Neisseria gonorrhoeae is the agent of gonorrhea, a sexually transmitted infection with an estimate from The World Health Organization of 78 million new cases in people aged 15–49 worldwide during 2012. If left untreated, complications may include pelvic inflammatory disease and infertility. Antimicrobial treatment is usually effective; however, resistance has emerged successively through various molecular mechanisms for all the regularly used therapeutic agents throughout decades. Detection of antimicrobial susceptibility is currently the most critical aspect for N. gonorrhoeae surveillance, however poorly structured health systems pose difficulties. In this review, we compiled data from worldwide reports regarding epidemiology and antimicrobial resistance in N. gonorrhoeae, and highlight the relevance of the implementation of surveillance networks to establish policies for gonorrhea treatment.
N. gonorrhoeae is the etiological agent of gonorrhea, the second most frequently reported sexually transmitted infection (STI) in the world. This bacterium typically colonizes and infects the genital tract in men and women, but may be found in additional body sites such as the rectal and oropharyngeal mucosa, with or without clinically evident infection.1
Gonorrhea is usually symptomatic in men, most often as urethritis, with pain or burning sensation during urination, urethral discharge, and painful testicles. In contrast, women develop symptomatic gonococcal cervicitis less frequently, presenting a slight increase in vaginal discharge, and rare vaginal bleeding unrelated to periods, or pain and a burning sensation when urinating. The absence of symptoms in men and women can lead to sustained infections. Complicated gonorrhea may cause infertility.2
Surveillance programs around the worldA report from the World Health Organization (WHO) indicated the occurrence of 78 million new cases of gonococcal infection in people aged 15–49 worldwide during 2012.3 Despite the high rate of incidence, just a few countries, including the United States (U.S.), Canada, members of the European Union (EU), and Australia conduct broad surveillance programs on gonorrhea. Additionally, some countries from Latin-America and the Caribbean region (LAC), and countries from Western Pacific Region (WPR) and South East Asian Region (SEAR) joined the Gonococcal Antimicrobial Surveillance Program (GASP) that was conducted by WHO in the early 1990s. Since antimicrobial resistance is the main challenge associated with this microorganism, published reports emphasize the antimicrobial susceptibility profile of isolates. However, analysis of published data can be challenging, considering the breakpoints to define resistance vary across different surveillance programs. Regardless of this limitation, resistance is clearly an emerging phenomenon.
United StatesIn the U.S., the Centers for Disease Control and Prevention (CDC) supports the Gonococcal Isolate Surveillance Project (GISP). This program analyzes the first 25–30 N. gonorrhoeae isolates collected from men with gonococcal urethral-syndrome in sentinel laboratories located in five regions of the U.S. monthly. Surveillance includes analysis of demographic and clinical data, and antimicrobial susceptibility.4 In recent years, the southern region reported the highest rate of gonorrhea, reaching 131.4 cases per 100,000 individuals in 2014.5 The CDC estimates 820,000 new gonorrhea cases per year throughout the country.6
The STI Surveillance Network in the U.S. indicated higher incidence rates of gonorrhea in men who have sex with men (MSM) of any age, followed by men who have sex with women (MSW), and women, in 2014.5 The network also reported that the risk of gonococcal infection declines with age, with most cases occurring in individuals under the age of 24.5 In regards to antimicrobial resistance, GISP data has shown that nearly 30% of the isolates obtained from MSM, and 12% of those obtained from MSW were resistant to ciprofloxacin, with a minimum inhibitory concentration (MIC)≥1μg/mL in 2014.7 Irrespective of the sexual partners gender, in that same year, 0.7% of the isolates (n=38) presented a decrease in cefixime susceptibility (MIC≥0.25μg/mL), and 2.5% showed azithromycin MIC alert values (MIC≥2μg/mL) (Table 1).7 Moreover, a genomic epidemiology study with 236 GISP isolates obtained in 2009–2010 has shown that an N. gonorrhoeae cluster with decreased susceptibility to extended-spectrum cephalosporins (ESC) spread predominantly among MSM during those years.8
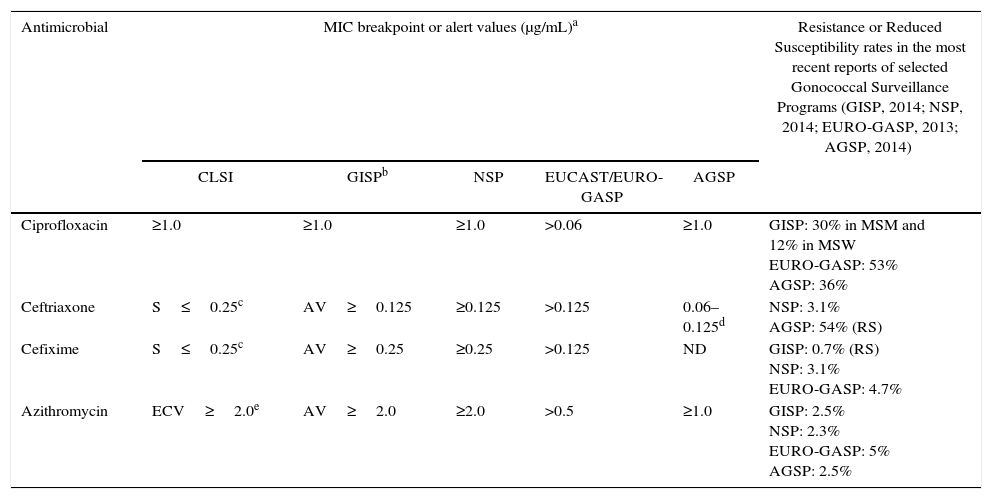
Breakpoints or alert values adopted for testing susceptibility of Neisseria gonorrhoeae to key antimicrobials by CLSI, GISP, NSP, EUCAST and AGSP and selected resistance rates.
| Antimicrobial | MIC breakpoint or alert values (μg/mL)a | Resistance or Reduced Susceptibility rates in the most recent reports of selected Gonococcal Surveillance Programs (GISP, 2014; NSP, 2014; EURO-GASP, 2013; AGSP, 2014) | ||||
|---|---|---|---|---|---|---|
| CLSI | GISPb | NSP | EUCAST/EURO-GASP | AGSP | ||
| Ciprofloxacin | ≥1.0 | ≥1.0 | ≥1.0 | >0.06 | ≥1.0 | GISP: 30% in MSM and 12% in MSW EURO-GASP: 53% AGSP: 36% |
| Ceftriaxone | S≤0.25c | AV≥0.125 | ≥0.125 | >0.125 | 0.06–0.125d | NSP: 3.1% AGSP: 54% (RS) |
| Cefixime | S≤0.25c | AV≥0.25 | ≥0.25 | >0.125 | ND | GISP: 0.7% (RS) NSP: 3.1% EURO-GASP: 4.7% |
| Azithromycin | ECV≥2.0e | AV≥2.0 | ≥2.0 | >0.5 | ≥1.0 | GISP: 2.5% NSP: 2.3% EURO-GASP: 5% AGSP: 2.5% |
Breakpoints define resistance, except otherwise described, adopted as reference by the Gonococcal Surveillance Programs listed.
GISP proposes minimum inhibitory concentration (MIC) alert values (AV) for ceftriaxone, cefixime and azithromycin.
CLSI indicates that isolates with MIC ≥2.0μg/mL for azithromycin have mutational and/or acquired resistance mechanisms, and defines as epidemiological cutoff value.
CLSI, Clinical Laboratory Standards Institution; S, susceptible; ECV, epidemiological cutoff value; ND, not determined; GISP, Gonococcal Isolate Surveillance Project; NSP, National Surveillance Program in Canada; EUCAST, European Committee on Antimicrobial Susceptibility Testing; EURO-GASP, European Gonococcal Antimicrobial Surveillance Program; AGSP, Australian Gonococcal Surveillance Program; MSM, men who have sex with men; MSW, men who have sex with women.
The National Surveillance Program (NSP) in Canada, implemented in 1985, provides data from different provinces across the country regarding N. gonorrhoeae antimicrobial susceptibility. Provincial public health laboratories (PL) send resistant isolates or isolates not submitted to antimicrobial susceptibility testing to the National Microbiology Laboratory (NML). In 2014, 2101 of 3089 N. gonorrhoeae isolates cultured in the PL were sent to NML. Interestingly, contrasting with other countries, NSP data showed a consistent diminishing trend in the ESC reduced susceptibility rates (from 7.6% in 2011 to 3.1% in 2014). However, regarding azithromycin, resistance rates rose from 0.4% in 2011 to 2.3% in 2014.9
EuropeIn Europe, the extent of N. gonorrhoeae surveillance varies in different countries, according to national public health policies. However, data compiled from 21 countries composing the European GASP (Euro-GASP) conducted by the European CDC (ECDC) reported 50,001 cases in 2013.10 Euro-GASP has two surveillance modules: one decentralized, based on the communication of antimicrobial susceptibility test (AST) results to ECDC; and the other centralized. In this case, participating laboratories send isolates to the Public Health England (London) for AST.10
In the most recent Euro-GASP report, each country was required to make a contribution of 100–200 isolates (depending on the number of gonorrhoeae cases detected by the national protocols), obtained during April/May and October/November 2013. With this sampling strategy, the number of isolates included in the study, compared to the total reported cases in each country, varied from less than 1% (in consequence of high incidence levels or very efficient surveillance) to more than 100% (when under-reporting from the national protocols occurred). For instance, the United Kingdom (U.K.), with a well-established national surveillance program, reported the most cases, 32,377 in 2013, followed by the Netherlands (n=4171), Spain (n=3314), and Hungary (n=1526); while Portugal reported only 116 cases in the same year, and Germany did not provide any information on this matter. Notwithstanding such differences, these six countries contributed to the Euro-GASP analysis with similar number of isolates, varying from 88 in Hungary to 240 in the U.K. Considering such a heterogeneous collection, data compiled showed in Europe, in addition to the U.S., MSM as the main group at risk of developing gonorrhea. However, in contrast to the predominant age group in the U.S., most cases reported for European citizens were with individuals over the age of 25.10
Concerning detection of resistance, the Euro-GASP adopts breakpoints set by The European Committee on Antimicrobial Susceptibility Testing (EUCAST), which are lower than the values adopted by the U.S.4,11,12 With this caveat in mind, the Euro-GASP reported ciprofloxacin and azithromycin resistance rates of 53% and 5%, respectively, among 1994 isolates studied in the program during 2013.10 In contrast, for cefixime, the Euro-GASP adopts the same resistance breakpoint used by the GISP. Still, cefixime resistance in Europe was higher than in the U.S., with 93 confirmed cases (4.7% of total isolates) in 2013 (Table 1).10
AustraliaThe Australian Gonococcal Surveillance Program (AGSP) showed a different epidemiological trend between remote regions and eastern states (Victoria, New South Wales, and Queensland) in 2012. In remote regions, notification rates were higher but stable compared to previous years, about 933 cases per 100,000 individuals, with low antimicrobial resistance rates. In contrast, in the eastern region, where an international community predominates, the incidence of gonorrhea was lower but had grown since 2009 (reaching 38.5 per 100,000 individuals), and the isolates showed higher resistance levels.13 Considering data from the whole country, and MIC breakpoint of ≥1μg/mL for both ciprofloxacin and azithromycin,14 the AGSP reported resistance rates of 36% and 2.5% for these drugs, respectively.13 Moreover, Australia does not conduct surveillance for cefixime, but detected 5.4% (n=258) reduced susceptibility to ceftriaxone (MIC 0.06–0.125μg/mL) among 4804 isolates in 2014.13 As a reference for comparison, with a slight difference taken into account in breakpoints adopted, this percentage rate is more than ten times higher than that observed by GISP (0.4%) in 2014, or Euro-GASP (0.1%) in 2013 (Table 1).7,10
AsiaSurveillance in N. gonorrhoeae resistance has been conducted in WPR and SEAR since 1992 and 2007, respectively, through a WHO-GASP initiative, under the Australian Health Department supervision. According to a GASP-WPR/GASP-SEAR report, 9744 N. gonorrhoeae isolates from 19 countries were submitted to antimicrobial susceptibility testing in 2010. Among these isolates, quinolone resistance or reduced susceptibility was widespread, reaching rates over 90% in 11 countries. Azithromycin resistance rates varied largely, from 0 to 1% in countries such as Cambodia and India to 34% in Mongolia. Different rates were also reported for decreased susceptibility to ceftriaxone: 1.3% in Singapore, 10.8% in India, 20.3% in Japan, 29.3% in Korea, and 55.8% in China.15
AfricaAccording to a WHO report published in 2015, a stable and efficient surveillance program for STI in Africa Region has not been implemented yet.16 In 2013, a good review about gonococcal antimicrobial resistance studies performed in Africa demonstrated that the small number of isolates tested and the lack of standardization in the sampling strategy adopted in different countries make measuring and comparisons of resistance rates difficult.17 In a general perspective, data obtained between 2004 and 2012 in the African continent demonstrated a rise in the antimicrobial resistance, especially with quinolones, and emphasizes the need for improving infrastructure and laboratory network to perform surveillance in that region.17,18
Latin America & CaribbeanIn addition to Asia and Africa, WHO-GASP has also been implemented in Latin America and the Caribbean region (GASP-LAC) since the 1990s.19 WHO-GASP-LAC published a report in 2013 that gathered information on the antimicrobial susceptibility of N. gonorrhoeae in 23 countries from 1990 to 2011. Among 12,730 isolates tested for ciprofloxacin susceptibility, a rising trend for resistance was observed during these years. Ciprofloxacin resistance rates stayed below 5% until 2004, ranged to more than 15% in 2006, finally reaching values of greater than 40% in 2010. Resistant N. gonorrhoeae isolates to azithromycin were not detected until 2000 and onwards, with the exception of Cuba, where 10 N. gonorrhoeae azithromycin resistant isolates were detected between 1995 and 1998. Azithromycin resistance rates remained above 6% from 2000 to 2009, rose to more than 25% in 2008, and reduced down to 1% in 2010. Among 5171 isolates tested for ceftriaxone between 2007 and 2011, 20 (0.4%) N. gonorrhoeae isolates obtained from Argentina, Brazil, Chile, Cuba and Uruguay exhibited reduced susceptibility to ceftriaxone (MIC≥0.125μg/mL).19
BrazilBrazil, the largest and economically most relevant country in South America, does not have a national gonococcal surveillance program. Over the last 20 years, three attempts of establishing laboratory networks with a focus in N. gonorrhoeae resistance were conducted by the Brazilian Health Ministry (BHM). The first one occurred in 1996 as an invitation of Pan American Health Organization (PAHO)/WHO. The second one, initiated in 2007, was denominated as Sengono Project, and engaged seven previously established laboratories and research groups. Both attempts failed, since no reports of the obtained results have been published. In 2013, a new edition of the Sengono Project was initiated. The project provided preliminary results published as a BHM communication note in the BHM website in 2016.20
The BHM estimated the occurrence of 9,285,000 cases per year amongst the age group of 15–49, in 2015. Unfortunately, this number comes from compiled data obtained in small studies performed with different subpopulations in seven Brazilian states, from 2002 to 2012. The data include cohort sampling reports and asymptomatic infections, differently from cases notified in surveillance programs associated with Health Care Systems.21 According to statistics currently posted on the BHM website, WHO estimates 1,541,800 new gonorrhea infections per year in Brazil. However, no information about the data source for the estimations were provided.22
In regards to antimicrobial resistance, a report presenting GASP-LAC outcomes for 2936 isolates obtained in Brazil from 2000 to 2009 indicated that during this time ciprofloxacin resistance rates were lower than 6%. Azithromycin resistance rates varied from 22% (9/41) in 2004 to 6% (7/110) in 2007, with one additional resistant isolate detected in 2009. However, it is not clear if such data are representative for the whole country, since no information about the geographic origin of the isolates or sampling strategy is available. The same report described seven isolates obtained in Manaus in 2007 exhibiting decreased susceptibility to ceftriaxone (MIC>0.25μg/mL).23 Beyond this report, a small number of studies were performed sporadically throughout different regions of the country. During 2004 and 2005, five ciprofloxacin resistant isolates (8%) were detected among 65 N. gonorrhoeae isolates recovered from patients with urethritis in São Paulo.24 A few years later, ciprofloxacin resistance reached 17% among 152 isolates obtained between 2006 and 2010 from patients with gonorrhea in Rio de Janeiro.25 More recently (2011–2012), among 201 N. gonorrhoeae isolates obtained from patients with urethritis and cervicitis in a Health Service Facility in Minas Gerais, 21% were resistant to ciprofloxacin and 5% were resistant to azithromycin.26
Neisseria gonorrhoeae genotypingUnderstanding clonal relationships among N. gonorrhoeae resistant isolates is an important strategy to control the spread and increase of resistance. There are two main DNA sequence-based typing methods performed in gonococcus with data stored on public website. The N. gonorrhoeae multiantigen sequence type (NG-MAST) is specific to this species and analysis two variable loci, porB and tbpB, which encode one of the two porins expressed by N. gonorrhoeae (PIB porin), and the B subunit of binding transferrin protein, respectively (http://www.ng-mast.net/). NG-MAST is a convenient tool for micro-epidemiological studies due to its discriminatory property.27 The other method is multilocus sequence typing (MLST), available for the gender Neisseria. This approach is based on the analysis of seven housekeeping genes, and is appropriate to track the spread of international clones (http://pubmlst.org/neisseria/). Both typing tools have demonstrated that infections may occur in clusters, and resistant clones may spread across continents.28 In addition to those methods, whole genome sequencing has brought new epidemiologic information in studies of transmission pathways.8,29
Current treatment recommendationsPublished guidelines usually state policies for syndromic treatment of gonorrhea, defined as symptomatic urethritis in men, and mucopurulent cervicitis in women, even while a microbiological diagnostic is not possible.6,21 The treatment recommendations vary slightly, especially concerning antimicrobial doses, and are described in Table 2. The BHM proposes two different strategies for Brazil. For most territories, ciprofloxacin should be prescribed with a combination of azithromycin. However, studies showing ciprofloxacin resistance rates over 5% in São Paulo, Minas Gerais, and Rio de Janeiro, encourage ciprofloxacin to be replaced by ceftriaxone in those states.21 This recommendation placed Brazil in the vast group of countries (U.S., European countries, Australia, and others) that recommend a combination of ceftriaxone-azithromycin for therapy.6,30–33 The WHO Guideline for the treatment of N. gonorrhoeae based on data from high, middle- and low-income countries, published in 2016, recommends ceftriaxone or cefixime plus azithromycin primarily for treatment of genital and anorectal gonococcal infections (Table 2).3
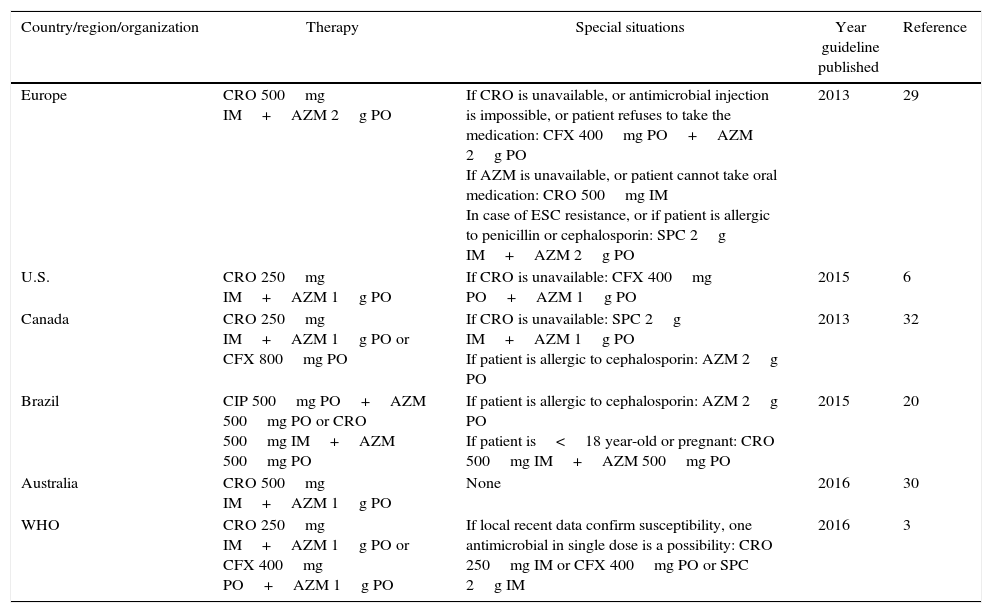
Antimicrobial therapy recommended for gonococcal infection.
| Country/region/organization | Therapy | Special situations | Year guideline published | Reference |
|---|---|---|---|---|
| Europe | CRO 500mg IM+AZM 2g PO | If CRO is unavailable, or antimicrobial injection is impossible, or patient refuses to take the medication: CFX 400mg PO+AZM 2g PO If AZM is unavailable, or patient cannot take oral medication: CRO 500mg IM In case of ESC resistance, or if patient is allergic to penicillin or cephalosporin: SPC 2g IM+AZM 2g PO | 2013 | 29 |
| U.S. | CRO 250mg IM+AZM 1g PO | If CRO is unavailable: CFX 400mg PO+AZM 1g PO | 2015 | 6 |
| Canada | CRO 250mg IM+AZM 1g PO or CFX 800mg PO | If CRO is unavailable: SPC 2g IM+AZM 1g PO If patient is allergic to cephalosporin: AZM 2g PO | 2013 | 32 |
| Brazil | CIP 500mg PO+AZM 500mg PO or CRO 500mg IM+AZM 500mg PO | If patient is allergic to cephalosporin: AZM 2g PO If patient is<18 year-old or pregnant: CRO 500mg IM+AZM 500mg PO | 2015 | 20 |
| Australia | CRO 500mg IM+AZM 1g PO | None | 2016 | 30 |
| WHO | CRO 250mg IM+AZM 1g PO or CFX 400mg PO+AZM 1g PO | If local recent data confirm susceptibility, one antimicrobial in single dose is a possibility: CRO 250mg IM or CFX 400mg PO or SPC 2g IM | 2016 | 3 |
AZM, azithromycin; CFX, cefixime; CIP, ciprofloxacina; CRO, ceftriaxone; SPC, spectinomycin; ESC, extend spectrum cephalosporin.
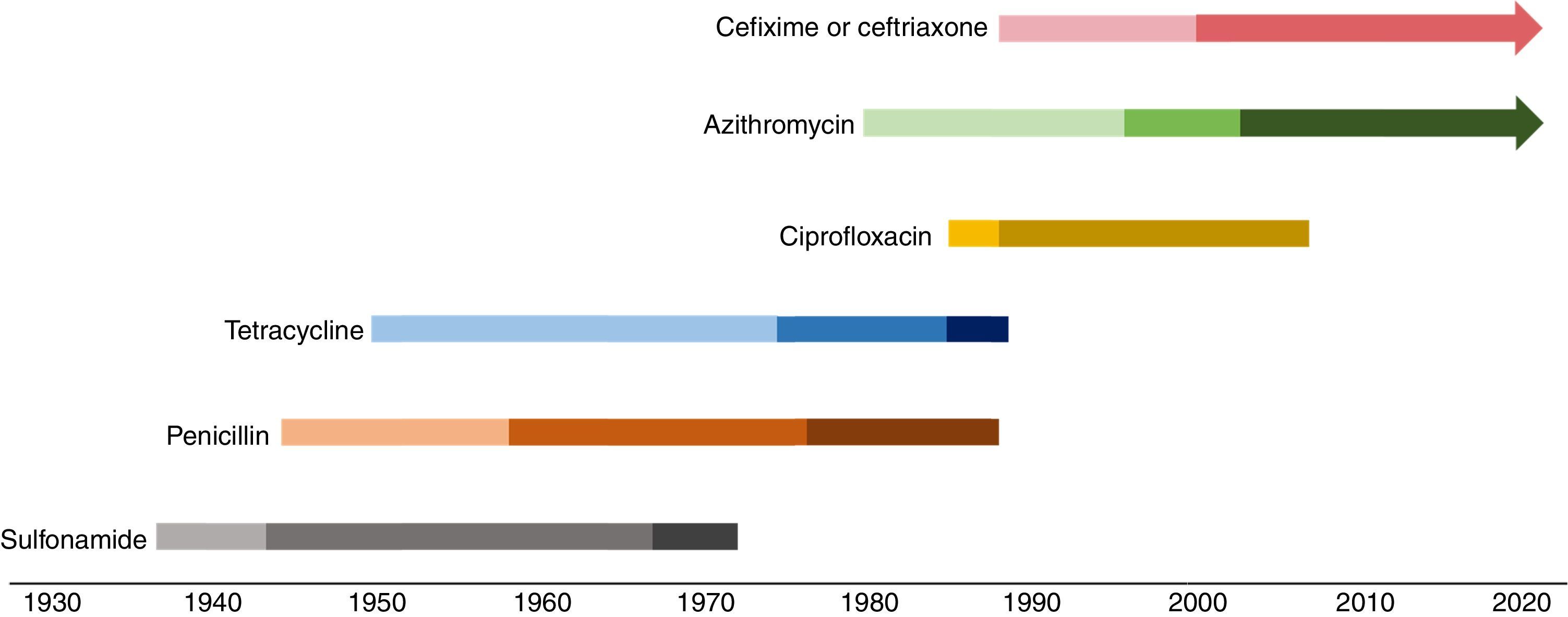
Over the last 80 years, N. gonorrhoeae has developed or acquired resistance mechanisms for sulfonamides, penicillins, tetracycline, ciprofloxacin, and more recently azithromycin and ceftriaxone, making this microorganism a candidate to cause an untreatable disease (Fig. 1).34–36 In the next section, we describe the evolution of N. gonorrhoeae resistance in a historical and molecular perspective, and provide the basis for understanding why this perception fits among the threats of the post-antibiotic era.
Evolution of Neisseria gonorrhoeae resistance to antimicrobials. Color changes indicate events that impacted the level of resistance along the time each antimicrobial was used. Sulfonamide: introduction, resistance reported, combined use with trimethoprim; penicillin and tetracycline: introduction, chromosomally-mediated resistance reported, plasmid-mediated resistance reported; ciprofloxacin: introduction, resistance reported; azithromycin: introduction, resistance reported, high level resistance reported; cefixime or ceftriaxone: introduction, reduced susceptibility reported.
Sulfonamides were introduced as a therapy for gonorrhea during the 1930s.37 However, by 1944, a rate of 75% treatment failure with sulphathiazole or sulphapyridine were reported amongst World War II soldiers in the Italian and Sicilian campaign.38 This class of antimicrobials acts as a competitive substrate to the dihydropteroate synthetase (DHPS) enzyme in the folic acid biosynthesis. In the gonococcus, resistance is achieved by increased production of the usual substrate, p-aminobenzoic acid, or synthesis of a mutated DHPS with low affinity to the antimicrobial.39
In the 1960s, in order to improve the efficacy of sulfonamide to treat uncomplicated gonorrhea, a combined therapy with trimethoprim was proposed as an option.40 This second compound inhibits an additional reaction in the same metabolic pathway catalyzed by the dihydrofolate reductase (DHFR) enzyme. However, N. gonorrhoeae DHRF has low affinity for trimethoprim, and may also be genetically modified, making the bacteria less sensitive to this antimicrobial.41 Still, the synergic combination of sulfonamides with trimethoprim was used to treat gonorrhea in high and multi-dose therapeutic schemes until the 1970s.42,43
PenicillinPenicillin, successfully used for decades, was introduced as antimicrobial treatment for gonorrhea in 1943, primarily in cases of sulfonamide treatment failure.44 However, penicillin decreased susceptibility in gonococcus starting in the 1960s.45 From then until the 1980s, the efficient penicillin dose for gonococcus infections increased 24-folds, from 200,000U to 4.8millionU. Meanwhile, penicillin MIC of clinical isolates varied from ≤0.015μg/mL to 2.0μg/mL.39
As a β-lactam antimicrobial, penicillin inhibits bacterial cell wall synthesis by binding to transpeptidase enzymes called penicillin-binding proteins (PBP) in the periplasm.46 Accordingly, over the first 50 years of use, penicillin resistance mechanisms in gonococcus were related to decreased susceptibility by cumulative chromosomal mutations in different genes related to cell wall biosynthesis (penA and ponA1), or structures affecting the periplasmic drug concentration (penB, penC and mtrR).
PBP2 is an important PBP in N. gonorrhoeae. Mutations such as an aspartate insertion after the 345 position, and a variable number of substitutions in the protein c-terminal have been detected in isolates with penicillin decreased susceptibility.47,48 Such new alleles are reported as numbered penA variants.49–55 A single base mutation in PBP1, called ponA1 (T to C in 1261bp, resulting in the alteration L421P), decreases the acylation with the β-lactam.56
Mutations in the porB gene are known as penB mutations. Specific substitutions G120K and A121D have been characterized to affect to penicillin and tetracycline MIC in gonococcus.57 Since these positions are presumably located in a region that forms a pore restriction zone, it was first hypothesized that these substitutions affect the penetration of penicillin across the outer membrane.57,58 However, further studies demonstrated that penB mutations affect the MIC of this drug only when MtrC-MtrD-MtrE efflux pump is over-expressed in the gonococcus, through a possible synergistic mechanism.59
The multiple transferable resistance (mtr) system of N. gonorrhoeae encodes proteins that resemble bacterial/efflux-transport molecules occurring in other bacterial species. In the gonococcus, its expression is affected by two transcriptional regulators, MtrR (repressor) and MtrA (activator), and impacts on the susceptibility of the microorganism to a variety of hydrophobic substances, including antimicrobials such as penicillin, tetracycline and macrolides, and detergent-like fatty acids.58,60 Many different mechanisms have been described to affect the expression of mtrCDE. These include mutations leading to dramatic amino acid changes A39T or G45D in the helix-turn-helix DNA binding structure of MtrR,61 and a single base pair (bp) deletion (T:A) or a dinucleotide insertion (TT:AA) within the mtrR promoter, which may impair mtrR expression.60–62 Other changes are increased mtrCDE expression related to a mutation outside the mtrR locus,61 and a cytosine to timine mutation 120bp upstream mtrC, which generates a second promoter to the gene insensitive to the repressive effect of mtrR.61,63
PilQ is an important gonococcal outer membrane component, member of secretin protein family, and involved in Type IV pilus formation. PilQ and Type IV pilus form together an SDS-resistant multimeric complex, which acts as a pore for antibiotics and small molecules. The replacement G666L in the pilQ gene, known as pilQ2 mutation or penC, hinders the creation of the PilQ multimeric, disrupting the pore formed, and hence decreasing the influx of penicillin.56,64
Through simultaneous occurences, chromosomally mediated mechanisms lead to a penicillin MIC up to 4μg/mL in gonococcus, which is twice the MIC reported in infections only treatable with very high penicillin doses.39,56 However, during the 1970s, N. gonorrhoeae isolates presenting MIC up to 128μg/mL emerged,65,66 and ended the penicillin era for gonorrhea treatment. The new resistance mechanism was a plasmid-mediated β-lactamase (bla) gene type TEM (bla-TEM), and the isolates became known as penicillinase-producing N. gonorrhoeae (PPNG).66,67N. gonorrhoeae blaTEM carried plasmids are genetically related, but present different sizes and insertion/deletion sites, and are named according to their epidemiological origin.68,69 The most frequently described bla-plasmids in N. gonorrhoeae are Asia (7426bp), Africa (5588bp), and Rio/Toronto (5154bp). Nevertheless, other types, such as Nimes (6798bp), New Zealand (9309bp), Johannesburg (4865bp), and Australian (3269bp) have been identified in gonococci.65–67,69–74
TetracyclineTetracycline was introduced as a treatment option for gonorrhea in patients allergic to penicillin in the 1950s.34 This antimicrobial agent affects protein synthesis by binding to the 30S ribosomal subunit.75 Similar to penicillin resistance, tetracycline resistance gradually evolved. In fact, some of the chromosomal mutations related to penicillin resistance, such as penB and mtr overexpression, impair the tetracycline activity, raising the MIC to 1μg/mL.39,47 Moreover, an additional single amino acid alteration V57M in the ribosomal protein 10S (rpsJ1) is enough to elevate tetracycline MIC to ≥2μg/mL,76 the current CLSI breakpoint for resistance.12
The first gonococcus isolates showing tetracycline high-level resistance (MIC 24–32μg/mL) were detected in the U.S. in 1985.77,78 The TetM protein was identified as the mechanism responsible for the resistance phenotype, protecting the ribosome from tetracycline binding.39 In N. gonorrhoeae, tetM is conservatively carried by two conjugative plasmids (Dutch and American), similar in size, but probably evolutionarily unrelated. Endonuclease restriction-fragment patterns of both plasmids demonstrate large differences, and even the sequences of the respective tetM encoded are not identical.79 Given the spread of resistance, the quinolone era in the gonorrhea treatment started when tetracycline was no longer recommended by the mid-1980s.80
The quinolone era in gonorrhea treatmentCiprofloxacin was developed in 1983 and was introduced to the U.K. and U.S. markets in the second part of the 1980s.81 Initially, gonorrhea treatment using ciprofloxacin was performed with a single dose of 250mg. However, because of early reported cases of decreased susceptibility, the first CDC recommendation for ciprofloxacin therapy for this STI was 500mg in a single dose.80,82 Although isolates exhibiting reduced susceptibility (MIC≥0.25μg/mL) had been detected in London before 1989,82 and with many therapeutic failures reported during the 1990s,83–85 ciprofloxacin therapy was continued to be used at the same dosage throughout the world, for additional 10–25 years depending on the country.
Quinolones affect the activity of DNA gyrase and topoisomerase IV, two topoisomerases essential for DNA replication, transcription, recombination, and repair. This class of antimicrobial agents acts by forming a drug–enzyme–DNA complex, with further release of double-strand-DNA breaks.86N. gonorrhoeae resistance to ciprofloxacin is mediated by mutations in the quinolone resistance-determining region (QRDR), located near the topoisomerases DNA binding site. Such mutations influence susceptibility of isolates cumulatively.87 Mutations leading to a single amino acid change in GyrA positions 91 or 95 lead to intermediary resistance level (MIC 0.1–0.5μg/mL); however, three or more amino acid changes in GyrA (at positions 91, 95 and 102) and ParC (87 and 91) and/or Par E (439) proteins may lead to MIC≥32μg/mL.25,88
One decade after being recommended as the first choice for gonorrhea treatment in the U.S., ciprofloxacin treatment was abandoned in the Asian Western Pacific, because of high resistance rates. In Japan, for example, the resistance percentage was 6% in 1993–1994 and rose to 24% in 1997–1998. Unfortunately, no information about the phylogeny of resistant strains was available.89 By then, ciprofloxacin-resistant gonococcus also emerged in Europe. Studies confirmed the genetic correlation among the isolates, highlighting the clonal pattern of ciprofloxacin resistant N. gonorrhoeae spread, mainly due to sexual networks.90–92 In a survey study conducted in Brazil from 2006 to 2010, 23 of the 25 resistant ciprofloxacin N. gonorrhoeae isolates were clustered into two clonal groups.25
In the U.S., the gonococcal ciprofloxacin resistance reached the west coast of the country and then spread through the rest of the territory. The first ciprofloxacin resistance isolates (MIC 1–16μg/mL) were detected in Hawaii in 1999, affecting 9.5% of the isolates.93 Soon afterwards, the same trend was seen in California (4.9% in 2001), which made the CDC withdraw the ciprofloxacin recommendation treatment in those states.94 Ciprofloxacin was removed from guidelines in Asia, Europe, and the U.S. in the early to mid-2000s.95
Extend spectrum cephalosporins and azithromycin: the current recommended antimicrobials to treat gonorrheaAzithromycinAzithromycin was included as a possible therapy for gonorrhea in the beginning of the 1980s.95 This macrolide interacts with the P site of the 50S ribosomal subunit, impairing the peptidyl transferase polypeptide chain elongation.96
Different mechanisms may impact azithromycin activity in N. gonorrhoeae. One of them is the overexpression of the efflux pump mtrCDE directed by the same molecular mechanisms reported to decrease the susceptibility of N. gonorrhoeae to penicillin, increasing the azithromycin MIC to 0.5μg/mL.62,97 An additional mechanism of azithromycin reduced susceptibility is the occurrence of mutations in the L4 ribosomal protein.97,98 This protein is in close contact with the peptidyl-transferase region in the V domain of the 23S rRNA, and mutations leading to dramatic amino acid changes, as the reported G70D in its extended loop, may indirectly impact the rRNA conformation.97,99–101
When mutations occur directly in this 23S rRNA domain, resistance to azithromycin emerges. In this case, two main mechanisms are described. A single adenine methylation, promoted by plasmid mediated erm enzymes in the 2058 position of the peptidyl transferase V domain, prevents antimicrobial target binding, and rises the azithromycin MIC to 1–4μg/mL.95,102,103 Moreover, the specific substitutions A2143G and/or C2599T present in one to four rrl gene alleles, encoding the 23S RNA, result in varied azithromycin MICs. Mutations in a single allele usually do not significantly impact the MIC103,104 however, MICs of 96μg/mL or >256μg/mL were reported for isolates showing C2599T or A2143G mutations in the four alleles, respectively.105,106
N. gonorrhoeae exhibiting a high level of resistance to azithromycin has emerged in the past few years. The first case occurred in Argentina in 2001.107 Since then, the detection of high level azithromycin resistance has occurred in different countries, such as Scotland, England, Italy, U.S. and China.108–110 An outbreak with isolates exhibiting MIC≥256μg/mL affected five men and three women, all heterosexual, in England, between November 2014 and March 2015. Seven isolates were from the same NG-MAST ST9768 and presented the A2143G substitution in all four 23S encoding gene alleles.111 Nonetheless, other studies characterizing collections of isolates presenting high MIC to azithromycin demonstrated that such resistance frequently occur in non-clonally related isolates, with a variety of NG-MAST STs being detected in a same country or region.104,106,112 In this case it is worth mentioning that the occurrence of NG-MAST ST649 presenting similar azithromycin MIC (≥256μg/mL) and 23S mutations in three different continents.104,106,109
CeftriaxoneThe ESC ceftriaxone presents high activity against gram-negative bacteria, such as N. gonorrhoeae, by high affinity binding to PBP2.113 Ceftriaxone introduction as a monotherapy for gonococcal infections was driven by the rise of ciprofloxacin resistance rates.35 Cefixime, an oral ESC, was in use before ceftriaxone; however, susceptibility to this antimicrobial decreased very quickly. For example, resistance rates reached 30% in six hospitals in the central territory of Japan in the beginning of the 2000s.49,114 Subsequently, treatment failures with cefixime monotherapy were reported in Europe.115–117
ESC resistance might be promoted by alterations in penB, mtrR, and penC gene, although mutations in penA gene, which encodes PBP2, seem to be the main ceftriaxone resistance determinant. The altered penA gene can result from point mutations, or from genetic recombination between commensal Neisseria spp. colonizing other human sites, the last generating a mosaic-like structure.95,118 The altered PBP2 presents diminished affinity to ceftriaxone.
Resistance to ceftriaxone is characterized by MIC>0.5μg/mL by CDC5 and >0.125μg/mL by EUCAST,11 and detection of resistant isolates is still rare, with one reported case in each Japan, France and Spain.55,119,120 ESC resistance has been related to the presence of different patterns of PBP2. Nevertheless, studies have associated the reduced susceptibility to the clonal spread of specific lineages.55,119–121 In Japan, the resistant isolate exhibited MIC 8μg/mL and 4μg/mL to cefixime and ceftriaxone, respectively, presenting PBP2 pattern highly similar to the PBP2 X, with four additional mutated amino acids residues A311V, T316P, A328T, and T484S.55 This isolate was assigned to ST7363 by MLST, the same ST of previous cefixime-resistant N. gonorrhoeae isolates circulating in Japan.54 The isolates detected in France and Spain were included in the same NG-MAST ST1407, a successful clone circulating in Europe and associated with decreased susceptibility to ESC. The isolates exhibited MIC 4μg/mL and 1.5μg/mL to cefixime, and 2μg/mL and 1.5μg/mL to ceftriaxone in France and Spain, respectively. The PBP2 XXXIV pattern with one additional amino acid substitution A501P was detected in the isolates from both countries.119,120 The occurrence of this clone is epidemiologically related to MSM.
The emergence of ESC resistance in gonococcus and the absence of a new perspective for treatment of gonorrhea have motivated the dual therapy protocols with ceftriaxone and azithromycin. According to agencies performing surveillance programs, this strategy may decelerate the increase of resistance rates to these antimicrobials.30,122
Concluding remarksCurrently, comparing N. gonorrhoeae resistance rates and epidemiological trends among countries is a difficult task, considering the different breakpoints and sampling strategies that have been adopted worldwide. Data obtained in different countries emphasize that surveillance is indeed essential to preserve the possibility of treatment based on syndromic diagnosis for gonorrhea.
The implementation of such surveillance programs may pose some challenges. For instance, in Brazil, the lack of interaction between research centers and the official health systems facilities face major obstacles. Moreover, continuing institutional and financial assistance is necessary to obtain, maintain and analyze N. gonorrhoeae isolates in a standard way for consecutive years. Collecting antimicrobial susceptibility testing results from diagnostic laboratories may prove useful to have an idea of current resistance rates. However, such approach is not applicable to epidemiological studies since patients’ data and even the isolates are not available for further studies
In conclusion, given the particularities of the disease, which is community based, and the difficulties of obtain and preserve N. gonorrhoeae isolates, extensive organization is necessary to implement adequate surveillance programs. In this sense, we believe that a good strategy would be to establish associations between STI medical centers and accredited laboratories that can report results to a governmental agency responsible for standardized protocols, centralize data and publish regular reports. The international agencies collaboration may prove necessary in achieving an efficient global action, especially in low income countries.