To investigate the optimal waist circumference (WC) cut-off points in the general Spanish-population and its relationships with insulin resistance (IR) and metabolic syndrome (MS) due to the disparity of values WC in the different regional areas.
MethodsA multicenter nationwide Spanish population based study on 3844 unrelated subjects, aged 35–74 years. Receiver operating characteristic (ROC) curve was analysed to identify the optimal WC cut-off points for detecting metabolic abnormalities related to the MS.
ResultsThe prevalence of MS, International Diabetes Federation (IDF) criteria: 29.1%/33.1% in males/females (P=.004), Adult Treatment Panel III (ATPIII) criteria: 23.5%/30.9% males/females (P<.001).
Optimal WC cut-off to obtain the maximal sensitivity and specificity for detecting two or more of the other metabolic abnormalities associated with MS or IR was 94.5/89.5cm in males/females. According to these cut-off points, the prevalence of abdominal-obesity was 57.2%/61.3% in males/females, P=.011 and prevalence of MS was similar in both genders (males/females: 27.9%/28.9%, P=.527).
The Spearman correlation coefficient relating WC to HOMA IR was 0.395. IR was similarly prevalent in males/females 24.1%/21.7% (P<.088). Prevalence of IR was 43% in subjects with MS by IDF criteria.
ConclusionsThe new WC cut-off points found in our population were 94.5/89.5cm for males/females. For males, the cut-off points were lower than those defined by ATPIII but similar to IDF criteria. For females these cut-off points were higher than those proposed by ATPIII/IDF criteria.
Investigar los puntos de corte óptimos para la circumferencia de la cintura (CC) en la población general española y su relación con resistencia a la insulina (RI) y Síndrome Metabólico (SM), debido a la disparidad de valores de la CC en las diferentes áreas.
MétodosEstudio multicéntrico a nivel nacional: se estudiaron 3,844 sujetos no relacionados, de 35-74 años. Se aplicó la curva COR (Curva de rendimiento diagnóstico) para identificar los puntos de corte óptimos para la CC para la detección de anormalidades metabólicas relacionadas con el SM.
ResultadosLa prevalencia de SM, criterio IDF: 29.1%/33.1% varones/mujeres (p= 0.004), criterio ATPIII: 23.5%/30.9% varones/mujeres (p< 0.001).
El punto de corte óptimo para la CC con máxima sensibilidad y especificidad para detectar 2 o más anormalidades metabólicas asociadas con SM o RI fue 94.5cm/89.5cm en varones/mujeres. De acuerdo con estos puntos de corte la prevalencia de obesidad-abdominal fue de 57.2%/61.3% en varones/mujeres, p= 0.011 y la prevalencia de SM fue similar en ambos sexos (varones/mujeres: 27.9%/28.9%, p = 0.527).
El coeficiente de correlación de Spearman en relación a CC con RI fue 0.395. La prevalencia de RI fue similar en varones/mujeres: 24.1%/21.7% (p < 0.088). La prevalencia de RI en sujetos con SM por criterio IDF fue 43%.
ConclusionesLos nuevos puntos de corte para la CC en nuestra población fueron 94.5/89.5cm para vaones/mujeres. Para varones los puntos de corte fueron más bajos que los definidos por criterio ATPIII, pero similar a los propuestos por criterio IDF. Para mujeres los puntos de corte fueron más elevados que los propuestos por criterios ATPIII/IDF.
Obesity is a major Public Health epidemic problem worldwide, both in adults and children.1 Obesity and overweight have been associated with an increased risk of diseases such as diabetes, heart disease, arthritis, and cancer.2–4 Furthermore, obesity, particularly abdominal type (AO), is associated with increased morbidity and mortality.5
The metabolic syndrome (MS) is considered to be a clustering of metabolic and non-metabolic risk factors that increase the risk for developing type 2 diabetes mellitus.6
In 2001, the National Cholesterol Education Program (ATPIII) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Expert Panel on Detection, Evaluation, and Treatment Panel III 2001)7 introduced a new definition for MS. One of the criteria of this definition, (fasting glucose≥100mg/dl), was modified in 2004.8
In 2005, the International Diabetes Federation (IDF) proposed a new definition9 in which the presence of AO was considered essential and the role of ethnicity in the relation of AO to other metabolic risk factors is taken into account. Many studies have indicated that AO10,11 and IR12 are major underlying mechanisms for MS.
Moreover, the new IDF definition requires ethnic specific cut-off points values for waist circumference (WC) (≥94 and ≥80cm for European men and women, respectively).
In 2009, a Joint Interim report13 by the IDF Task Force on Epidemiology and Prevention in collaboration with other expert Committees of the National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity declared that obesity should not be an obligatory component, but that “national or regional cut-off points for WC can be used”.Overall, those reported values for waist circumference are very variable in different geographical areas in Spain.14,15 Due to the disparity of WC values in Spain, we decided to search for WC cut-off point values for our population through an extensive multicentre nationwide Spanish population based study in our country to detect IR and MS.
Design, populationWe studied 4097 subjects from the general population of Spain. Details of recruitment and study protocols of this population-based survey were previously described.16,17 In brief, all men and non-pregnant women aged 35–74 years were invited to participate. Two hundred and fifty-four subjects were excluded, because they met one or more of the following exclusion criteria: type 1 diabetes, overt heart or hepatic failure, surgery during the previous year, weight changes >5kg within the previous 6 months, and hospitalization. A total of 3844 subjects completed the study, 1754 males and 2090 females. We used standard procedures adapted from the WHO MONICA protocol,18 which was approved by the Institutional Review Board. All participants gave written informed consent.
Interviewers trained to obtain the following data administered a medical questionnaire: age, sex, parity, menopausal status, family history of diabetes, and treatment of diabetes, hypertension, and other relevant chronic diseases. Weight, height (BMI was calculated: kg/m2) and WC (cm) at the level of the umbilicus were measured by trained personnel. The reliability of the anthropometric measurements was established by comparing values obtained by three interviewers in a sample of individuals.
Procedures and laboratory studiesAfter an overnight fasting period, 20ml of blood were obtained from an antecubital vein without compression. Plasma glucose was determined in duplicate by a glucose-oxidase method adapted to an Autoanalyzer (Hitachi 704, Boehringer Mannheim, Germany). Total cholesterol, triglycerides and high-density lipoprotein cholesterol (HDL-C) were determined by enzymatic methods using commercial kits (Boehringer, Mannheim, Germany). Low-density lipoprotein cholesterol (LDL-C) was calculated by the Friedewald formula.
A 75-g oral glucose tolerance test (OGTT) was performed and interpreted according to the revised 2003 criteria of the American Diabetes Association.19 Diabetes mellitus was diagnosed when fasting plasma glucose was ≥7.0mmol/l or 2-h post glucose≥11.1mmol/l. Subjects on antidiabetic medications were also considered to have diabetes. In non-diabetic subjects, a fasting plasma glucose 5.6–6.9mmol/l was indicative of impaired fasting glucose (IFG) and a 2-h glucose of ≥7.8–11.0mmol/l of impaired glucose tolerance (IGT).
Serum insulin concentrations were determined by RIA (Human Insulin Specific RIA kit, Linco Research Inc., St Louis, MO, USA). This assay had a lower detection limit of 2μU/ml with within and between assay coefficients of variation of <1% and <7.43%, respectively. Cross reactivity with proinsulin was less than 0.2%.
IR was estimated by homeostasis model assessment of IR (HOMA-IR) using the following formula20: fasting insulin (μU/ml)×fasting glucose (mmol/l)/22.5. In subjects without clinical or biological parameters of IR, the 90th percentile for the HOMA-IR was equal to or greater than 3.8, and this value was considered diagnostic of IR.21 Obesity was defined as BMI≥30kg/m2. We used the following definitions of the MS: modified ATPIII8 and IDF.9 As detailed in the ATPIII report, participants with 3 or more of the following criteria were defined as having the MS: AO: WC>102cm in males and >88cm in females; hypertriglyceridemia (HTG), triglyceride (TG) levels≥150mg/dl (1.7mmol/l); low HDL-C: <40mg/dl (0.9mmol/l) in males and <50mg/dl (1.1mmol/l) in females; elevated blood pressure: systolic blood pressure (SBP)≥130mmHg, diastolic blood pressure (DBP)≥85mmHg, and/or treatment with antihypertensive medications; and elevated fasting glucose (FG)≥100mg/dl (5.6mmol/l) and/or treatment with antidiabetic medications.
The IDF definition of MS9 required central obesity (defined as WC≥94cm for males and ≥80cm for females) plus any two of the following four criteria: HTG, TG levels>150mg/dl (1.7mmol/l), or specific treatment for this lipid abnormality; low HDL-C: <40mg/dl (0.9mmol/l) in males and <50mg/dl (1.1mmol/l) in females, or specific treatment for this lipid abnormality, elevated blood pressure: SBP≥130mmHg, DBP≥85mmHg, and/or treatment of previously diagnosed hypertension; and elevated FG≥100mg/dl (5.6mmol/l) or previously diagnosed type 2 diabetes.
Statistical methodsThe Student's t test or ANOVA was used to compare continuous variables expressed as means±standard deviation (SD). We used logarithmic transformation of variables that were not normally distributed. The Bonferroni significance correction was used when comparing more than two means. Dichotomous variables were compared using the Chi-square test. Spearman's rank correlation was applied to study the associations between WC and IR. The subjects with treated type 2 diabetes mellitus (n=120) were excluded. The receiver operator characteristic (ROC) curve was used to determine the discriminative point for WC. We used the efficiency point of maximum specificity and maximum sensitivity (Youden index). We used the area under of curve (AUC) with 95% confidence intervals (CI) as the parameter of performance of the cut-off points for WC. Logistic regression models were adjusted by HOMA IR, sex and age. The null hypothesis was rejected in each statistical test with a P<.05. The statistical analyses were performed using Windows SPSS version 15.0 software.
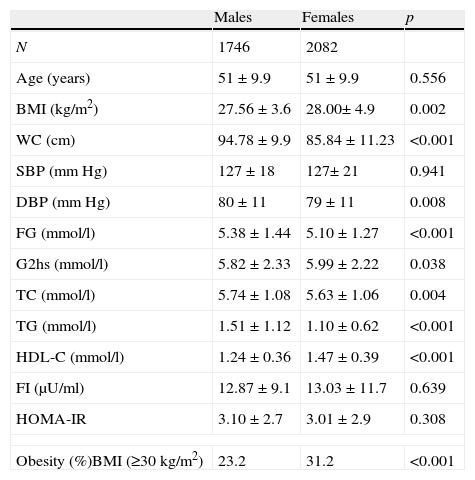
ResultsThe characteristics of subjects are shown in Table 1. WC, DBP, FG, total cholesterol (TC), TG, and LDL-C values were significantly higher in males than in females. However, values of BMI, 2-h glucose, HDL-C, and obesity were significantly higher in females.
Characteristics of population by sex.
| Males | Females | p | |
| N | 1746 | 2082 | |
| Age (years) | 51±9.9 | 51±9.9 | 0.556 |
| BMI (kg/m2) | 27.56±3.6 | 28.00±4.9 | 0.002 |
| WC (cm) | 94.78±9.9 | 85.84±11.23 | <0.001 |
| SBP (mmHg) | 127±18 | 127±21 | 0.941 |
| DBP (mmHg) | 80±11 | 79±11 | 0.008 |
| FG (mmol/l) | 5.38±1.44 | 5.10±1.27 | <0.001 |
| G2hs (mmol/l) | 5.82±2.33 | 5.99±2.22 | 0.038 |
| TC (mmol/l) | 5.74±1.08 | 5.63±1.06 | 0.004 |
| TG (mmol/l) | 1.51±1.12 | 1.10±0.62 | <0.001 |
| HDL-C (mmol/l) | 1.24±0.36 | 1.47±0.39 | <0.001 |
| FI (μU/ml) | 12.87±9.1 | 13.03±11.7 | 0.639 |
| HOMA-IR | 3.10±2.7 | 3.01±2.9 | 0.308 |
| Obesity (%)BMI (≥30kg/m2) | 23.2 | 31.2 | <0.001 |
BMI: Body Mass Index; WC: waist circumference; SBP: systolic blood pressure; DBP: diastolic blood pressure; FG: fasting glucose; G2hs: glucose post 2h; TC: total cholesterol; TG: triglycerides; HDL-C: high density lipoprotein-cholesterol; FI: fasting insulin; HOMA-IR: homeostasis model assessment of insulin resistance.
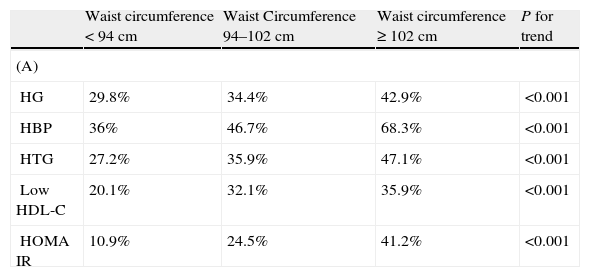
The prevalence of MS was 23.5% in males and 30.9% in females (P<.001) by ATPIII criteria and 29.1% in males and 33.1% in females, (P<.004) by IDF criteria. The AO prevalences by ATPIII and IDF criteria were 24.5% and 64% in males and 55.6% and 68.9% in females, respectively. In males, AO prevalence by IDF criteria was more than twice the prevalence by ATPIII criteria. Sex differences in the prevalence of the other metabolic abnormalities were as follows: HTG 28.7% in males and 13.2% in females, P<.001; low HDL-C 26.8% in males and 32.7% in females, P<0.001; high blood pressure (HBP), 48.3% in males and 47.7% in females, P=.369; high FG, 35.1% in males and 27.9% in females, P<.001. Two or more metabolic abnormalities (IDF criteria) were present in 48.7% of males and 37.4% of females. The prevalence of individual metabolic abnormalities using WC categories is shown in Table 2. As expected, the prevalence of individual metabolic abnormalities increased with worsening WC in both genders. The prevalence of normal glucose tolerance (NG), IFG, IGT and diabetes was 67.6%, 16.6%, 8.3%, and 7.5%, respectively.
Prevalence of individual metabolic abnormalities by waist circumference categories in males (A) and females (B).
| Waist circumference<94cm | Waist Circumference 94–102cm | Waist circumference≥102cm | P for trend | |
| (A) | ||||
| HG | 29.8% | 34.4% | 42.9% | <0.001 |
| HBP | 36% | 46.7% | 68.3% | <0.001 |
| HTG | 27.2% | 35.9% | 47.1% | <0.001 |
| Low HDL-C | 20.1% | 32.1% | 35.9% | <0.001 |
| HOMA IR | 10.9% | 24.5% | 41.2% | <0.001 |
| Waist circumference<80cm | Waist circumference 80–88cm | Waist circumference≥88cm | P for trend | |
| (B) | ||||
| HG | 12.9% | 16.3% | 34.2% | <0.001 |
| HBP | 16.7% | 28.1% | 59.6% | <0.001 |
| HTG | 10.4% | 13.9% | 25.7% | <0.001 |
| Low HDL-C | 16.8% | 30.2% | 39.6% | <0.001 |
| HOMA IR | 6.5% | 10.2% | 28.1% | <0.001 |
HG: high glucose: fasting glucose≥100mg/dl; HBP: high blood pressure; HTG: hypertriglyceridemia; low HDL-C: low high density lipoprotein cholesterol; HOMA IR: homeostasis model assessment of insulin resistance.
The Spearman correlation coefficient relating WC to HOMA IR was 0.395. IR was similarly prevalent in males 24.1% and females 21.7% (P<.088). IR prevalence was 43% in subjects with MS by IDF criteria.
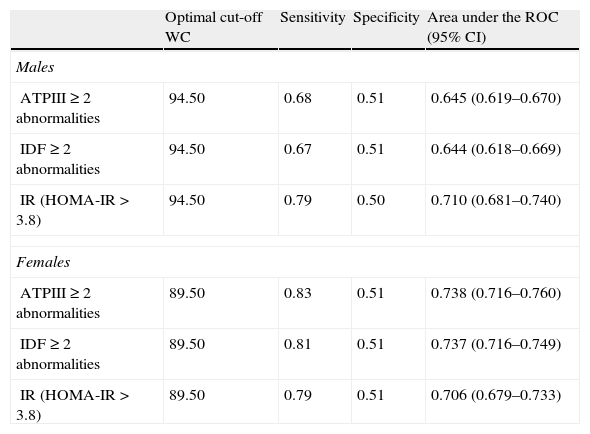
ROC curves were plotted to determine the cut-off point values for WC to predict the presence of two or more metabolic abnormalities (Table 3). The optimal cut-off for WC to obtain the maximal sensitivity and specificity for detecting two or more of the other metabolic abnormalities associated with MS or IR was 94.5cm in males and 89.5cm in females. According to these cut-off points, the prevalence of AO was 57.2% in males and 61.3% in females, P=.011 and the prevalence of MS were similar in males and females (27.9% vs. 28.9%, P=.527).
Optimal cut-off point of WC for identifying subjects with 2 or more metabolic abnormalities or insulin resistance.
| Optimal cut-off WC | Sensitivity | Specificity | Area under the ROC (95% CI) | |
| Males | ||||
| ATPIII≥2 abnormalities | 94.50 | 0.68 | 0.51 | 0.645 (0.619–0.670) |
| IDF≥2 abnormalities | 94.50 | 0.67 | 0.51 | 0.644 (0.618–0.669) |
| IR (HOMA-IR>3.8) | 94.50 | 0.79 | 0.50 | 0.710 (0.681–0.740) |
| Females | ||||
| ATPIII≥2 abnormalities | 89.50 | 0.83 | 0.51 | 0.738 (0.716–0.760) |
| IDF≥2 abnormalities | 89.50 | 0.81 | 0.51 | 0.737 (0.716–0.749) |
| IR (HOMA-IR>3.8) | 89.50 | 0.79 | 0.51 | 0.706 (0.679–0.733) |
WC: waist circumference; 95% CI: confidence intervals; IR: insulin resistance; HOMA-IR: homeostasis model assessment of insulin resistance.
Applying our optimal cut-off points to the IDF criteria, MS was 4.4 points higher in males relative to ATPIII-defined MS and 1.2 points lower relative to IDF-defined MS; in females, 2.0 and 4.2 points lower, respectively. The prevalence of ATPIII-defined MS was lower than that of IDF-defined MS (P=.007) and the prevalence of IDF-defined MS was higher than that of IDF-defined MS using our cut-off points (P=0.011). However, the differences in prevalence between ATPIII- and IDF-defined MS using our cut-off points were not statistically different (P=.388).
In logistic regression models with MS as the dependent variable (ATPIII criteria, IDF, and IDF using the new cut-off points) and HOMA IR, sex and age, as independent variables, HOMA IR was directly associated with MS (data not shown).
DiscussionThis study is the first nation-wide one aimed to describe new cut-off points for WC in a Spanish population and its impact on the prevalence of MS as defined by IDF criteria. Our results indicate that optimal WC cut-off points for detecting multiple metabolic abnormalities are 94.5cm in males and 89.5cm in females, whereas the prevalence of MS is similar in both genders according to WC cut-off points IDF criteria.
Our optimal cut-off points for WC differ from those proposed by the International Day for the Evaluation of Abdominal Obesity (IDEA) study.22 In this multicentre study significant differences for AO were observed between Southern European countries (including Greece, Italy, Portugal, Spain and Turkey) (WC 99.4cm for males, 91.3cm for females) and other Central and Northern European countries (Austria, Belgium; Denmark; Finland, France, Germany, Ireland, The Netherlands, Norway, Sweden, Switzerland) (WC 97cm for males, 88.3cm for females).
Other studies in Spain23 also concluded that the prevalence of AO measure by WC is more strongly associated with CVD and diabetes mellitus than BMI. Otherwise, our study confirms that IR prevalence is positively correlated with increased values of WC. IR is an important risk factor associated with MS and diabetes,24 and atherosclerosis25 and abnormalities linked to IR are frequently observed in subjects with AO.26 However, the odds of MS for detecting IR are similar, regardless of the cut-off point used for WC (ATPIII and IDF, as well as our cut-off points). In fact the relationship between WC and IR is consistent across many publications.27,28 Also some studies29,30 reported a stronger association between IR and WC in women as compared to men.
Furthermore, prevalence found of MS differ from those proposed both by ATPIII (WC>102/88cm for males/females)8 and IDF criteria for the Caucasian population (WC≥94/80cm for males/females).9 Interestingly, and in agreement with the concept that obesity is not a “sine qua non” condition for the MS,31 it should be pointed out that a proportion (15–20%) of obese individuals do not fulfill any current definition of MS. Paradoxically, those metabolically healthy obese often demand aggressive therapy.32
The impact of ethnic origin in different populations33 on the prevalence of MS is illustrated by comparative studies carried out in the USA. These studies reveal that the prevalence in non-Hispanic whites is lower than in Mexican-American individuals. In men, the prevalence is lower in African-Americans than in non-Hispanic whites and Mexican-Americans in women, it is lower in non-Hispanic whites than in any of the other two ethnic groups. Also Kue Young et al. in the three Canadian ethnic (Indians, Inuit, non Aboriginal Canadians) have suggested that uniform criteria for defining the MS may not be appropriate.34 Nevertheless, ethnic-specific cut-off points for WC, as per the IDF and ATPIII definitions need to be validated.13,33–36
In a cross-sectional study in Japanese individuals, Narisawa et al.,35 reported that appropriate cut-off point values for WC in persons with multiple cardiovascular risk factors were 87cm in males and 83cm in females. Furthermore, in Asian Indians, the appropriate WC was 87cm for males and 82cm for females.36
Results from the INTERHEART study suggest that AO is a high risk factor for myocardial infarction.37,38 Also the distribution pattern of adiposity correlates with the presence of CVD risk factors.39 Central distribution of body fat is also an important predictor of CVD risk,39,40 diabetes, and mortality, independently of the effect of other traditional risk factors.32,41 Therefore, optimal cut-off points for AO are important in order to determine which individuals are at significant risk of CVD. Park et al. concluded that WC or waist to height ratio may be a better predictor of CVD risk factors.42
However, Alberti et al.,13 have recently emphasized that there should be a mandatory component. Thus WC measurement will remain a “useful tool for preliminary screening”. In this context the authors recommend that WC to be used as a useful screening tool, particularly in the primary care setting.
A limitation of our study is that our results are based on cross-sectional data. Prospective studies are needed to determine whether our cut-off points have an advantage over ATPIII and IDF cut-off points for predicting CVD and diabetes in the Spanish population.
In conclusion, our populations presented WC cut-off points of 94.5cm for males and 89.5cm for females that are optimal for identifying IR and multiple metabolic abnormalities in the Spanish population. According to our study these new cut-off points detect more male subjects with the MS and IR than the ATPIII criterion of central obesity, values for WC in females are higher than those proposed in the ATPIII (modified) and IDF cut-off points. More prospective studies are needed to determine whether these cut-off points improve the identification of individuals at an increased risk of future diabetes and CVD.
FundingThis research was supported by grants from FIS (Fondo de Investigación Sanitario) 95/0029, FEDER2FD 1997/2309 from the European Regional Development Fund, Red de Centros RCMN (C03/08), and FIS 03/1618, from Instituto de Salud Carlos III-RETICRD06/0015/0012, Madrid, Spain. CIBERDEM. Partial support also came from Fundation Mutua Madrileña (FMM08), Educational Grants from Eli Lilly Lab, Spain and Bayer Pharmaceutical Co., Spain; and DANONE Institute.
Conflict of interestThe authors declare no conflict of interest.
We acknowledge Milagros Pérez Barba for dedicated and careful technical assistance. We acknowledge members of the VIVA Study Group and of the Segovia Study Group.