This study is aimed at analyzing the impact of the main factors contributing to short and long-term mortality in patients at final stages of heart failure (HF).
SettingPatients attended at any of the 279 primary health care centers belonging to the Institut Català de la Salut, in Catalonia (Spain).
ParticipantsPatients with Advanced HF.
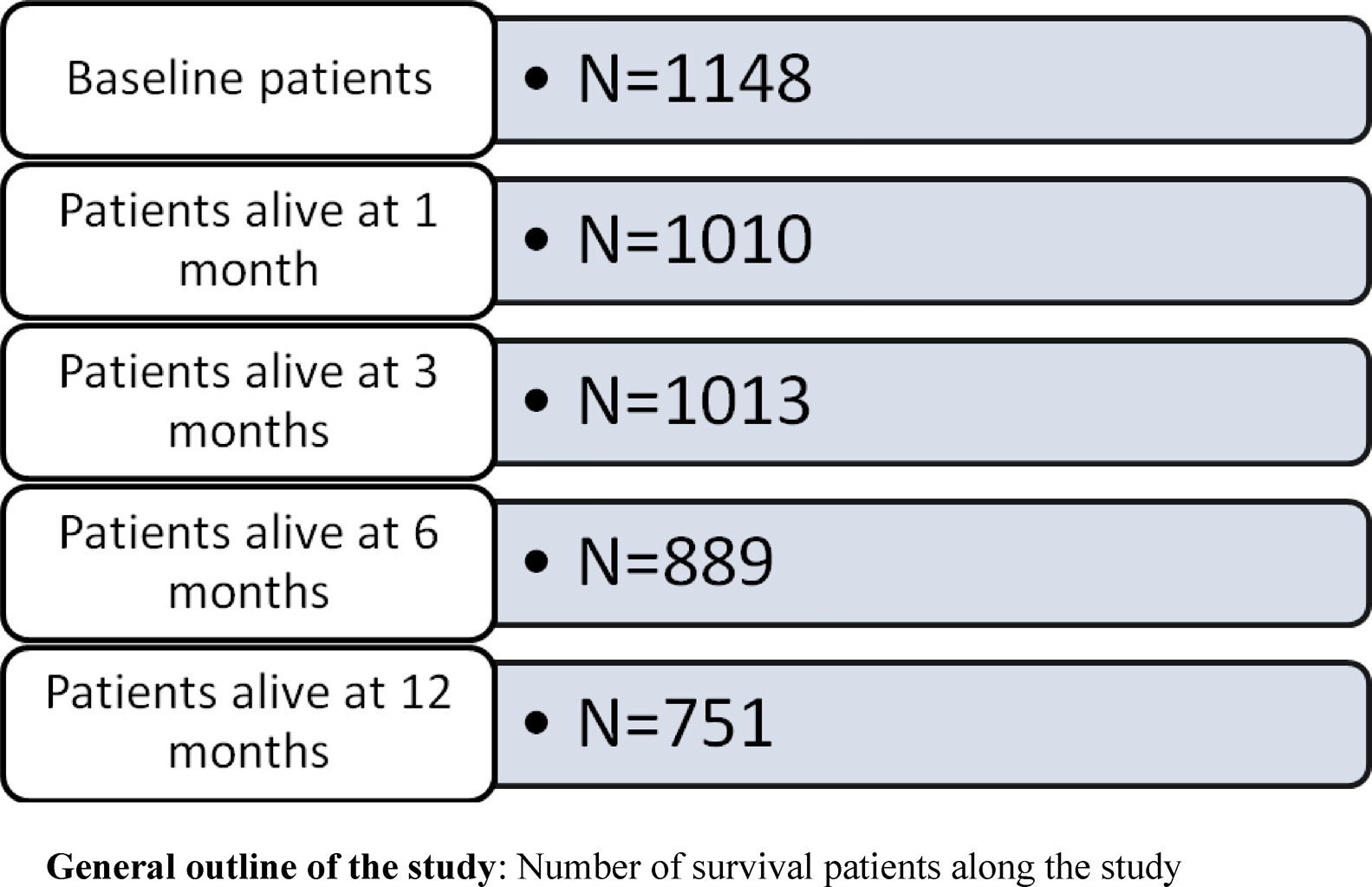
DesignMulticenter cohort study including 1148 HF patients followed for one-year after reaching New York Heart Association (NYHA) IV.
Main measurementsThe primary outcome was all-cause mortality. Multivariate logistic regression models were performed to assess the outcomes at 1, 3, 6, and 12 months.
ResultsMean age of patients was 82 (SD 9) years and women represented 61.7%. A total of 135 (11.8%) and 397 (34.6%) patients died three months and one year after inclusion, respectively. Male gender, age, and decreased body mass index were associated with higher mortality at three, six and twelve months. In addition, low systolic blood pressure levels, severe reduction in glomerular filtration, malignancy, and higher doses of loop diuretics were related to higher mortality from 6 to 12 months.
The most important risk factor over the whole period was presenting a body mass index lower than 20kg/m2 (three months OR 3.06, 95% CI: 1.58–5.92; six months OR 4.42, 95% CI: 2.08–9.38; and 12 months OR 3.68, 95% CI: 1.76–7.69).
ConclusionsWe may conclude that male, age, and decreased body mass index determined higher short-term mortality in NYHA IV. In addition, low systolic blood pressure, reduced glomerular filtration, malignancy, and higher doses of loop diuretics contribute to increasing the risk of mortality at medium and long-term. Such variables are easily measurable and can help to decide the best way to face the most advances stages of the disease.
Analizar los factores que contribuyen a la mortalidad de pacientes en las etapas finales de la insuficiencia cardiaca (IC).
ÁmbitoCentros de atención primaria del Institut Català de la Salut, Cataluña, España.
ParticipantesPacientes con IC avanzada.
DiseñoEstudio de cohortes multicéntrico. Incluyó 1.148 pacientes de IC seguidos durante un año tras el registro de estadio funcional NYHA IV.
Mediciones principalesEl resultado principal fue la mortalidad por todas las causas. Se realizaron modelos de regresión logística multivariada (1, 3, 6 y 12 meses).
ResultadosEdad media 82 años (DE 9), las mujeres representaron el 61,7%. Un total de 135 (11,8%) y 397 (34,6%) pacientes murieron 3 meses y un año después de su inclusión. El sexo masculino, la edad y el índice de masa corporal (IMC)<20kg/m2 se asociaron con una mayor mortalidad a los 3, 6 y 12 meses. Bajos niveles de presión arterial sistólica, reducción severa en el filtrado glomerular, malignidad y dosis altas de diuréticos fueron relacionadas con una mortalidad más alta de 6 a 12 meses.
El factor de riesgo más importante fue un IMC<20kg/m2 (3 meses OR: 3,06; IC 95%: 1,58-5,92; 6 meses OR: 4,42; IC 95%: 2,08-9,38 y 12 meses OR: 3,68; IC 95%: 1,76-7,69).
ConclusionesLos varones, la edad avanzada y un IMC disminuido determinaron una mortalidad a corto plazo más alta en pacientes NYHA IV. La baja presión arterial sistólica, la reducción del filtrado glomerular, la malignidad y las dosis altas de diuréticos aumentan el riesgo de mortalidad a medio y largo plazo. Estas variables son fáciles de obtener, y pueden ayudar a decidir las mejores estrategias para afrontar los estadios más avanzados de la enfermedad.
Heart failure (HF) affects more than 10% of those aged over 70 years, and in individuals over 65 it is the major reason for hospitalization and the third leading cause of death.1,2 The clinical course of HF is progressive and eventually results in advanced stages leading to transplantation or death.3
Although HF symptoms, burden, and mortality are similar to the most prevalent cancers,2,3 little has been published regarding the final stages of advanced HF patients managed mainly in primary care setting. Due to their particular characteristics, such as old age and comorbidities, most of these patients are not candidates for transplantation or implantable devices, and palliative measures are commonly needed. Accurate knowledge regarding the factors related to a curtailed life expectancy would guide health professionals, together with patients and families, in deciding where and when to provide treatment, as well as how to carry out the appropriate palliative and comfort measures.4,5 At these final stages, the relevance of such issues might fluctuate rapidly as the patient's condition becomes increasingly deteriorated.
There are no specifically designed models to describe the prognosis of terminal HF patients, consequently, some of them underestimate the risk of dying, and often include a number of variables not easily obtained in general practice. Such models do not thus properly represent most of the advanced HF population attended in primary care.6–9
The aim of our study was to identify variables accessible in general practice which can determine the impact of the main prognostic factors contributing to mortality at short and long-term in patients at final stages of HF.
MethodsWe carried out a multicenter cohort study based on information from the SIDIAP database (Information System for the Development of Research in Primary Care System). This database contains the daily clinical activities registered by primary care nurses and family physicians. It includes information from over five million patients’ electronic records (EMR).10
Patient populationStudy population were patients older than 44 years registered as having HF (International Classification Diseases 10th version: I.50) at stage IV of the New York Heart Association (NYHA) on 31st December, 2013. To be included in the analysis, patients must have been attended at least once during the study period at any of the 279 primary health care centers.
Patients were included when they were registered as having NYHA IV in the patient's EMR in the period between 1st January, 2010, and 31st December, 2013. All patients had a follow-up for at least one year or until they died during this period.
Variables included in the analysis were: socio demographic (gender, age), cardiovascular risk factors and comorbidities (hypertension, diabetes, coronary heart disease, stroke, and atrial fibrillation), other comorbidities (chronic renal disease, chronic pulmonary disease, and cancer), laboratory tests (sodium, potassium, creatinine, and hemoglobin), considering anemia an hemoglobin <13g/dL in men and 12g/dL in women, and Hyponatremia if Na<140mmol/L, daily living activities assessment, and treatment related to HF (beta-blockers (BB), angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARB), mineral corticoid antagonists (MRA), and loop diuretics). In order to perform the analysis ACEi and ARB were grouped together.
Blood pressure, body mass index, and heart rate were categorized as hypotension (systolic blood pressure (SBP) <90mmHg), low body mass index (<20kg/m2), and high heart rate (>100b/min), respectively.
The main outcome was all-cause mortality (at one, three, six, and twelve months) after NHYA IV registration in EMR.
Statistical analysisContinuous variables were expressed as mean and standard deviation while categorical variables were described as total number and percentages. Univariate analysis between patients’ characteristics (socio-demographic, comorbidities, clinical and analytic information, daily living activities, and medical treatment related to HF) and mortality outcome were performed using student's t test and chi-square test or Fisher's exact test as appropriate. The unadjusted odds ratio was also computed. Multivariate logistic regression analyses were performed to analyze the association of mortality (at one, three, six, and twelve months after reaching the NYHA IV stage of HF) and the patients’ characteristics. A multiple imputation with the predictive mean matching method was carried out to deal with the missing values of the variables considered in the multivariate model. To generate the imputations all the patients’ information was taken into account. Twenty imputations were generated and the values combined using Rubin's rules.11 The significance level was fixed at 0.05. Analysis was performed using R software for Windows version 3.3.2 (R project for statistical computing; Vienna, Austria). The multiple imputations were carried out with the aregImpute function in the Hmisc package.
EthicsAll ethical aspects were taken into consideration. The study protocol was approved by the local Clinical Ethic Committee (reference number P13/052).
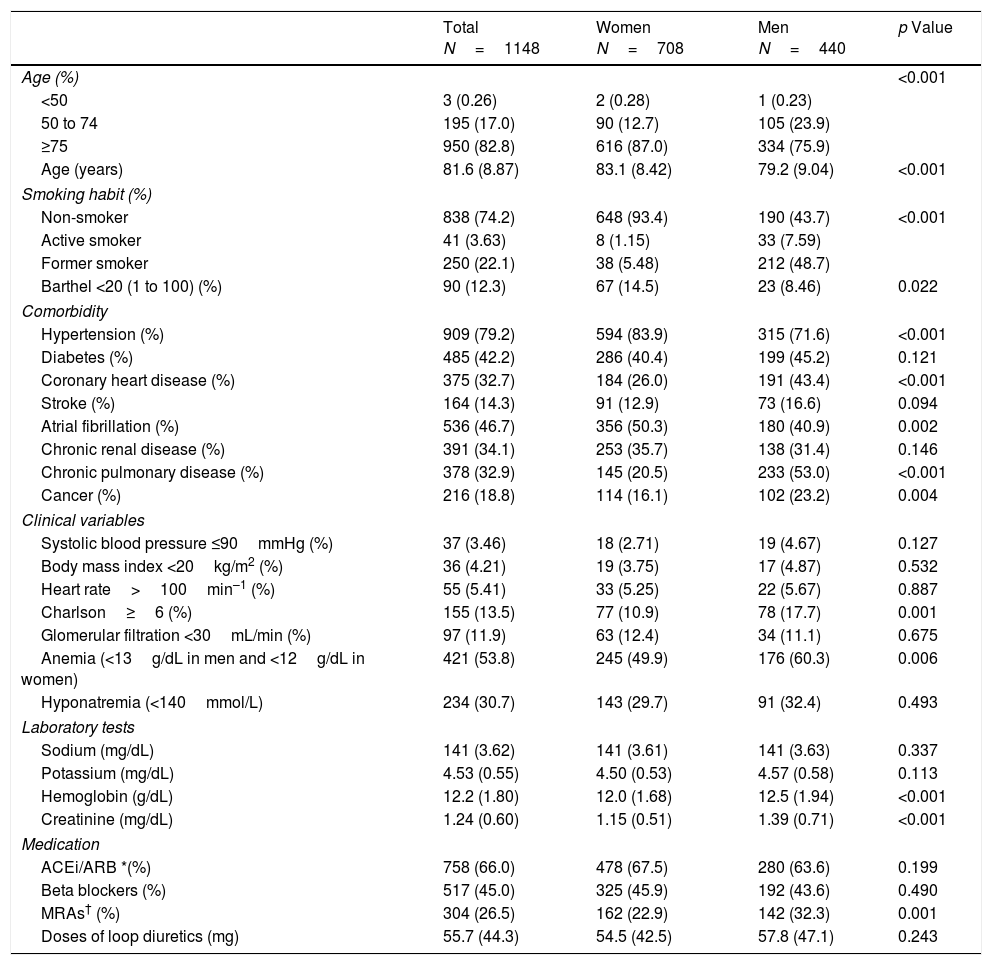
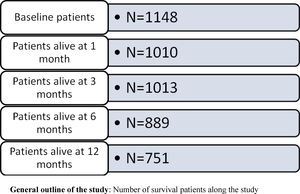
ResultsDescriptive analysisBaseline characteristics of patients are shown in Table 1. Among the 1148 patients, women represented 61.7%, mean age was 82 (SD 9) years, and 82.8% were more than 75 years old. The most prevalent cardiovascular comorbidities at baseline were hypertension (79.2%), atrial fibrillation (46.7%), and diabetes (42.2%). One third of the patients had a history of coronary heart disease and 14.3% had had a previous stroke. Among the non-cardiovascular comorbidities, the most frequent was chronic kidney disease (34.1%). The most commonly prescribed medication were ACEi/ARB (66.6%) followed by BB (45%), and MRA (26.5%).
Baseline characteristics of heart failure patients.
| Total N=1148 | Women N=708 | Men N=440 | p Value | |
|---|---|---|---|---|
| Age (%) | <0.001 | |||
| <50 | 3 (0.26) | 2 (0.28) | 1 (0.23) | |
| 50 to 74 | 195 (17.0) | 90 (12.7) | 105 (23.9) | |
| ≥75 | 950 (82.8) | 616 (87.0) | 334 (75.9) | |
| Age (years) | 81.6 (8.87) | 83.1 (8.42) | 79.2 (9.04) | <0.001 |
| Smoking habit (%) | ||||
| Non-smoker | 838 (74.2) | 648 (93.4) | 190 (43.7) | <0.001 |
| Active smoker | 41 (3.63) | 8 (1.15) | 33 (7.59) | |
| Former smoker | 250 (22.1) | 38 (5.48) | 212 (48.7) | |
| Barthel <20 (1 to 100) (%) | 90 (12.3) | 67 (14.5) | 23 (8.46) | 0.022 |
| Comorbidity | ||||
| Hypertension (%) | 909 (79.2) | 594 (83.9) | 315 (71.6) | <0.001 |
| Diabetes (%) | 485 (42.2) | 286 (40.4) | 199 (45.2) | 0.121 |
| Coronary heart disease (%) | 375 (32.7) | 184 (26.0) | 191 (43.4) | <0.001 |
| Stroke (%) | 164 (14.3) | 91 (12.9) | 73 (16.6) | 0.094 |
| Atrial fibrillation (%) | 536 (46.7) | 356 (50.3) | 180 (40.9) | 0.002 |
| Chronic renal disease (%) | 391 (34.1) | 253 (35.7) | 138 (31.4) | 0.146 |
| Chronic pulmonary disease (%) | 378 (32.9) | 145 (20.5) | 233 (53.0) | <0.001 |
| Cancer (%) | 216 (18.8) | 114 (16.1) | 102 (23.2) | 0.004 |
| Clinical variables | ||||
| Systolic blood pressure ≤90mmHg (%) | 37 (3.46) | 18 (2.71) | 19 (4.67) | 0.127 |
| Body mass index <20kg/m2 (%) | 36 (4.21) | 19 (3.75) | 17 (4.87) | 0.532 |
| Heart rate>100min–1 (%) | 55 (5.41) | 33 (5.25) | 22 (5.67) | 0.887 |
| Charlson≥6 (%) | 155 (13.5) | 77 (10.9) | 78 (17.7) | 0.001 |
| Glomerular filtration <30mL/min (%) | 97 (11.9) | 63 (12.4) | 34 (11.1) | 0.675 |
| Anemia (<13g/dL in men and <12g/dL in women) | 421 (53.8) | 245 (49.9) | 176 (60.3) | 0.006 |
| Hyponatremia (<140mmol/L) | 234 (30.7) | 143 (29.7) | 91 (32.4) | 0.493 |
| Laboratory tests | ||||
| Sodium (mg/dL) | 141 (3.62) | 141 (3.61) | 141 (3.63) | 0.337 |
| Potassium (mg/dL) | 4.53 (0.55) | 4.50 (0.53) | 4.57 (0.58) | 0.113 |
| Hemoglobin (g/dL) | 12.2 (1.80) | 12.0 (1.68) | 12.5 (1.94) | <0.001 |
| Creatinine (mg/dL) | 1.24 (0.60) | 1.15 (0.51) | 1.39 (0.71) | <0.001 |
| Medication | ||||
| ACEi/ARB *(%) | 758 (66.0) | 478 (67.5) | 280 (63.6) | 0.199 |
| Beta blockers (%) | 517 (45.0) | 325 (45.9) | 192 (43.6) | 0.490 |
| MRAs† (%) | 304 (26.5) | 162 (22.9) | 142 (32.3) | 0.001 |
| Doses of loop diuretics (mg) | 55.7 (44.3) | 54.5 (42.5) | 57.8 (47.1) | 0.243 |
Values are given as mean (standard deviation) or frequency (percentage).
Women were more commonly older, hypertensive, and had atrial fibrillation whilst men suffered more frequently from coronary heart disease, chronic obstructive pulmonary disease, and cancer. Mineral corticoid receptors antagonist were more frequently prescribed in men and no differences were found regarding the rest of the medication.
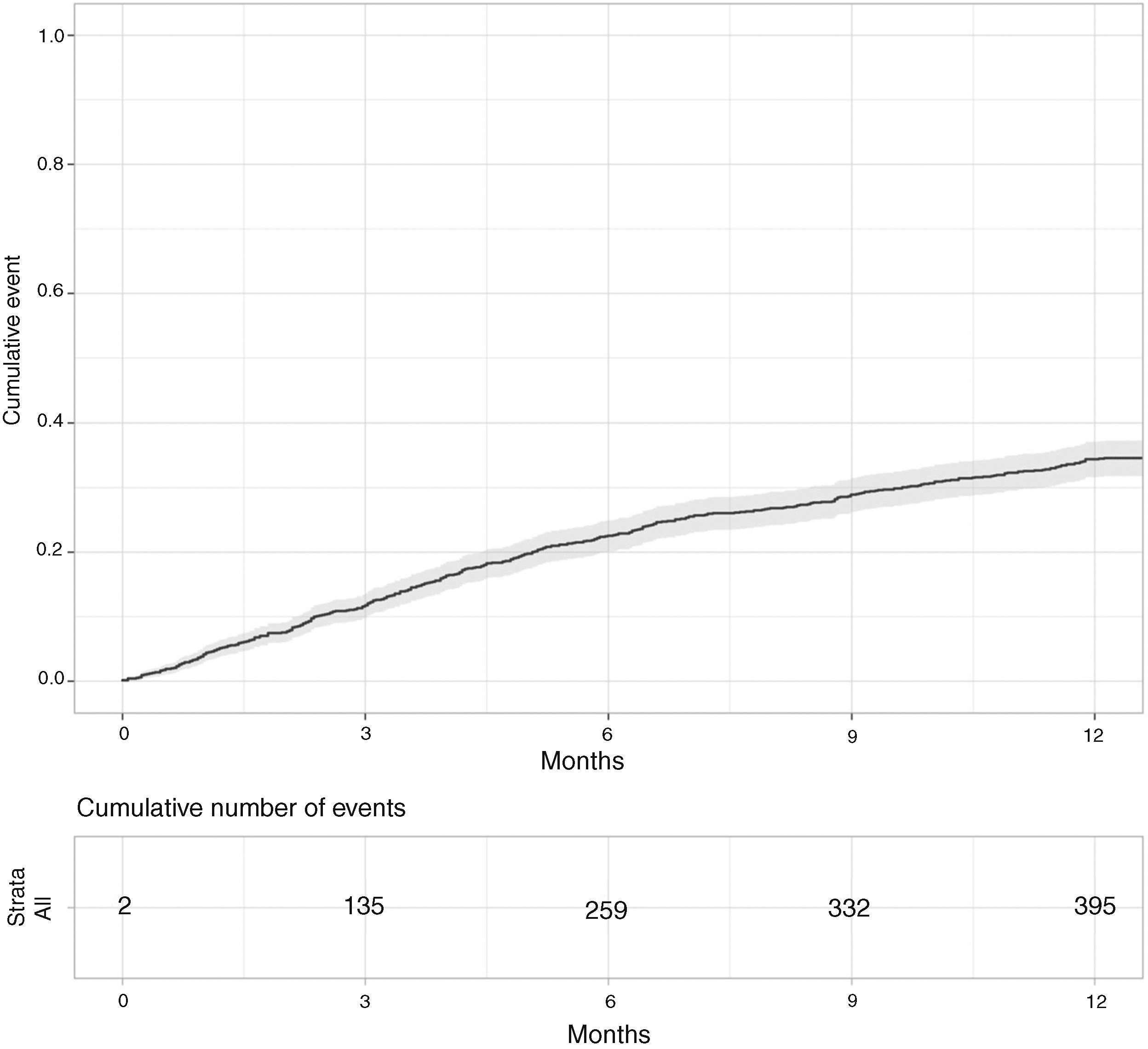
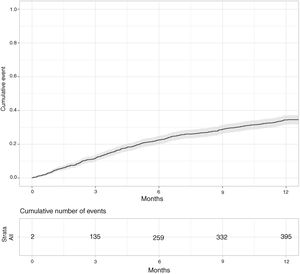
Fig. 1 depicts the evolution in mortality during the first year after reaching NYHA IV. It can be observed that the slope of the curve was higher in the first six months.
Univariate analysisDuring the first year mortality constantly increased (4.2% at 1 month, 11.8% at 3 months, 22.6% at 6 months, and 34.6% at 12 months, respectively).
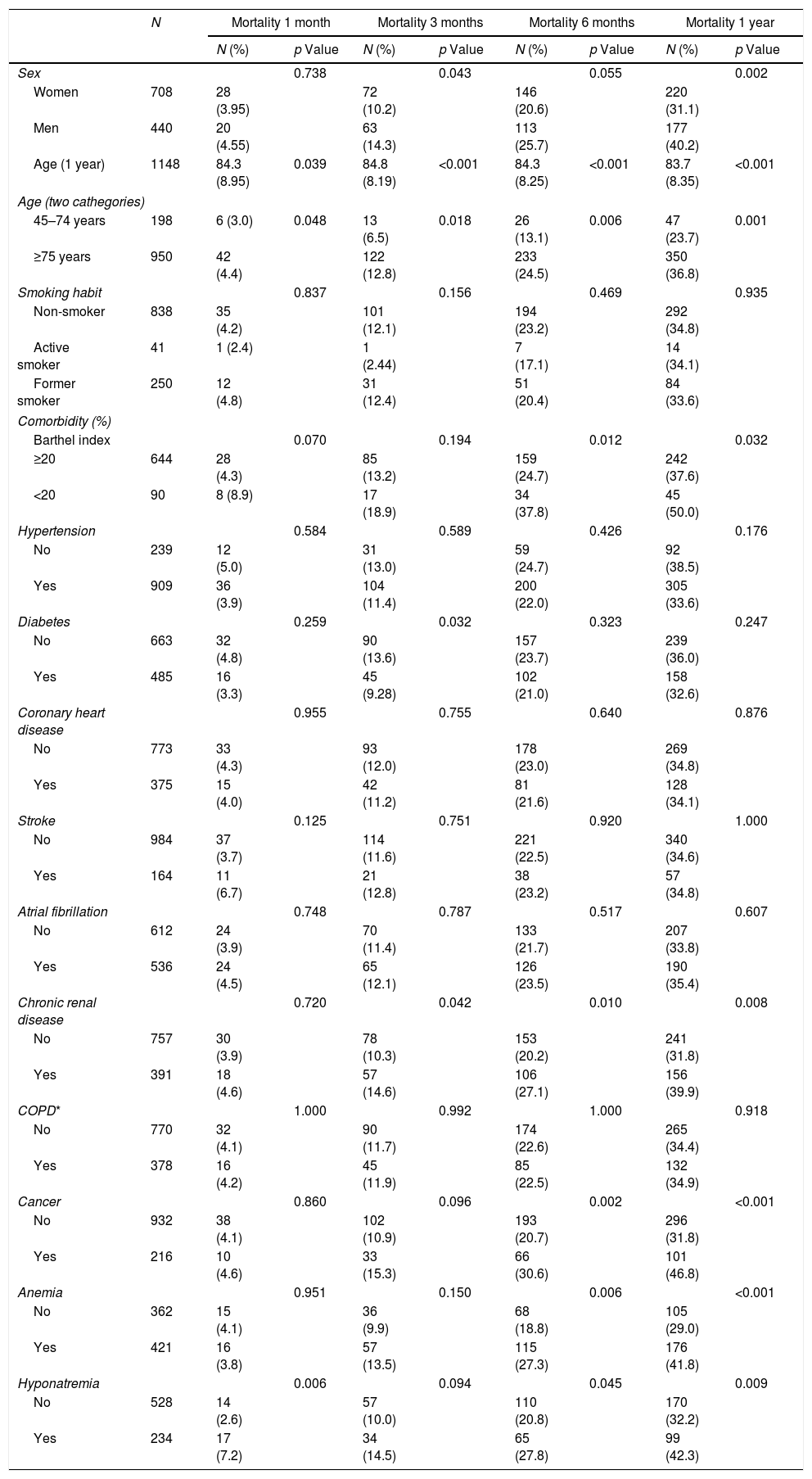
Cardiovascular comorbidity did not significantly affect mortality at any time during the first year after reaching NYHA IV. Only age and hyponatremia were related to higher mortality at very early stages (one month). From the sixth month onwards, male gender, age, high dependency on daily activities, cancer, hyponatremia, chronic renal disease, low SBP, and decreased body mass index were associated with a higher risk of mortality. Reduced levels of hemoglobin and raised loop diuretic doses were related to mortality at six and twelve months (Table 2).
Risk factors related to mortality at 1, 3, 6, and 12 months.
| N | Mortality 1 month | Mortality 3 months | Mortality 6 months | Mortality 1 year | |||||
|---|---|---|---|---|---|---|---|---|---|
| N (%) | p Value | N (%) | p Value | N (%) | p Value | N (%) | p Value | ||
| Sex | 0.738 | 0.043 | 0.055 | 0.002 | |||||
| Women | 708 | 28 (3.95) | 72 (10.2) | 146 (20.6) | 220 (31.1) | ||||
| Men | 440 | 20 (4.55) | 63 (14.3) | 113 (25.7) | 177 (40.2) | ||||
| Age (1 year) | 1148 | 84.3 (8.95) | 0.039 | 84.8 (8.19) | <0.001 | 84.3 (8.25) | <0.001 | 83.7 (8.35) | <0.001 |
| Age (two cathegories) | |||||||||
| 45–74 years | 198 | 6 (3.0) | 0.048 | 13 (6.5) | 0.018 | 26 (13.1) | 0.006 | 47 (23.7) | 0.001 |
| ≥75 years | 950 | 42 (4.4) | 122 (12.8) | 233 (24.5) | 350 (36.8) | ||||
| Smoking habit | 0.837 | 0.156 | 0.469 | 0.935 | |||||
| Non-smoker | 838 | 35 (4.2) | 101 (12.1) | 194 (23.2) | 292 (34.8) | ||||
| Active smoker | 41 | 1 (2.4) | 1 (2.44) | 7 (17.1) | 14 (34.1) | ||||
| Former smoker | 250 | 12 (4.8) | 31 (12.4) | 51 (20.4) | 84 (33.6) | ||||
| Comorbidity (%) | |||||||||
| Barthel index | 0.070 | 0.194 | 0.012 | 0.032 | |||||
| ≥20 | 644 | 28 (4.3) | 85 (13.2) | 159 (24.7) | 242 (37.6) | ||||
| <20 | 90 | 8 (8.9) | 17 (18.9) | 34 (37.8) | 45 (50.0) | ||||
| Hypertension | 0.584 | 0.589 | 0.426 | 0.176 | |||||
| No | 239 | 12 (5.0) | 31 (13.0) | 59 (24.7) | 92 (38.5) | ||||
| Yes | 909 | 36 (3.9) | 104 (11.4) | 200 (22.0) | 305 (33.6) | ||||
| Diabetes | 0.259 | 0.032 | 0.323 | 0.247 | |||||
| No | 663 | 32 (4.8) | 90 (13.6) | 157 (23.7) | 239 (36.0) | ||||
| Yes | 485 | 16 (3.3) | 45 (9.28) | 102 (21.0) | 158 (32.6) | ||||
| Coronary heart disease | 0.955 | 0.755 | 0.640 | 0.876 | |||||
| No | 773 | 33 (4.3) | 93 (12.0) | 178 (23.0) | 269 (34.8) | ||||
| Yes | 375 | 15 (4.0) | 42 (11.2) | 81 (21.6) | 128 (34.1) | ||||
| Stroke | 0.125 | 0.751 | 0.920 | 1.000 | |||||
| No | 984 | 37 (3.7) | 114 (11.6) | 221 (22.5) | 340 (34.6) | ||||
| Yes | 164 | 11 (6.7) | 21 (12.8) | 38 (23.2) | 57 (34.8) | ||||
| Atrial fibrillation | 0.748 | 0.787 | 0.517 | 0.607 | |||||
| No | 612 | 24 (3.9) | 70 (11.4) | 133 (21.7) | 207 (33.8) | ||||
| Yes | 536 | 24 (4.5) | 65 (12.1) | 126 (23.5) | 190 (35.4) | ||||
| Chronic renal disease | 0.720 | 0.042 | 0.010 | 0.008 | |||||
| No | 757 | 30 (3.9) | 78 (10.3) | 153 (20.2) | 241 (31.8) | ||||
| Yes | 391 | 18 (4.6) | 57 (14.6) | 106 (27.1) | 156 (39.9) | ||||
| COPD* | 1.000 | 0.992 | 1.000 | 0.918 | |||||
| No | 770 | 32 (4.1) | 90 (11.7) | 174 (22.6) | 265 (34.4) | ||||
| Yes | 378 | 16 (4.2) | 45 (11.9) | 85 (22.5) | 132 (34.9) | ||||
| Cancer | 0.860 | 0.096 | 0.002 | <0.001 | |||||
| No | 932 | 38 (4.1) | 102 (10.9) | 193 (20.7) | 296 (31.8) | ||||
| Yes | 216 | 10 (4.6) | 33 (15.3) | 66 (30.6) | 101 (46.8) | ||||
| Anemia | 0.951 | 0.150 | 0.006 | <0.001 | |||||
| No | 362 | 15 (4.1) | 36 (9.9) | 68 (18.8) | 105 (29.0) | ||||
| Yes | 421 | 16 (3.8) | 57 (13.5) | 115 (27.3) | 176 (41.8) | ||||
| Hyponatremia | 0.006 | 0.094 | 0.045 | 0.009 | |||||
| No | 528 | 14 (2.6) | 57 (10.0) | 110 (20.8) | 170 (32.2) | ||||
| Yes | 234 | 17 (7.2) | 34 (14.5) | 65 (27.8) | 99 (42.3) | ||||
| Total | Mortality 1 month | Mortality 3 months | Mortality 6 months | Mortality 1 year | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | N (%) | p Value | N (%) | p Value | N (%) | p Value | N (%) | p Value | |
| Clinical variables (%) | |||||||||
| Systolic blood pressure | 0.070 | 0.072 | 0.001 | 0.006 | |||||
| >90mmHg | 1033 | 42 (4.07) | 120 (11.6) | 227 (22.0) | 347 (33.6) | ||||
| ≤90mmHg | 37 | 4 (10.8) | 8 (21.6) | 17 (45.9) | 21 (56.8) | ||||
| Body mass index | 0.137 | <0.001 | <0.001 | <0.001 | |||||
| ≥20kg/m2 | 819 | 28 (3.42) | 74 (9.04) | 149 (18.2) | 244 (29.8) | ||||
| <20kg/m2 | 36 | 3 (8.33) | 11 (30.6) | 19 (52.8) | 23 (63.9) | ||||
| Heart rate | 0.728 | 0.207 | 0.294 | 0.809 | |||||
| ≤100min–1 | 961 | 41 (4.27) | 111 (11.6) | 212 (22.1) | 325 (33.8) | ||||
| >100min–1 | 55 | 3 (5.45) | 10 (18.2) | 16 (29.1) | 20 (36.4) | ||||
| Charlson | 0.384 | 0.942 | 0.078 | 0.012 | |||||
| <6 | 993 | 39 (3.93) | 116 (11.7) | 215 (21.7) | 329 (33.1) | ||||
| ≥6 | 155 | 9 (5.81) | 19 (12.3) | 44 (28.4) | 68 (43.9) | ||||
| Glomerular filtration | 1.000 | 0.032 | 0.001 | <0.001 | |||||
| ≥30mL/min | 719 | 29 (4.03) | 76 (10.6) | 150 (20.9) | 233 (32.4) | ||||
| <30mL/min | 97 | 3 (3.09) | 18 (18.6) | 36 (37.1) | 52 (53.6) | ||||
| Medication (%) | |||||||||
| ACEi/ARB† | 0.004 | 0.040 | 0.009 | <0.001 | |||||
| No | 390 | 26 (6.67) | 57 (14.6) | 106 (27.2) | 162 (41.5) | ||||
| Yes | 758 | 22 (2.90) | 78 (10.3) | 153 (20.2) | 235 (31.0) | ||||
| Beta blockers | 0.741 | 0.672 | 0.085 | 0.016 | |||||
| No | 631 | 28 (4.44) | 77 (12.2) | 155 (24.6) | 238 (37.7) | ||||
| Yes | 517 | 20 (3.87) | 58 (11.2) | 104 (20.1) | 159 (30.8) | ||||
| MRAs‡ | 0.550 | 1.000 | 0.759 | 0.450 | |||||
| No | 844 | 33 (3.91) | 99 (11.7) | 188 (22.3) | 286 (33.9) | ||||
| Yes | 304 | 15 (4.93) | 36 (11.8) | 71 (23.4) | 111 (36.5) | ||||
| Doses of loop diuretics (Mean, SD§) | 1148 | 57.9 (49.4) | 0.763 | 60.0 (48.9) | 0.287 | 61.7 (48.8) | 0.025 | 60.8 (47.3) | 0.008 |
| Laboratory tests (Mean, SD) | |||||||||
| Sodium (mg/dL) | 1148 | 140 (4.12) | 0.094 | 140 (4.13) | 0.512 | 141 (4.08) | 0.361 | 140 (3.99) | 0.143 |
| Potassium (mg/dL) | 1148 | 4.53 (0.59) | 0.982 | 4.48 (0.63) | 0.466 | 4.54 (0.62) | 0.799 | 4.56 (0.59) | 0.304 |
| Creatinine (mg/dL) | 1148 | 1.25 (0.58) | 0.932 | 1.39 (0.69) | 0.026 | 1.36 (0.73) | 0.007 | 1.34 (0.65) | 0.001 |
| Hemoglobin (g/dL) | 1148 | 12.1 (2.02) | 0.869 | 11.8 (1.91) | 0.083 | 11.8 (1.90) | 0.003 | 11.9 (1.89) | 0.001 |
Some variables had missing values, especially the Barthel index (36%), hemoglobin levels (32%), and glomerular filtration rate (29%). We present the final adjusted multivariate models after performing multiple missing imputation.
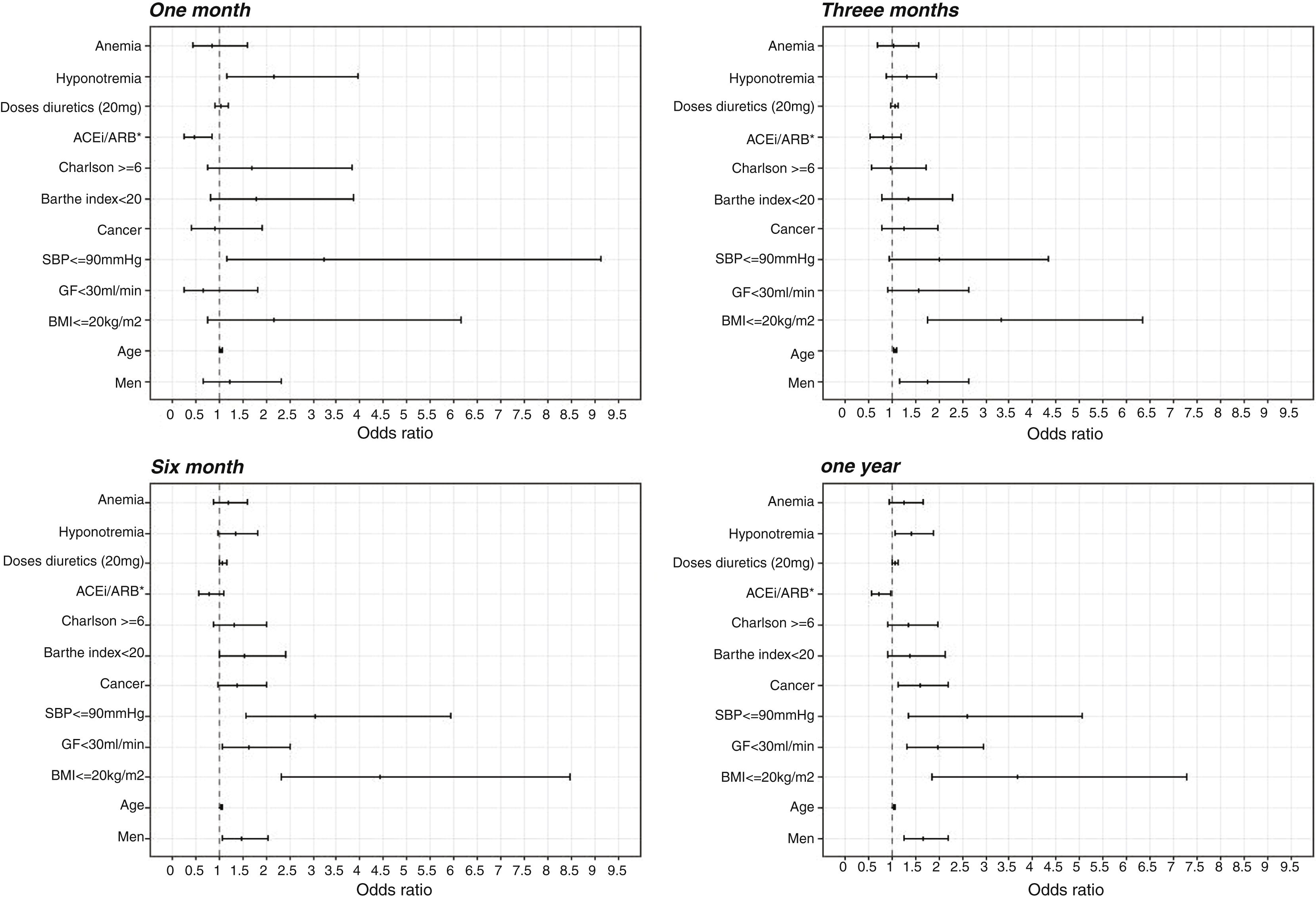
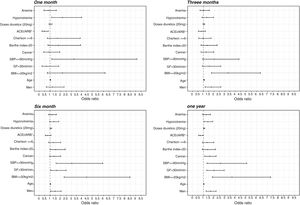
In the first month the prescription of ACE inhibitors or ARB resulted in a protective effect (OR 0.46 95% CI 0.26–0.84). This effect remained along the first year but was not statistically significant from the third month onwards (Fig. 2).
Three months after reaching NYHA IV the main factors related to higher mortality were male gender, increased age, and reduced body mass index. Six months after reaching NYHA IV the effect of the former variables remained significant and other variables presented statistical significance in the multivariate model, such as reduced glomerular filtration, low SBP, cancer, and the loop diuretic doses used to manage HF.
DiscussionOur study, based on real world data from clinical records, adds information to previous evidence about HF prognosis. It describes the evolution and impact short-term, and at one year, of the main prognostic factors after reaching NYHA IV, and is specifically focused on stable, advanced HF patients managed in primary care. At three months, only age, male gender, and reduced body mass index resulted in higher mortality. At six and 12 months these factors were still related to higher mortality with the addition of decreased glomerular filtration, low SBP, cancer, and higher doses of furosemide.
A number of scores have been developed to predict the prognosis of HF patients, in most of them, however, survival was analyzed at long-term (one year or more), participants were recruited in hospitals, the proportion of NYHA IV was lower than 10%, and many findings were based on variables generally complicated to obtain in non-hospital settings.12–15 With respect to mortality, the PRAISE study was conducted in patients with NYHA IIIb and IV and showed an annual rate of 11%. The CHARM study included a sample of 173 patients with NYHA IV and observed a figure of 29%, which was closer to our findings.16,17 Ambardekaer et al. also reported a high rate of mortality after a 16-month follow-up in a cohort of ambulatory advanced HF patients who were not eligible for transplantation/left ventricular assisted device.18 Currently, morbidity and mortality for individuals with advanced HF continues to be elevated and comorbidity common, since most patients are elderly, as previously described.19
Comparative analysis of mortality determinantsRegarding gender, it has already been argued that women present higher comorbidity (with the exception of coronary heart disease such as HF) and symptom severity than men, but better survival. It is plausible to think that this could have influenced prognosis, nevertheless, it does not completely explain differences in mortality between genders.20
Patients with SBP levels lower than 90mmHg had a more than two-fold risk of death in comparison with the others, as previously observed by Vidán et al. They justified this result by the fact that patients with higher SBP on hospital admission were younger, healthier, and received more aggressive treatment. In addition, the pathophysiology might differ in patients with HF and higher blood pressure.21 Similar results were also reported in advanced chronic systolic HF patients although the SBP cut-off was 120mm Hg. The authors hypothesized that their findings could be related to a greater incidence of ischemic heart disease in patients with lower blood pressure.22 In our study such an effect on mortality was found to be independent of age, treatment, and comorbidity. The latest version of the European Society of Cardiology Clinical Practice Guidelines includes hypotension as a factor of poor ischemic cardiomyopathy prognosis and proposes these patients be candidates for the implantation of assistive devices.23
In relation to the patients’ weight, in our study, the risk of dying for patients with a body mass index <20kg/m2 was more than three times higher than in the rest. Considerable research has been devoted to the role body mass index plays in HF patients.24 It has been hypothesized that individuals with chronic HF and obesity have significantly lower sympathetic activation. This may partially explain the obesity paradox described in chronic HF patients.25 The higher mortality related to low body mass index has been associated with higher Tumoral Necrosis Factor-alfa, adiponectin, troponin T and pulmonary arterial systolic pressure.26 Whilst we could not measure either cachexia or sarcopenia in our cohort we did make an approximation of the obesity effect by measuring body mass index. It has been shown to accurately correlate with the thickness of the subcutaneous fat layer and is accepted as a measure of obesity in HF.27
Most HF prognostic scores consider glomerular filtration as an independent factor of death. We found an almost two-fold odds ratio of mortality in patients presenting a glomerular filtration <30mL/min. When attempts to alleviate congestion are limited by impaired renal function the cardiorenal syndrome appears.28 It consists of a neuro hormonal adaptation leading to renal-reduced perfusion and right ventricular dysfunction. Its prevalence is around 60% in patients with acute decompensation and up to 51% mortality has been reported when glomerular filtration is <53mL/min.29
Although it may appear obvious that HF patients requiring higher loop diuretic doses might be more unwell than those receiving lower ones, the powerful and independent association with mortality warrants further consideration. Loop diuretic activation of the renin-angiotensin-aldosterone and sympathetic nervous systems has been shown to play a major role in the pathophysiology of HF and may be associated with HF progression.30
The protective effect on early mortality of ACEi/ARB disappeared along the follow up. We cannot ascertain why this effect is not maintained, but probably may be due to the fact of the advanced stage of the disease, where could be hard to lengthen the survival with any intervention.
An association between anemia and increased all-cause and short-term cardiovascular mortality has been reported.31 In our study, the trend regarding anemia was toward a higher risk although it was not statistically significant at multivariate analysis.
Strengths and limitationsOur main limitation is the retrospective design and the fact that data were gathered from electronic medical records. Nevertheless, SIDIAP has already proven to be a valid source for cardiovascular disease research.10 Some variables presented missing values which were imputed in order to avoid substantial loss of precision and power in the statistical analysis. A strength is the large cohort of patients with stable, advanced HF managed in the community.
It would be necessary further studies to properly explain some of the findings, such as the role of ACEi/ARB on the survival of patients at advances stages of HF.
Nevertheless the initial objective of our study was not to test the efficacy of treatments in enlarging the survival but to identify factors related with a poorer prognosis in order to be able to discuss with the patients and their families the best way to face the end of life.
ConclusionsAmong stable, ambulatory HF patients, male gender, age, and low body mass index are involved in higher mortality at both short and long-term after reaching NYHA IV stages. In addition, low SBP, reduced glomerular filtration, malignancy, and higher necessity in the doses of loop diuretics contribute to increasing the risk of mortality at medium and long-term. Such variables are easily measurable in primary care and may help health professionals identify HF patients with worse outcomes and decide with patients and their families the best way to face the most advanced stages of the disease.
- 1.
Clinical course of HF is progressive and, in advanced stages leads to a number of hospitalizations, transplantation or death.
- 2.
Palliative measures can help to better manage patients at final stages.
- 3.
Since the uncertainty of the evolution at final stages it is difficult to ascertain which patients could benefit from homecare and palliative measures.
- 4.
To know the factors related to a worse short term prognostic may help to take decisions about their care.