Introduction
The success of pharmacological treatment depends on the active participation of the patients, who will require the necessary cooperation of health professionals to obtain maximum therapeutic benefit and to prevent secondary effects of the treatment.1-4 When patients use medications different results are possible. Normally patients benefit from pharmacotherapeutic intervention. However, events associated with lack of efficacy or safety of drug treatment can be observed.1-4 Any deviation from the desired beneficial effects of medicines causes a problem related to medication (PRM).3-5 PRM have been defined as health problems, understood as clinically negative results derived from the
pharmacotherapy which, due to different reasons, may lead to the therapeutic objective not being achieved or the appearance of undesired effects.5 These demonstrate that PRM are the cause of morbility and mortality associated with medicines5-11 and strategies need to be implemented to carry out an appropriate approach to this problem.10,11
By using the activities of pharmaceutical care, the pharmacists, in collaboration with the patients and doctors, improve the results of pharmacotherapy by preventing, detecting and resolving PRM, thus avoiding the morbility and mortality associated with medicines.2-4,12-15 One of the activities derived from pharmaceutical care is pharmacotherapeutic follow-up (PFU), which can be defined as the professional practice in which the pharmacist is responsible for the needs of the patient related with their medication by the continuous, systematic and documented detection, prevention and resolving of PRM, in collaboration with the patient him/herself and other health professionals, with the aim of achieving concrete results which will improve the quality of life of the patient.15 The results of PFU have been demonstrated in different scenarios of pharmaceutical professional practice, achieving an effective solution of PRM.16-24
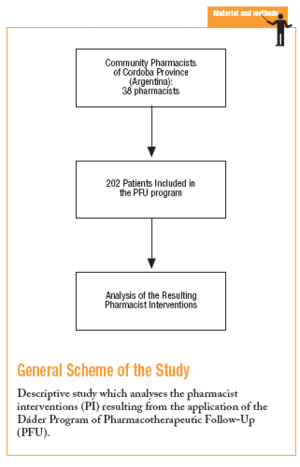
The Dáder Program of PFU was created by the Pharmaceutical Care Research Group of the University of Granada (Spain), and is essentially a teaching program for practising pharmacists.16,17,25,26 The implementation of the Dáder Program in the Province of Cordoba (Argentina) was carried out by a meeting between the Pharmaceutical Care Research Group of the University of Granada, the Faculty of Chemical Sciences of the National University of Cordoba, and the College of Pharmacists of the Province of Cordoba.26 In this study the results obtained during the first year of activity with this work method are presented and analysed.
Patients and Methods
The Dáder Program has produced protocols for the procedures and the ways of recording which must be adopted to provide PFU to patients, with the aim of being more effective in the identification and resolving of PRM.25 It is based on obtaining the pharmacotherapeutic history of the patients, that is, their health problems and the medications used, and the evaluation of the state of the situation at a determined date, to identify and resolve the possible PRM that the patient may be suffering from. Then the necessary pharmacist interventions (PI) are carried out to resolve the PRM, patient involvement being necessary and in the majority of cases, the patient´s doctor.2,3,14,15,25
A PI is considered effective when the PRM, which the pharmacist has identified, has been resolved favourably.25
The PFU method consists of the following phases: a) offering the service; b) first interview; c) state of situation; d) study phase; e) evaluation phase; f) intervention phase; g) result of the intervention; h) new state of the situation; and i) successive interviews.
The participating pharmacists are community pharmacists and practice their activity under the auspices of the College of Pharmacists of the Province of Cordoba. As the Dáder Program is a formative program, the registering of drugs is carried out continuously under the guidance of a Scientific Committee created for this purpose. It also coordinates the activities of the program which are basically carried out by having clinical sessions where cases are presented and discussed; the reception and registering of the PI which are generated, and the quarterly sending out of a report to the participating pharmacists where the progress made by everyone is reported and evaluated.13,26 The inclusion of pharmacists participating in the program was voluntary and was carried out after an initial training period of 10 hours26.
The inclusion of patients in the PFU program was based on the observed needs of all the participating pharmacists, and involved all their patients and their carers who attended the pharmacy throughout the study talking about any worries or requesting some information as regards their state of health or pharmacotherapy. Those patients were offered the PFU service, and who accepted the appointment for a first interview.25,26
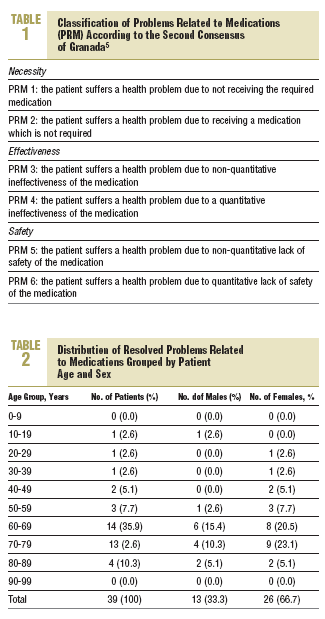
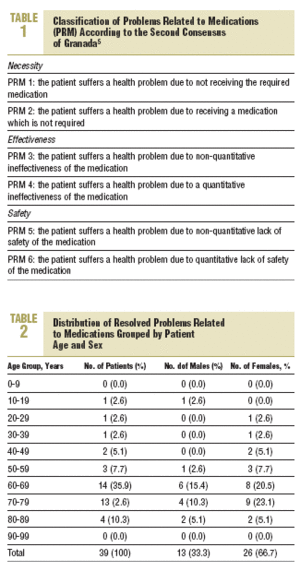
The data which is presented was obtained from the PI records passed on by the community pharmacists and covered the period between September 200 and August 2001, inclusively. A descriptive statistical analysis was carried out using the STATISTICA program (Version 6 StatSoft, Inc. 2001). To classify the PRM the Second Consensus of Granada on the Problems Related to Medications was used (Table 1), which considered the 3 basic needs to complete the whole pharmacological treatment: necessity, efficacy, and safety.5
The causes of PRM are listed, such as: interactions, non-compliance, duplication and others. The sex and age of the patients were recorded with PI, the number of medications used and the number of visits to the pharmacy necessary to resolve the PRM. The drugs involved in the PRM were analysed according to the ATC (Anatomical Therapeutic Chemical) classification.
Results
A total of 38 pharmacists took part in the PFU program, of which 68.4% (n=26) recorded PI. A total of 202 patients were included, and PI was recorded in 108 (53.5%) of them. The total number of PI climbed to 280, with a mean PI per pharmacist who notified results of 10.8 (95% CI, 4.4-5.0; range, 0-10) and the number of visits to the pharmacy to resolve a PRM was 4.0 (95% CI, 3.6-4.3).
The number of PRM resolved was 218, and the effectiveness of PI was 77.9% (95% CI, 70.6-86.2).
The distribution of resolved PRM, grouped by age and sex (in patients where the age and sex were recorded) is shown in Table 2. The distribution of the PRM based on the 3 basic requirements of pharmacotherapy was as follows: necessity, 25%; effectiveness, 37%; and safety, 38%.
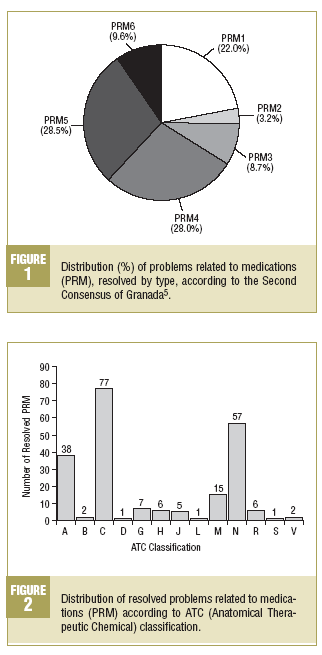
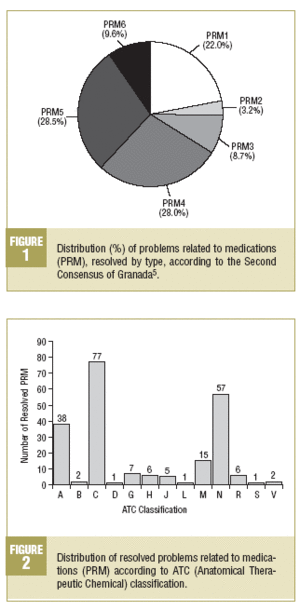
The percentage distribution of the resolved PRM is shown in Figure 1. Among the related PRM, a predominance of PRM4 (28.0%) is observed in the effectiveness and of PRM5 (28.4%) in the safety.
As regards the causes of resolved PRM, 4.1% (n=9) was due to medication interactions and 16.1% (n=35) to non-compliance of treatment, 1.4% (n=3) due to duplication and 74.8% (n=171) to other causes which were not categorised.
In relation to the means of communication employed for the resolving of PRM, 25% were resolved between the pharmacist and the patient, while 75% required the involvement of the doctor, achieving in the latter case an acceptance of PI of 74.6% (95% CI, 67.8-82.6).
The distribution of PRM according to the active agent involved, following the ATC classification is also shown. In Figure 2, where the resolved PRM according to this classification are described, a significant predominance-in descending order-is observed of drugs which act on the cardiovascular system, nervous system and digestive system and metabolism.
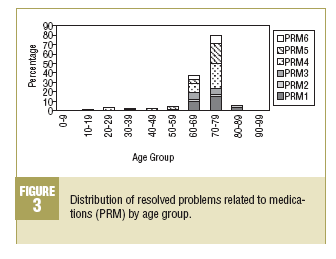
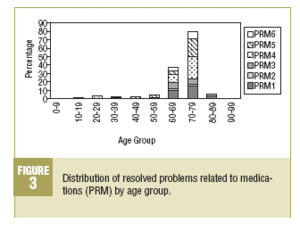
In the 2 majority age groups (60-69 and 70-79 years) a high incidence of PRM1 (lack of necessary medication) is observed, as well as in the safety PRM, which are fundamentally related to adverse reactions of medications (PRM5). It is also worth pointing out that PRM6 (quantitative lack of safety of the medication) and PRM4 (quantitative ineffectiveness of the medication) have a higher frequency in the 70-79 years age group (Figure 3).
Discussion
The carrying out pharmaceutical care activities related to PRU is the first experience in Argentina linked to a group of community pharmacists who worked in an organised way and followed a single work method in identifying and resolving of PRM in their patients. The integration of the universities in association with professionals for the implementation of these types of activities enables these initiatives to be channelled appropriately, which, on the whole, will benefit society. It is important to highlight the collaboration offered by the Medicines Information Centre of the National University of Cordoba (CIME), for the objective and up to date information which it provided in order that the pharmacists could carry out PI with solid foundations and backed by scientific evidence.
The high percentage of resolved PRM. (77.9%) is similar to other published works21,22 which have used the Dáder Program, and could demonstrate that the general use of this activity constitutes a valid means for the prevention of morbility and mortality associated with medications and has, as a consequence, a positive impact on the health of the population who go to community pharmacists.
The predominance of females (66.7%) among the group of patients could be related to the fact that women form the majority who visit this type of establishment, so there is a direct contact by which the pharmacists can offer the PFU service.
As regards the distribution of the PRM, taking into account the three basic necessities of all pharmacotherapy, a very similar proportion is seen between the PRM related to effectiveness and safety of medications. Among the PRM related to effectiveness, the predominance of type 4 (28%) is highlighted which is basically related to non-compliance. These PRM were resolved between the patients and the pharmacist in 76.2% of cases, which demonstrates the importance of the community pharmacist in resolving these types of problems related to adherence to treatment.
In the field of safety a predominance of PRM5 is seen, related to qualitative insecurity, an aspect which demonstrates the possibility of promoting and carrying out pharmacovigilance activities which could be coordinated perfectly with this PFU program by the voluntary notification to the National Pharmacovigilance System.
The analysis of resolved PRM, taking into account the drugs involved according to the ATC classification, shows a high of proportion of those that act on the cardiovascular systemas the main anatomical group, followed, in descending order, by those that act on the nervous system and digestive system and metabolism. It is important to point out that, as regards the main therapeutic group, it is seen that in more than 50% of the resolved PRM, 7 different types of drugs prevail, which are, in descending order: drugs active on the renin-angiotensin system, psycholeptics, anti-diabetics, calcium channel blockers, anti-inflammatories and anti-rheumatics, analgesics, and psychoanaleptics. This data should indicate that the patients who use these types of drugs are most susceptible to the appearance of PRM, and as such that the promotion and development of more intensive PFU strategies would be required for them.
The problems of safety of medicines, qualitative ones as well as quantitative, are those which show up more often in patients of more advanced ages (60-69 and 70-79 years), which indicates the need for carrying out PFU in this population, since it seems to be more susceptible to these problems.
On analysing the PI which required the participation of the doctor for resolving them, a high percentage of acceptance is observed (74.6%), which agrees with other published works.16,17 This demonstrates that teamwork is possible for the PFU of patients. The professionals of the health team have to be made aware of the need of this type of multidisciplinary approach which has a beneficial bearing on the results for the patient and helps to integrate the health care services.
Among the limitations of this study it should be pointed out that the drug and patient samples are not representative, which, as a consequence, limits the extrapolation of the results. Also, there is no control group, since it is not ethically acceptable to deny the PFU service to a particular group of patients.
This experience shows the need to add a larger number pharmacists to carry out PFU activities, with the intention of being able to demonstrate, as such, its significant impact on the morbility and mortality associated with medicines in society.28 The commitment from Institutions, private and public, who promote health must also be obtained, to support and maintain, in the long term, these PFU activities, which are feasible from the community pharmacists
Acknowledgements
To the College of Pharmacists of the province of Cordoba, to the Lecturer Sonia Uema for reviewing the manuscripts, and to the community pharmacists who participated: Rubio de Morales, Felisa; Álvarez de Besso, Inés; Antonello, Andrea; Armando, Pedro; Badra, Silvia; Bauducco, Martha; Birolo, David; Borgogno, Rossana; Cariddi, Stella; Cavalli, Mariel; Cestilli, María Inés; Coiset, Laura; Coppetti, María Angélica; Cravero, María Emilia; Checa, Patricia; Dutto, Javier; Gabaglio, Alicia; Garrera, María; Grosso, Cristina; Hernández, María Francisca; Hernández, María Mercedes; Herrera, Viviana; Lenarduzzi, Stella; Luna, Carlos Alberto; Minatta, Silvia; Mondino, Carina; Peralta, Laura; Pessina, Graciela; Picossi, Patricia; Quiroga de Arce, Laura; Ricciuti, Cristina; Roberts, Matilde; Robledo, Jorge; Semeria, Nora; Tenllado, María Isabel; Terbonati, Aldo; Trusendi, Fernando, and Vilca, Claudia.