7.9% of the participants in whom menarche had not yet occurred. In addition, a dietary factor may also have influenced our findings: in the post-menarche subgroup of young women, %BMI was significantly lower (possibly because of concerns over body image) than in the pre-menarche subgroup.
In summary, nutritional iron deficiency is a relatively frequent disorder in the adolescent population in our setting, and represents a serious public health problem. The prevention, detection and management of iron deficiency should be dealt with specifically in child and adolescent health programs developed within the scope of primary care efforts.
Correspondence: T. Durá. Centro de Salud Estella, Paseo Inmaculada 39, 31200 Estella (Navarra), Spain.This work was supported by the Department of Health of the Navarra Government (Resolution 1036/1999). Manuscript accepted for publication 3th August 2001.