Clostridium perfringens is the causative agent for necrotic enteritis. It secretes the major virulence factors, and α- and NetB-toxins that are responsible for intestinal lesions. The TpeL toxin affects cell morphology by producing myonecrosis, but its role in the pathogenesis of necrotic enteritis is unclear. In this study, the presence of netB and tpeL genes in C. perfringens type A strains isolated from chickens with necrotic enteritis, their cytotoxic effects and role in adhesion and invasion of epithelial cells were evaluated. Six (27.3%) of the 22 C. perfringens type A strains were harboring the tpeL gene and produced morphological alterations in Vero cells after 6h of incubation. Strains tpeL (−) induced strong cell rounding after 6h of incubation and produced cell enlargement. None of the 22 strains harbored netB gene. All the six tpeL (+) gene strains were able to adhere to HEp-2 cells; however, only four of them (66.6%) were invasive. Thus, these results suggest that the presence of tpeL gene or TpeL toxin might be required for the adherence of bacteria to HEp-2 cells; however, it could not have any role in the invasion process.
Clostridium perfringens spore-forming gram-positive bacterium is the causative agent of gaseous gangrene in humans, enterotoxaemia in cattle, and necrotic enteritis in chicken. Based on the type of potent and lethal toxins produced, they are classified into five toxinotypes: from A to E.1–3
Large clostridial cytotoxins (LCTs) comprise C. difficile toxin A (TcdA) and toxin B (TcdB), C. sordellii lethal toxin (TcsL) and hemorrhagic toxin (TcsH), C. novyi alpha toxin (TcnA), and toxin C. perfringens large cytotoxin (TpeL), are important virulence factors in pathogenesis of myonecrosis and intestinal diseases.4–6
C. perfringens is able to produces other potent toxins and enzymes, including NetB related to human and veterinary diseases. However, the role of the NetB-toxin in the necrotic enteritis is still controversial because it has been detected in both healthy and sick animals.7
TpeL toxin was initially detected in C. perfringens type C isolated from swine and in C. perfringens type A strains isolated from chicken. It is lethal to mice and cytotoxic to Vero cells, causing an enlargement, rounded cells, and forming aggregates, eventually detached cells from the plate.4,8,9
In early study, chickens inoculated with C. perfringens TpeL (+) showed higher capacity to cause severe intestinal lesions and an earlier onset of symptoms than chickens not inoculated with TpeL.10 This suggests that TpeL may potentiate or contribute to the pathogenesis of necrotic enteritis caused by C. perfringens type A strains.9
Adhesion process to epithelial cells is the first step to the bacterial colonization, and this is mediated by fimbriae or non-fimbriae appendices.11C. prefringens is able to produce biofilm on cell surfaces and the presence of pili as well as the production of sialidase can collaborate with this process.12 McClane13 showed that adherent C. perfringens strains increase the neuraminidase and toxin production.
In this study, the presence of netB and tpeL genes in C. perfringens type A strains isolated from chickens with necrotic enteritis and their cytotoxic effects and role in the adhesion and invasion of the epithelial cells were evaluated.
Chickens belonged to five Brazilian states, Ceará (CE, 1 chicken), Sao Paulo (SP, 2 chickens), Paraná (PR, 3 f chicken), Santa Catarina (SC, 2 chickens), and Rio Grande do Sul (RS, 1 chicken). Twenty-two C. perfringens strains previously isolated from the intestinal samples of nine chickens with necrotic enteritis, identified and toxinotyped as C. perfringens type A by biochemical tests. The presence of the cpa gene codifying the toxin α in all C. perfringens strains was also evaluated by PCR. This study was approved by the Ethics Committee of the Biomedical Sciences Institute, University of Sao Paulo (No. 104/CEEA).
Bacterial DNA was obtained in accordance with Sambrook et al.14 Briefly, the bacteria grown into 5mL brain heart infusion (BHI) were harvested by centrifugation (14,000×g, 10min), and the aliquots of supernatant were maintained at −80°C until use. The pellets were washed twice with 0.1M phosphate-buffered saline (PBS, pH 7.2), and incubated with 10mg/mL lysozyme at 37°C for 3h. Then, 20% SDS and 20mg/mL proteinase K were added and incubated at 55°C for 2h. The DNA was extracted by using equal volumes of phenol–chloroform and eluted in 100μL of TE. PCR assays were performed to detect netB and tpeL genes with final reaction volumes of 25μL containing 10× PCR buffer, 1.5mM MgCl2, 0.2mM dNTP mix, 0.5U Platinum Taq DNA polymerase (Invitrogen), 0.4mM of each primer5,15 and 1ng of DNA. Thermocycler (PE Applied Biosystems Gene Amp PCR System 9700) was programmed to: initial denaturation at 94°C (5min), followed by 30 cycles of 94°C (1min), 55°C (1min) and 72°C (1min), and a final extension at 72°C (7min). The PCR products were analyzed on 1% agarose gel stained with ethidium bromide (0.5mg/mL) and photographed by using Kodak Digital System DC-120. DNA from C. perfringens JGS 5369 strain was used as control. DNA was sequenced using MegaBACE 1000 system. Sequencing data were analyzed by using Nucleotide BLAST (NCBI, Bethesda, Maryland, USA).
The cytotoxic effect at different times (6, 24, 48, and 72h) was evaluated on Vero African Green Monkey kidney cell line (Vero, ATCC CRL-81) cultured in Dulbecco's modified Eagle's medium (DMEM; Vitrocell, Embriolife, SP, Brazil) supplemented with 2% (v/v) fetal bovine serum (FBS). In each well of a 24-well microplate, 1mL of cell suspension (1×105cells/mL) was dispensed, and incubated at 37°C in 5% CO2 for 24h. The Vero cells were treated with the supernatant of the bacteria cultured in BHI for 24h. Morphological alterations in cells were assessed by using a light microscope. All assays were independently performed twice in duplicate.
The bacterial adherence assays were performed by using HEp-2 cells (1×105cells/mL), as previously described by Nakano et al.8 Briefly, 900μL of DMEM supplemented with 2% FBS was added to HEp-2 cells, which was further inoculated with 100μL of 24-h bacterial culture (c.a. 1.5×108cells/mL). Plates were incubated (in 5% CO2, 6h), washed three times with PBS, fixed with absolute methanol, and stained with May–Grünwald–Giemsa stain. The enteroaggregative strain Escherichia coli O42 displaying an aggregative adhesion pattern was used as a control. All assays were performed in duplicate.
The invasion assay was performed in accordance with Nakano et al.,8 as follows: 100μL of bacterial suspension was added to HEp-2 cells, and plates were incubated (in 5% CO2, 2h). The non-adherent bacteria were killed by incubating them in 1mL of DMEM supplemented with 200mg/L of cefoxitin (for C. perfringens) or 300mg/L of gentamicin (for E. coli) at 37°C, in 5% CO2 for 1h; the antibiotic concentrations did not affect the morphology of the epithelial cells. Then, cells were washed in PBS (three times) and lysed with 400μL of Triton X-100 (1%, v/v), thoroughly and transferred to tubes containing 1.6mL BHI broth. Aliquots of 100μL of this were plated on Brucella blood agar (Difco Laboratories, UK), incubated in anaerobiosis (90% N2+10% CO2) at 37°C for 48h, and the colony forming unit (CFU) was determined. An enteroinvasive strain E. coli serotype O124:NM, and a non-invasive strain E. coli HB101 were used, as positive and negative control, respectively. All assays were performed in duplicate. An invasion was expressed as the percentage of bacteria recovered from the initial inoculum after the antibiotic treatment and lysis of the epithelial cells as reported by Tang et al. (1993).18 The statistical analysis was performed by using GraphPad Prism version 6.0 (GraphPad Software, La Jolla California, USA) and a p-value of 0.05 was considered as statistically significant. The CFU were compared by using unpaired T-test, and the other data were statistically evaluated by Chi-square test.
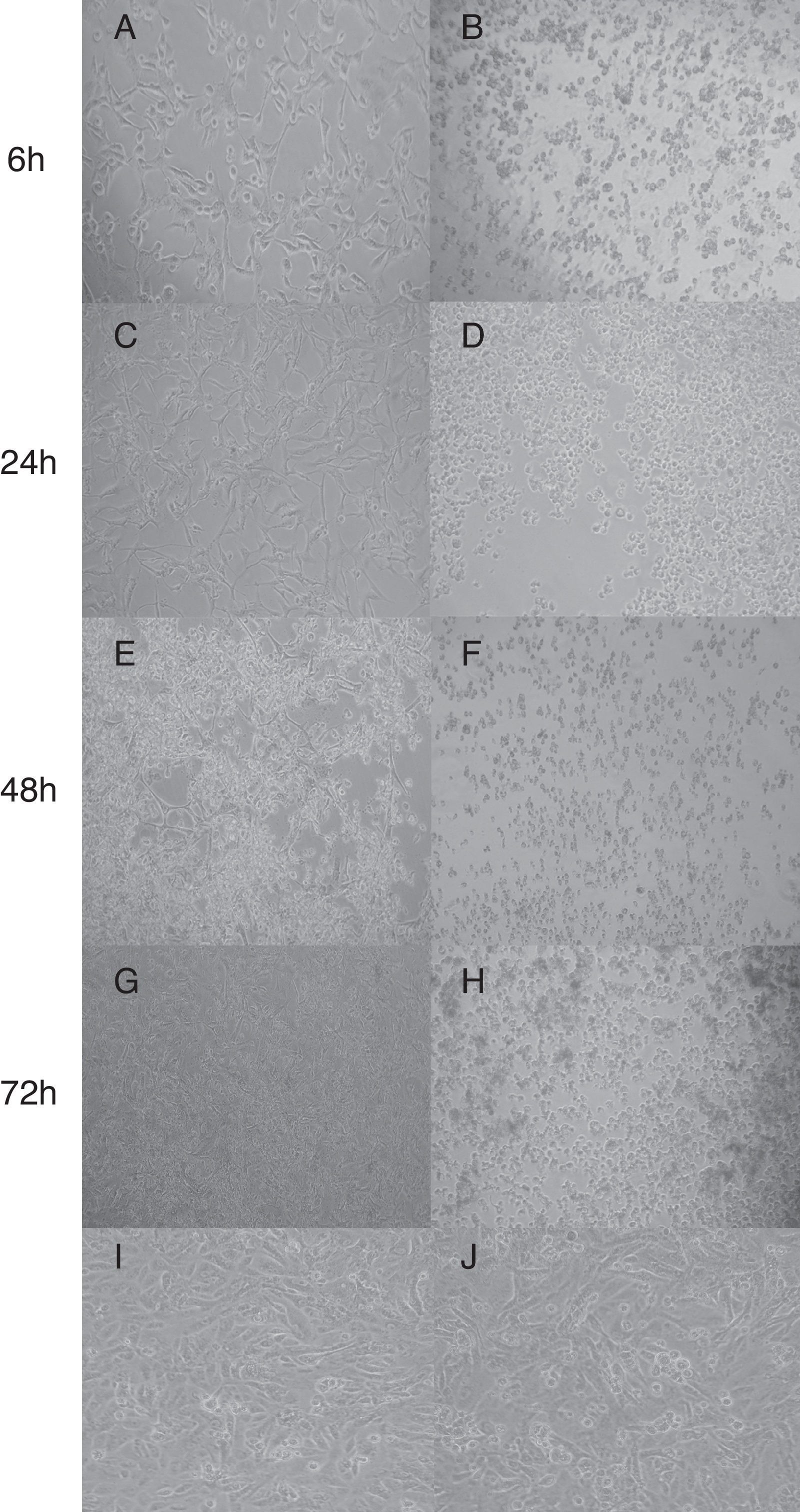
In this study, none of the strains harbored the netB gene in accordance with Llanco et al.5; although, it is important to note that the production of this toxin does not necessarily gene-dependent.16 The partial sequencing of the tpeL gene from six (27.3%) out of 22 strains showed 99% sequence similarity with C. perfringens (AB262081.1) tpeL (+). These strains altered morphology of Vero cells which displayed characteristic cell enlargement (Fig. 1A) and rounded cells (Fig. 1E) as early as 6h of incubation, and this effect was pronounced after 48h of incubation. On the other hand, the supernatant from strains tpeL (−) gene induced a strong cell rounding after 6h of incubation; and after 72h the cells were found to be detached completely without forming any aggregates (Fig. 1H). This effect of tpeL (−) strains might be due to the toxin-α. The initial stretching or elongation of Vero cells (Fig. 1A) noticed with tpeL (+) strains was due to the characteristic action of the toxin TpeL or by any synergistic or competitive effect, since the supernatant was used, and in accordance with previous studies.3,17
Cytotoxic of C. perfringens type A strain on Vero cells in different times. (A, C, E, G) C. perfringens tpeL (+) gene; (B, D, F, H) C. perfringens tpeL (−) gene; (I) Vero cells (control); (J) Vero cells and BHI. Times of Incubation: A and B, 6h; C and D, 24h; E and F, 48h; G and H, 72h. Magnification: 1000×.
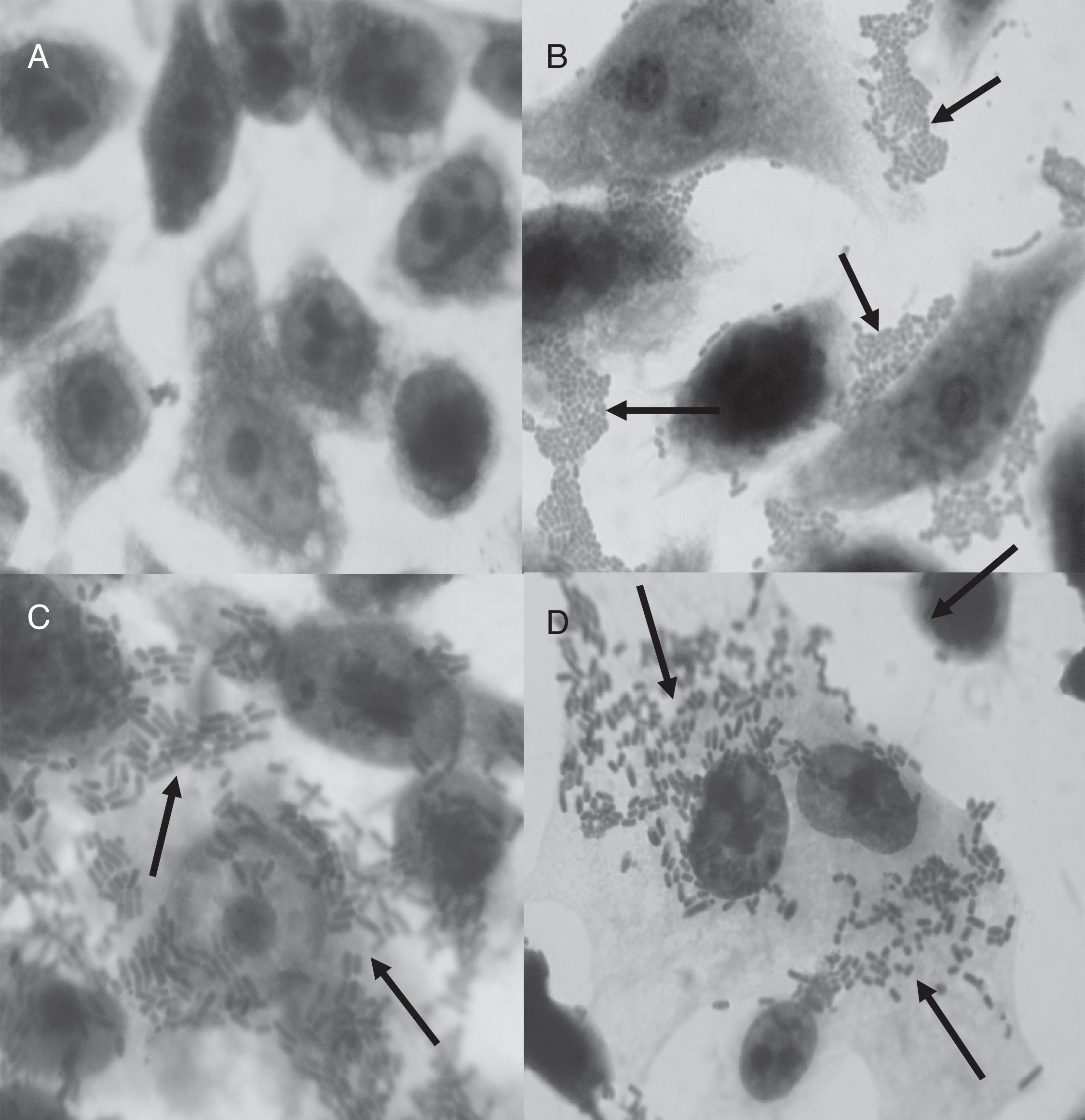
All the tpeL (+) strains and two tpeL (−) strains were able to adhere to HEp-2 cells; however, no significant difference was observed (p=0.1336). The adhesion profile displayed non-localized clusters (Fig. 2C and D). The invasive capacities observed in four of the six adherent tpeL positive strains and two tpeL negative strains were comparable (p=1.0000). All non-adherent strains also did not invade HEp-2 cells. The tpeL negative C. perfringens ATCC 13124 and enteroinvasive E. coli O143 were able to adhere to and invade HEp-2 cells. Although the higher level of invasion was recorded with tpeL (+) (log103.26CFU/mL) than in tpeL (−) strains (log102.89CFU/mL), there was no statistically significant difference (p=0.9080).
There are few reports from Brazil in which the presence of TpeL toxin-producing C. perfringens type A isolated from chickens with necrotic enteritis are studied. TpeL toxin belongs to LCTs that exhibits lethality in mice and cytotoxicity in various cell lines including Vero cells.18 Some of the strains studied here were able to adhere to and invade HEp-2 cells which might have contributed in the pathogenicity of these strains. Studies have shown by using RAPD-PCR, MLST, and PFGE methods that some clones of C. perfringens isolated from different origins might be associated to enteritis necrotic and they can persist for long time at the diseased sites.19,20
The ability of bacteria to adhere epithelial cells is considered as important virulence factor in various pathogens, such as C. difficile, Salmonella Typhimurium, Shigella spp., and Helicobacter pylori.21 Since most studies have been exclusively focused on the toxin production, the adhesion in C. perfringens has not been adequately evaluated.22 In histopathological studies of the intestinal tissue from necrotic enteritis, C. perfringens was found to be adhered to intestinal cells and necrotic areas, suggesting that this organism produces toxins after intestinal colonization.21
Once the six adherent strains evaluated in this study belonged to chickens from different Brazilian states, it is possible to suggest that these strains tpeL-positive are distributed in most region of Brazil; however, more studies are needed evaluating a high number of chickens and strains. In addition, since six strains were adherent and four of them were invasive, it is suggested that the TpeL toxin has any role in the adhesion, but not to invasion. Studies have shown that the adhesion process of C. perfringens to HEp-2 cells could be mediated by the presence of pili type IV, which has an important role in the biofilm formation on the epithelial cells.23 The presence of pili or other appendices was not evaluated.
In this study, we have identified tpeL harboring C. perfringens type A strains isolated from chickens with necrotic enteritis, and our results suggests the possible contribution of this toxin in aggravation of this disease. In conclusion, the presence of tpeL gene or TpeL toxin would be associated with adhesion but not with invasion process. Further studies are warranted to characterize the mechanisms involved in the adhesion and invasion of epithelial cells by tpeL positive and negative C. perfringens.
Conflicts of interestThe authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The authors thank to Miss Marcia H. Fukugaiti for her technical support. Clostridium perfringens JGS 5369 was kindly provided by Dr. J.G. Songer at University of Arizona, USA. This study was supported by grant of CNPq No. 158799/2012-7 and FAPESP2013/17739-9.