Polyhydroxyalkanoates (PHA) are efficient, renewable and environment friendly polymeric esters. These polymers are synthesized by a variety of microbes under stress conditions. This study was carried out to check the suitability of waste frying oil in comparison to other oils for economical bioplastic production. Six bacterial strains were isolated and identified as Bacillus cereus (KF270349), Klebsiella pneumoniae (KF270350), Bacillus subtilis (KF270351), Brevibacterium halotolerance (KF270352), Pseudomonas aeruginosa (KF270353), and Stenotrophomonas rhizoposid (KF270354) by ribotyping. All strains were PHA producers so were selected for PHA synthesis using four different carbon sources, i.e., waste frying oil, canola oil, diesel and glucose. Extraction of PHA was carried out using sodium hypochlorite method and maximum amount was detected after 72h in all cases. P. aeruginosa led to maximum PHA production after 72h at 37°C and 100rpm using waste frying oil that was 53.2% PHA in comparison with glucose 37.8% and cooking oil 34.4%. B. cereus produced 40% PHA using glucose as carbon source which was high when compared against other strains. A significantly lesser amount of PHA was recorded with diesel as a carbon source for all strains. Sharp Infrared peaks around 1740–1750cm−1 were present in Fourier Transform Infrared spectra that correspond to exact position for PHA. The use of waste oils and production of poly-3hydroxybutyrate-co-3hydroxyvalerate (3HB-co-3HV) by strains used in this study is a good aspect to consider for future prospects as this type of polymer has better properties as compared to PHBs.
Polyhydroxyalkanoic acids (PHA) present inside prokaryotic cells are carbon and energy reserved granules stored during environmental stress conditions. About 300 different bacterial strains are identified to accumulate PHA.1 All Gram positive, Gram negative, archea, halophilic, halotolerant, root nodule bacteria are able to produce PHA.2,3
Bacteria has the ability to produce PHA from a diverse range of carbon sources such as complex waste effluents to plant oils, carbohydrates, short chain fatty acids, and alkanes. Wastes are discharged in large amounts from food processing industries and agricultural wastes that have the potential to be used as carbon sources for PHA production.4
Carbohydrates are a good source but the production cost remains high as compared to synthetic plastics, so the search must be continued. Carbon substrates including organic fatty acids have also been reported for PHA production.5 Therefore to make PHA production more economical, one of the foremost steps is to isolate naturally occurring bacterial strains that utilize less expensive carbon sources such as molasses that are obtained either from sugar beets or sugarcane. Molasses normally sold for about 33–50% of the cost of glucose.
In order to reduce the production cost researchers are trying to produce PHA from plant oils. These oils have been projected to be more efficient carbon sources for industrial PHA production than sugars.6 Industrial-scale processes for the production of PHA from plant oils are currently being developed.7 Different plant oils such as palm kernel oil, palm olein, crude palm oil, palm acid oil, canola oil, soya bean oil, corn oil and olive oil have been reported.8,9Comamonas testosteroni has been studied for its ability to synthesize medium chain length mcl-PHA from vegetable oils such as castor seed oil, coconut oil, mustard oil, cotton seed oil, groundnut oil, olive oil and sesame oil. Kahar and coworkers reported that the recombinant strains of Ralstonia eutropha are able to use plant oil such as soybean oil,10 although the use of plant oils for plastic production may create a sense of competition for food sources. Various agro-industrial wastes like wheat bran, potato starch, sesame oil cake, groundnut oil cake, cassava powder, jackfruit seed powder and corn flour were tested for PHA production by Bacillus sphaericus which showed that jackfruit seed powder led to the production of maximum 19% PHBs. Many other agricultural and industrial wastes have also been reported as carbon sources including cassava bagasse hydrolyzate, babassu, soy cake, cane molasses and whey for PHB production.11 Some scientists have tried waste plant oils as substrate. It has been shown that Cupriavidus necator produced 1.2g/L PHA from waste frying oil that is in accordance with previous results obtained with glucose.12
The aim of this research work was to check the PHA production ability of purified strains using cheap carbon sources. We analyzed production ability of lipase producing bacterial strains using four different carbon sources including glucose, waste frying oil, diesel and cooking oil. Study of growth kinetics of bacterial strains and product accumulation kinetics using different oils parallel with glucose as standard carbon source was carried out. Then chemical analysis of the produced PHA using Fourier transform infrared spectroscopy (FTIR) was performed to identity monomer type of PHA our bacterial strains are able to produce by using these carbon sources.
Materials and methodsBacterial strainsStrains were obtained from Department of Microbiology and Molecular Genetics, University of the Punjab, Lahore and were named accordingly using the abbreviation for strain (STN) STN-6 to STN-11. All the strains were streaked on L-agar plates and incubated at 37°C for 48h to get isolated colonies. PHA detection agar (PDA)13 containing 5g/mL Nile blue was used for the direct screening of PHA producers. Medium (PDA) was supplemented with 20g/L glucose. Plates were streaked and incubated at 37°C for 24–48h.14
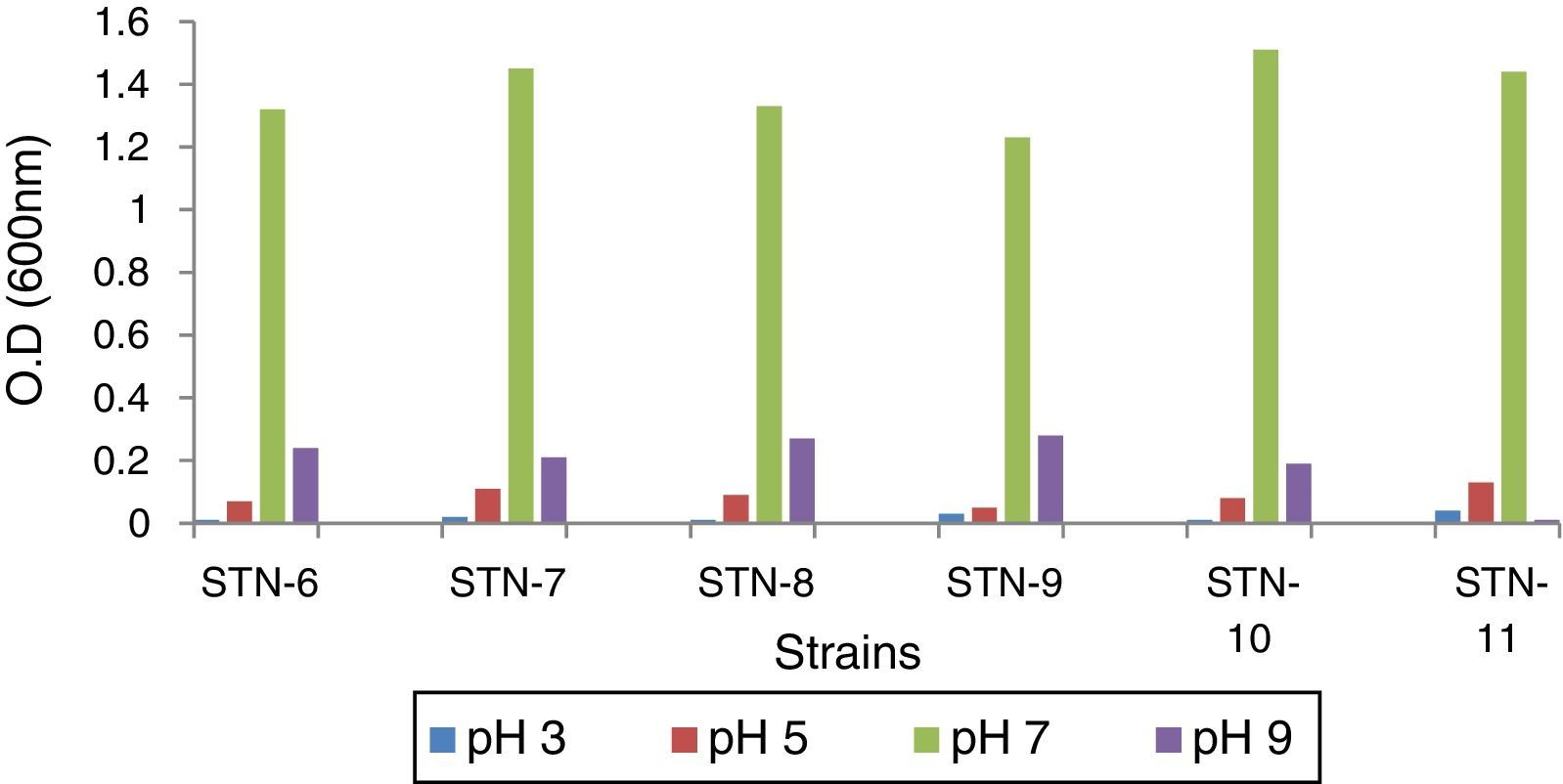
Optimization of bacterial strains for pHIn order to find optimal pH for bacteria to grow and accumulate PHA granules, strains were grown on PDA medium having pH range 3–9. Optical density (O.D.) was recorded after every 24h till 72h at 600nm and graphs were plotted.
Analysis of lipase enzyme activity of bacterial isolatesPurified strains were tested for their ability to utilize oils and the activity of lipase enzyme was checked by growing them on tributyrine agar.15
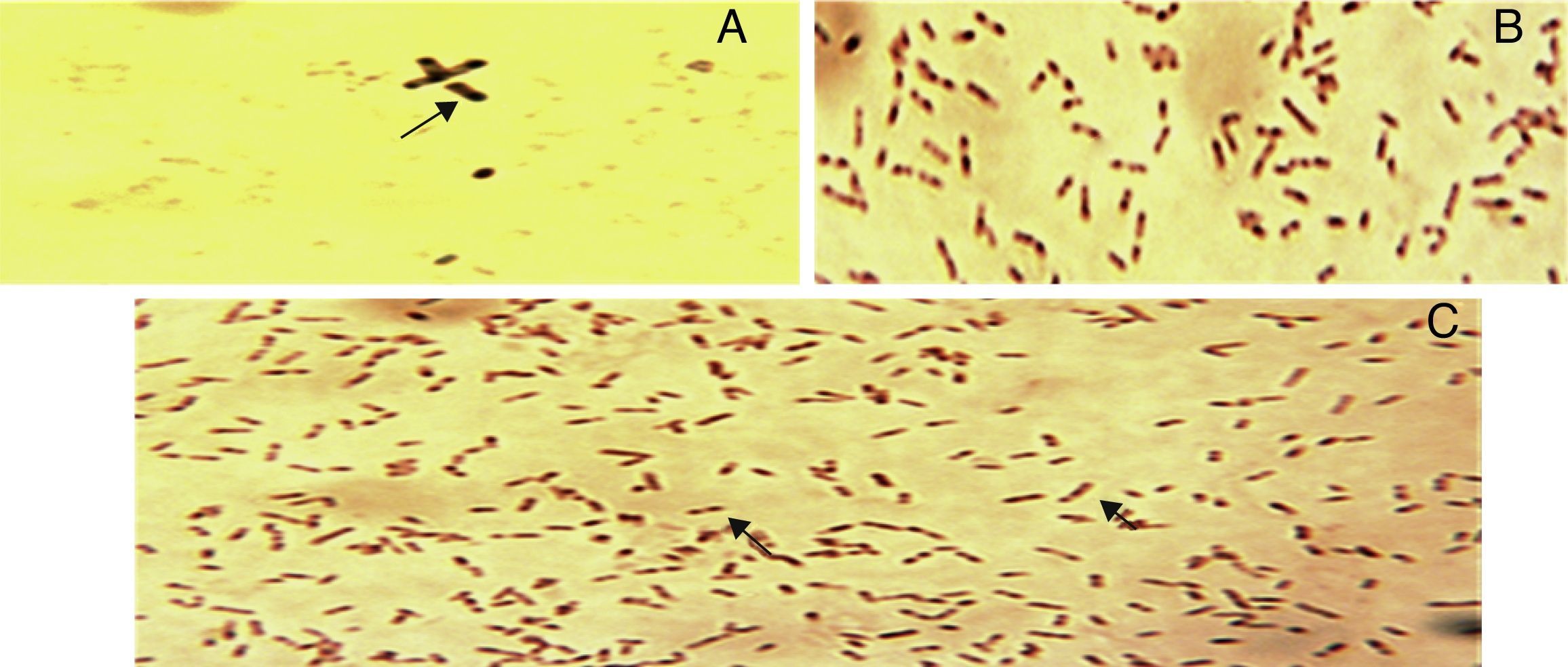
Sudan black B stainingSudan black B (0.3%) in (ethyl alcohol) was used for detection of PHA inclusion bodies in cells. Heat fixed smears of strains were prepared and stained with Sudan black B for 15min.14 Slides were examined under Olympus CX31 microscope (Olympus, Inc, USA) using 100× objective lens with oil immersion.
Fluorescent stainingFor fluorescent microscopy, 10μL of 72h old bacterial culture and 50μL of acridine orange (0.1%) were taken in an eppendorf tube and incubated at 30°C for 30min. Pellet was collected by centrifugation at 4000×g for 5min.16 On clean microscopic slide smear was prepared and observed under 100× objective lens of Leica BZ-01 fluorescence microscope (Leica Microsystems, Germany).
Growth kinetic studies and growth conditionsShake flask fermentation process was carried out using 250mL Erlenmeyer flasks containing 100mL of PDA medium with glucose, waste frying oil (WFO), cooking oil and diesel as carbon substrates at 2% concentration and sterilized by autoclaving at 121°C and 15lb/in.2 for 20min. The oil molecules were separated using sonicator12 and then shaking in incubator so that bacteria were able to utilize it. Experimental design includes four flasks for each carbon source at 2% concentration and was inoculated with 24h old bacterial culture from seed culture medium. One flask was kept as control and all were incubated at 37°C and 100rpm in a shaker. The aliquots (2mL) of incubated medium were taken out at regular intervals of 8h till 72h from both the control and inoculated medium. Bacterial growth was determined by recording optical density at 600nm. Along with it, 15mL sample was collected every 24h till 72h and biomass was obtained by centrifugation at 4000×g for 20min. Pellet was lyophilized and biomass weight was calculated.
Extraction of PHA using sodium hypochlorite methodPHA was extracted from dried biomass after 72h using sodium hypochlorite digestion method.17 In 8g biomass, 100mL sodium hypochlorite (30%) was added and incubated for 90min at 37°C. Pellet was collected by centrifugation and washed with alcohol, dissolved in chloroform and transferred to clean and pre-weighed serum tubes. Later chloroform was allowed to evaporate and weight of PHA was calculated.
Fourier transform infrared spectroscopy (FTIR) spectroscopyFor FTIR analysis biomass was collected aseptically after 72h of incubation. Medium was removed by washing it thrice with autoclaved distilled water and centrifugation. The resulting washed biomass was lyophilized and further dried by placing it in a hot air oven for 3h. One part of biomass was combined with three parts of KBr and grinded well so that a transparent pellet was obtained. This pellet was placed in IR spectrometer and peaks were obtained. The selected range was 400–4000wavenumbercm−1.
Isolation of genomic DNAIsolation of bacterial DNA from 5mL culture of selected strains was carried out using DNA isolation method,18 suspended the obtained pellet in 100μL TE buffer and stored at −20°C. Gel electrophoresis was carried out for detection of DNA by loading 5μL of genomic DNA in wells of the gel and allowed to run at 70V.
Polymerase chain reaction (PCR) amplification of PhaC gene sequencesGene amplification was performed using PCR for PhaC gene. For this purpose, extracted genomic DNA was used as template. PCR reaction mixture was prepared in sterile condition. This PCR PhaC gene amplification program was run for 30 cycles. Annealing temperature was 60°C. PCR product was purified by using liquid PCR product purification kit (Fermentas, Inc.). All steps were performed according to instructions given in manufacturer protocol. Purified products were run on 1% agarose gel. PCR purified products were submitted to Center of Excellence in Molecular Biology (CEMB), University of the Punjab, Lahore, Pakistan for analysis. Deoxyribonucleic acid DNA sequencing was carried out by dideoxy method (chain termination) using apparatus applied biosystem DNA sequencer. All the sequence results for amplified fragments were analyzed using BLAST algorithm (http://www.ncbi.nlm.nih.gov/blast/Blast.cgi). Multiple sequence alignments, and sequences were submitted to NCBI GenBank at http://www.ncbi.nlm.nih.gov/genbank/submit.html.
ResultsBacterial strainsPreviously isolated strains were obtained from Department of Microbiology and Molecular Genetics, University of the Punjab, Lahore, Pakistan and identified by sequencing. These were grown on PDA medium with pH range 3–9 which showed that neutral pH (7) is the optimal pH for growth and PHA production (Fig. 1). No growth was observed at pH 3 while very little at pH 9 which indicated that the microorganisms could not survive at acidic or basic pH.
The strains were analyzed for PHA production by growing them on PHA detection agar (2% glucose) with 5μg/mL Nile blue dye, out of six, three strains could produce blue fluorescence under UV-transilluminator. All isolates contained black granules in them when observed with Sudan Black B (Fig. 2). Genomic DNA was successfully isolated from all 6 strains. These were identified as STN-6 Bacillus cereus (KF270349), STN-7 Klebsiella pneumoniae (KF270350), STN-8 Bacillus subtilis (KF270351), STN-9 Brevibacterium halotolerance (KF270352), STN-10 Pseudomonas aeruginosa (KF270353), and STN-11 Stenotrophomonas rhizoposid (KF270354) by ribotyping. PhaC gene was successfully amplified from P. aeruginosa.
Analysis of lipase enzyme activity of bacterial isolatesStrains STN-8, STN-10 and STN-11 were able to form clear halo after 24h of incubation while STN-7 and STN-6 formed slightly visible halo after 48h of incubation which confirmed that strains have active lipase enzymes and hence are able to utilize oils. One of the isolates, STN-9 was unable to indicate lipase enzyme activity as it did not form halo around the growth even after 48h of incubation.
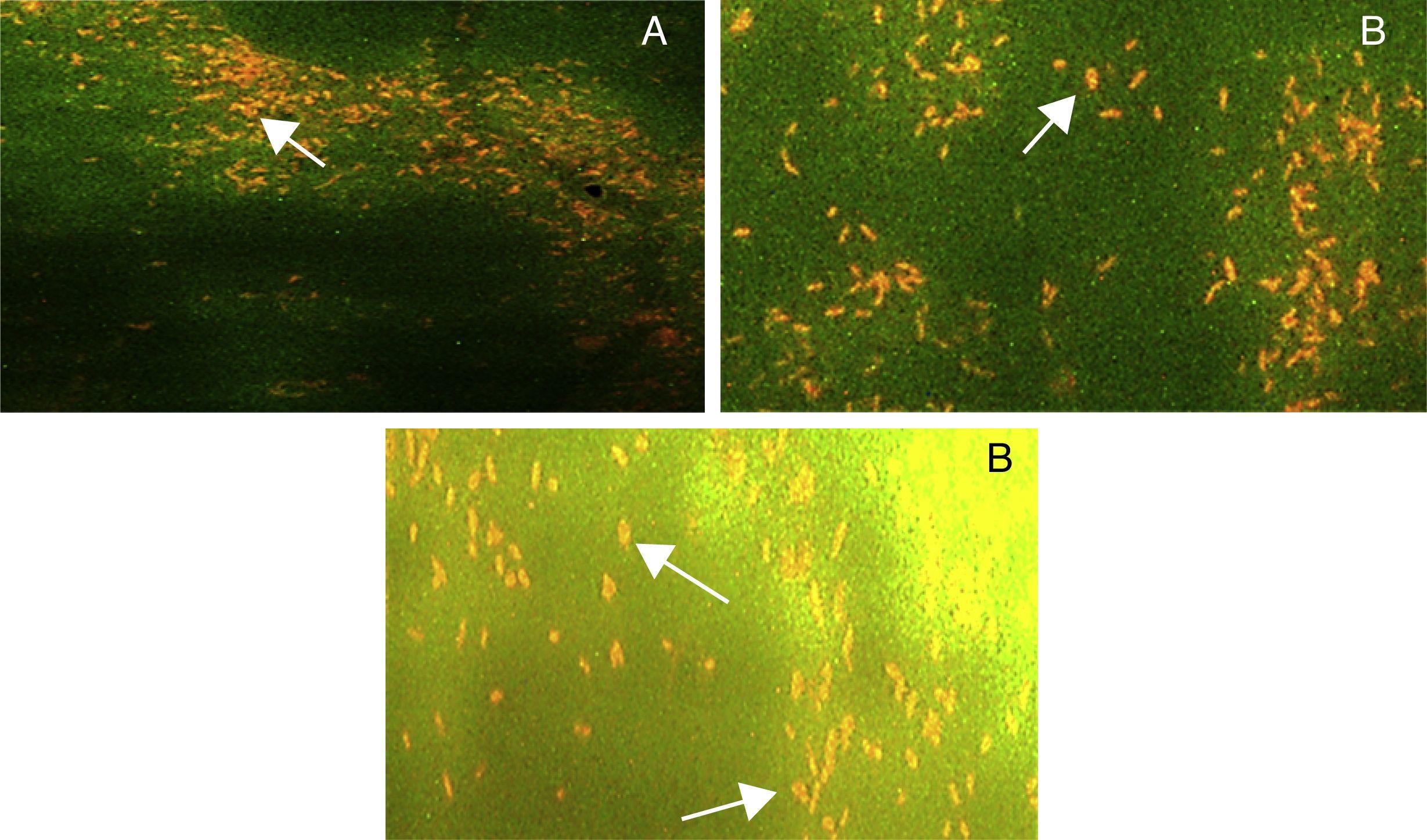
Fluorescent microscopyAcridine orange stained slides of three selected strains STN-7, STN-8 and STN-10, showed orange fluorescence with all the given carbon sources. Results showed that lesser number of cells was fluorescing under UV light as compared to white light. Maximum numbers of fluorescent cells were observed for STN-10 when waste frying oil (WFO) was used in the media. However number of fluorescent cells for all three strains was comparable with different carbon sources. Results indicated a clear difference in number of fluorescent cells when incubation time increased from 24 to 72h. An increase in size of fluorescent cells was noticed and it seemed that larger cells were producing more fluorescence (Fig. 3). Slight difference in external morphology or shape of fluorescent cells was also noticed.
Fluorescence microscopy of strain STN-10 with 0.1% acridine orange showing presence of PHA granules after 72h of incubation (A) on PHA biosynthesis media supplemented with WFO as carbon source, (B) on media supplemented with cooking oil as carbon source and (C) on media supplemented with glucose as a carbon source (magnification 100×).
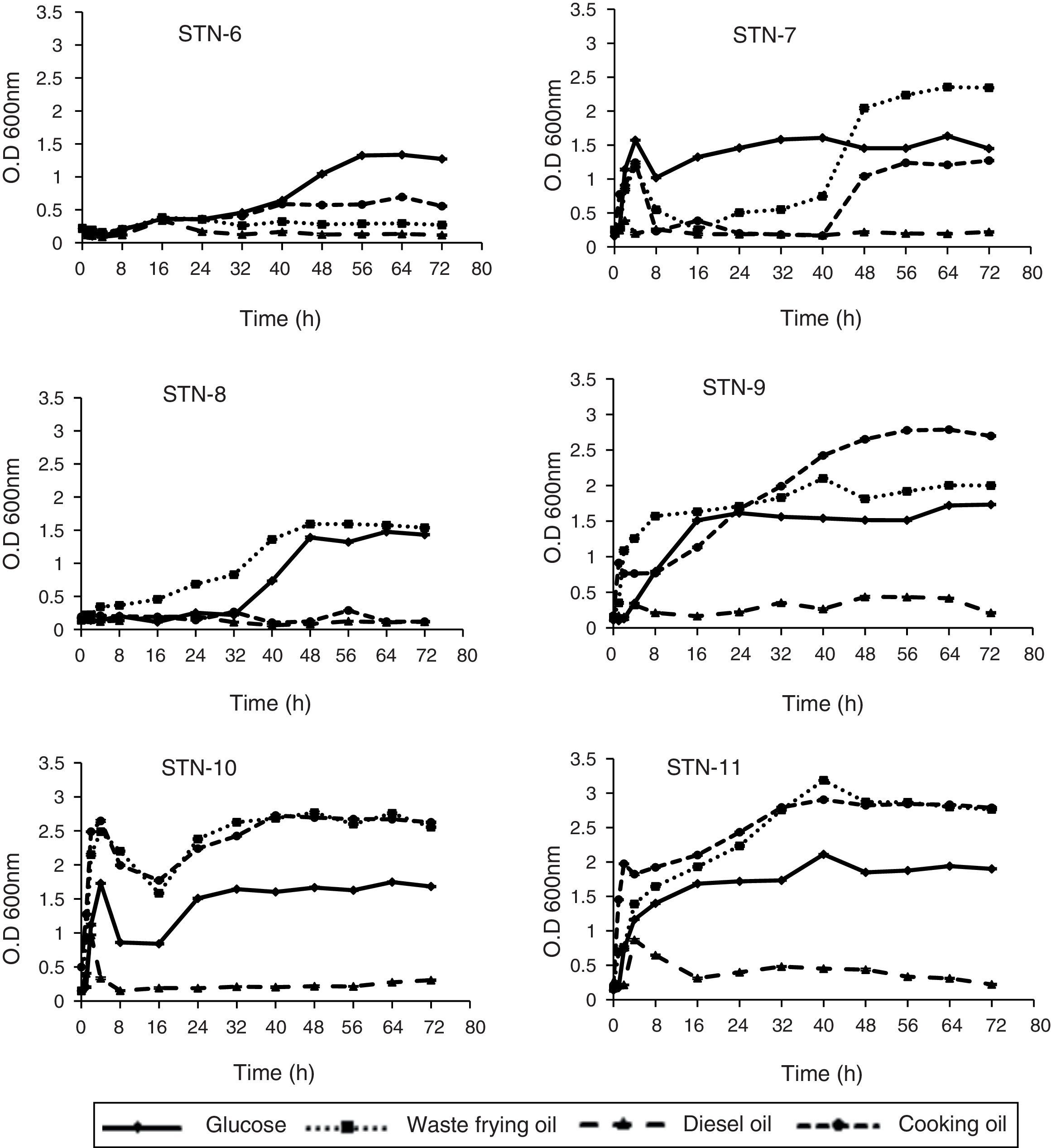
Growth curves indicated that all strains proliferated easily on all sources except diesel. In almost all cases growth steadily increased with increasing time of incubation from 2 to 60h. The strains log and lag phases were observed at variable incubation time however; stationary phase for all strains was around 60–72h. Strain STN-7, STN-8 and STN-10 produced maximum growth with WFO after 48h. Both STN-6 and STN-9 showed maximum growth after 56h of incubation with glucose and cooking oil as carbon source respectively. Strain STN-11 indicated maximum absorbance at 40th hour with WFO supplemented PHA media and utilized waste frying oil and cooking oil equally well for its growth (Fig. 4).
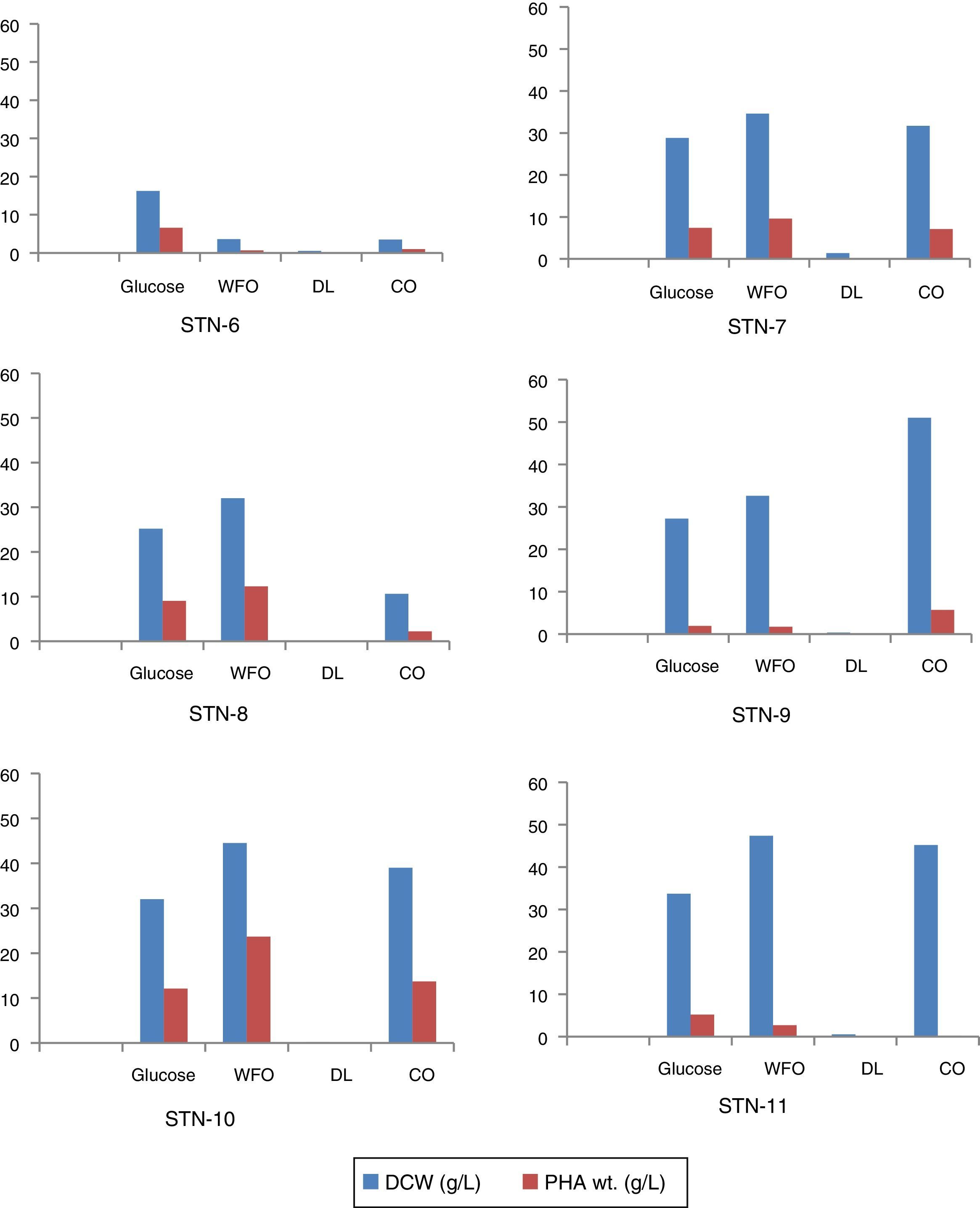
Kinetics of product (PHA) accumulationAlmost all strains showed similar pattern of PHA accumulation, i.e., amount of PHA increased gradually through 24, 48 and 72h of incubation. Among all the isolates strain STN-10 produced maximum amount of PHA 53.2% (23.7g/L) using WFO as carbon source after 72h. The amount of PHA accumulation with cooking oil as carbon source was 34.4% (13.7g/L) shown by strain STN-10 and proved to be the best strain to utilize oils. Strain STN-8 accumulated 35.7% (9.0g/L) from glucose and 38.4% from WFO (12.3g/L) after 72h incubation period. Strain STN-7 produced maximum 9.6g/L (27.8%) PHA from WFO and strain STN-6 accumulated 40% PHA using glucose as carbon source and was noted as the only strain to utilize glucose the most as compared to all other sources used. The maximum amount obtained with diesel was 0.02g/L which is 10% of total biomass content produced by strain STN-10 after 72h. Biomass production and the comparative accumulation of PHA in 100mL media after 72h of incubation are shown in Fig. 5.
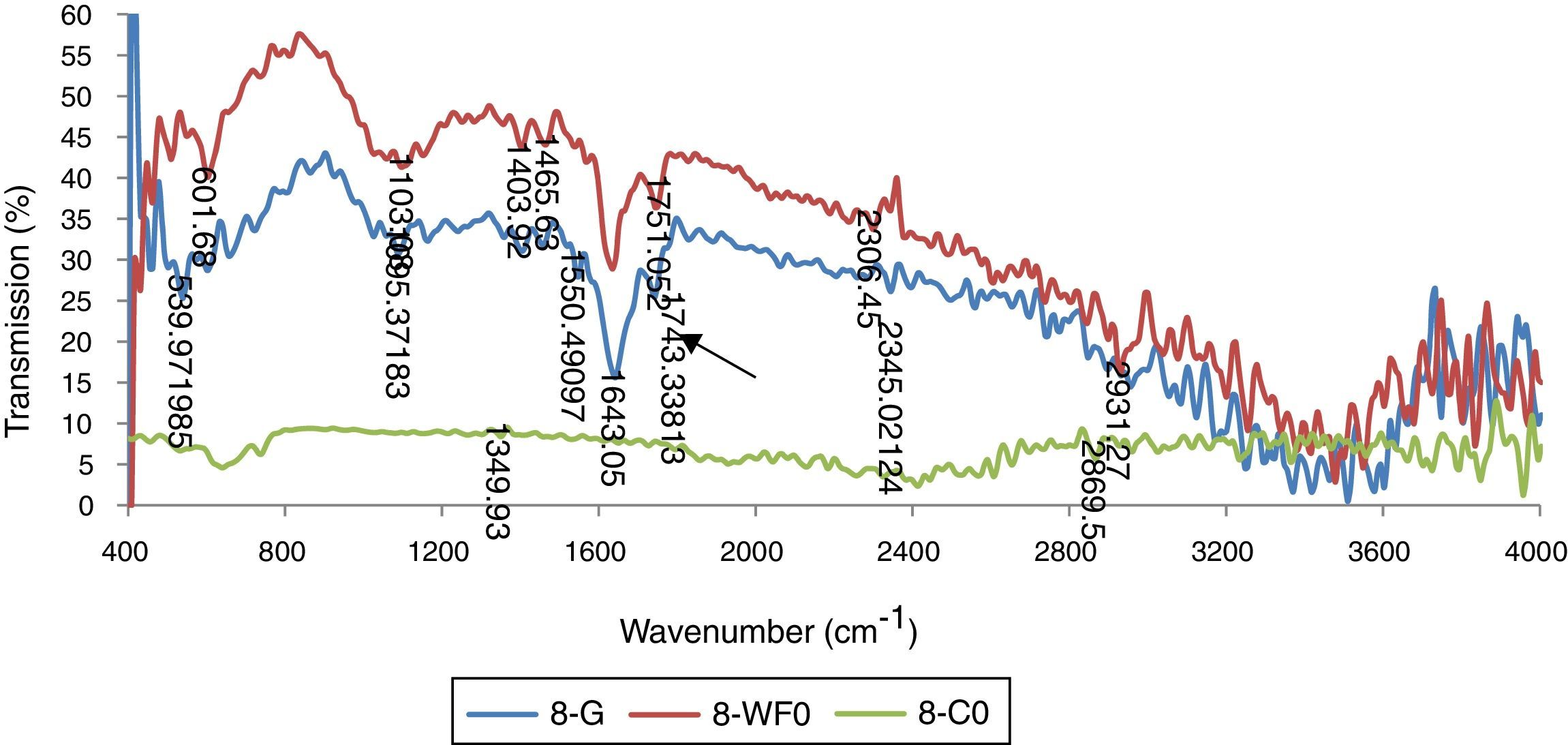
Chemical analysis by FTIR spectroscopyResults for FTIR analysis showed different peaks representing the presence of diverse functional groups. The presence of different peaks showed that bacterial biomass has complex accumulations. Many sharp peaks were present in the range of 1000 wave number (cm−1) to 3000 wave number (cm−1). This is the specific region of PHA specific functional groups. The spectra for Bacillus subtilus (STN-8) P. aeroginosa (STN-10) and Klebsiella pneumoniae (STN-7) revealed that sharp absorption bands at 1741cm−1, 1635cm−1 and 1249cm−1 were present which correspond to ester carbonyl group (CO), ester group stretching C–O and –CH group respectively and correspond to the peak positions for PHA (Fig. 6). Bacillus strain showed these functional groups by utilizing glucose, waste frying oil and cooking oil. It has been reported that B. cereus showed almost the same peak positions by growing on nutrient broth and these peaks are characteristic peaks for PHBs.19
DiscussionPurified carbon sources used for PHA accumulation are costly that hinders PHA production at large scale, therefore we tried to identify cheap and readily available carbon sources glucose, waste frying oil, diesel and cooking oil. We used cooking oil and waste frying oil specifically of canola as it is hardly reported for this kind of biological (microbial) transformation. Although studies using waste frying oil have exposed that it is a good carbon source but less work has been done.
The highest PHA producing strain P. aeruginosa (STN-10) produced 53.2% PHA using waste frying oil that is higher than that produced using glucose (37.2%), a standard commercially available carbon source. Pseudomonas spp. has been shown to produce 5 and 23.52% PHA of their DCW by utilizing waste frying oil20,21 and has high growth rate among oil degrading bacteria by following β-oxidation pathway.22 Waste sesame oil has also been reported as carbon source for PHB accumulation by C. necator with maximum yield of 4.6g/L.23 Other strains used in this study, Klebsiella pneumoniae (STN-7) and B. subtilis (STN-8) also produced good yields with 27.8 and 38.4% PHA respectively using waste frying oil. The PHA content in B. subtilis decreased after 48h as it may be consumed for sporulation process.24 The DCW did not follow the same trend as a clear decrease in the amount of DCW occurred after 48h which may be due to nutrients depletion.
Strain STN-7 showed that for all the carbon sources used PHA accumulation increased with increase in DCW over time except for WFO where DCW remained constant. Comparatively the utilization of WFO was less in case of STN-6 and STN-11 that produced 18.1 and 5.7% PHA using waste frying oil respectively but produced good yield using glucose and CO. These strains may lack the enzymes for conversion of fatty acids into PHA as the environment of their proliferation were free of oils but enriched with carbohydrates and other nutrients. Strain STN-9 showed a variable pattern for PHA production with all carbon sources. By utilizing glucose and diesel as carbon source it produced maximum PHA after 48h but decreased afterwards which may be because of the re-utilization of these storage granules. It has been reported that at cessation of log phase or early stationary phase the PHA accumulation reaches its maximum but after that it decreases due to sporulation.24
All strains did not utilize diesel and not even accumulated it in the form of PHA. The reason may be its composition containing high concentrations of many aliphatic and aromatic hydrocarbons. These hydrocarbons change the membrane structure by changing the membrane fluidity and protein conformations. This overall alteration disrupts membrane integrity, energy transduction mechanisms and membrane associated enzyme activity. This is how diesel kills microorganisms and proves to be toxic for the microbes25 while other reason may be its volatile nature which makes it unavailable through one week experimental procedure.
On the whole the results of kinetics of PHA accumulation showed that strains STN-10, STN-7 and STN-8 grow best by utilizing waste frying oil (WFO) as carbon source and accumulate more PHA using WFO than cooking oil. This may be due to change in composition of oil after frying as it has been reported that after being used, a change in composition occurs and have very less quantity of polar compounds.26 After frying the canola oil it has 78% of oleic acid level and very low level of total polar compounds. Other than oleic acid, 20% linoleic acid was also present.27 The composition of oil before and after frying changes the percentage of different compounds (lauric acid, palmitic acid, oleic acid, linoleic acid and stearic acid, etc.) either increases or decreases, and overall fried oil has low molecular weight acids,28 free fatty acids and the constituents (proteins, carbohydrates and lipids) of the fried food get transferred to that oil,23 which seems to be beneficial for the efficient production of PHA. Our strains not only produced PHBs but they produced PHBs and PHVs copolymers. The cost effective production of co-polymers using waste oil as carbon source has been achieved previously23 and it has been reported that a polymeric material which is a blend of PHBs and PHVs is more strong and flexible than PHBs29 hence more suitable for practical applications.
The findings of this research work recommend the use of waste frying oils because PHA production using waste oil was high as compared to cooking oil and glucose. Bacteria under research not only produced PHB but also produced 3(PHB-co-PHV) which more strongly states the potential of these bacteria and the waste frying oil as source for industrial use17 as this copolymer is more promising material for practical applications. It also showed that utilization of waste frying oil not only makes bioplastic material (PHA) economical and improves its market but it will also make management of oily waste very easy. Future prospects include the optimization of these bacterial strains for PHA production at large scale fermentation for volumetric production of this Eco-Green material using waste frying oil as cheap substrate.30
Conflicts of interestIt is stated that the authors have no conflict of interest in either way such as personal, political, financial, academic, or religious.
The authors would like to thank University of the Punjab and Higher Education Commission, Pakistan (Project No: NRPU-20-3443) for providing financial assistance in this work.