Antibodies and antibody fragments are nowadays among the most important biotechnological products, and Pichia pastoris is one of the most important vectors to produce them as well as other recombinant proteins. The conditions to effectively cultivate a P. pastoris strain previously genetically modified to produce the single-chain variable fragment anti low density lipoprotein (−) under the control of the alcohol oxidase promoter have been investigated in this study. In particular, it was evaluated if, and eventually how, the carbon source (glucose or glycerol) used in the preculture preceding cryopreservation in 20% glycerol influences both cell and antibody fragment productions either in flasks or in bioreactor. Although in flasks the volumetric productivity of the antibody fragment secreted by cells precultured, cryopreserved and reactivated in glycerol was 42.9% higher compared with cells precultured in glucose, the use of glycerol in bioreactor led to a remarkable shortening of the lag phase, thereby increasing it by no less than thrice compared to flasks. These results are quite promising in comparison with those reported in the literature for possible future industrial applications of this cultivation, taking into account that the overall process time was reduced by around 8h.
Monoclonal antibodies and antibody fragments are regarded as biopharmaceuticals with huge potential to boost novel treatments of cancer, inflammatory and infectious diseases.1,2 In 2010, about 7% of the global market of pharmaceuticals (US$ 597 billion) was constituted by monoclonal antibodies and 10% by recombinant proteins.3–5 Antibodies have emerged as successful targeted therapeutics owing to the specificity of their antigen binding fragment and potent immune effector functions via the crystallizable fragment.6,7
Essentially, the single-chain variable fragment (scFv), where VH and VL domains are connected via a flexible polypeptide, retains the specificity of the parent antibody with improved pharmacokinetics for tissue penetration and is stable for use in immunodiagnostic kits.8 Recombinant antibody (rAb) fragments have been expressed in a wide variety of microorganisms including Escherichia coli,9Bacillus subtilis, Saccharomyces cerevisiae10 and Pichia pastoris.2
P. pastoris has become a popular host for industrial protein production because of its ability to produce foreign proteins at high levels.11 Alcohol oxidase 1 promoter (AOX1) is one of the most widely utilized among all the available promoters for P. pastoris.12,13 It is tightly repressed during yeast growth on glucose or ethanol, but effectively induced by methanol,14 which, however, is toxic at high levels.15 Thus, the use of glycerol as a cosubstrate could be an interesting, alternative strategy,13,16 provided that it does not repress AOX1 promoter at relatively low level.14 Based on this information, a typical rAb production by P. pastoris consists of three distinct phases: (a) glycerol batch phase to stimulate growth, (b) glycerol fed-batch phase for AOX1 derepression, and (c) methanol induction phase to express the recombinant protein.17–19 In the last phase methanol is typically pulsed repeatedly after complete depletion of substrate.20
For therapeutic purposes, large doses of antibodies are required, which in some cases exceed one gram per patient per year. Thus, there is a need to develop new processes to produce these molecules efficiently and cost effectively.21 An optimal expression system depends on the type, purity and quantity of the rAb fragment to be expressed. However, to make the production of monoclonal antibodies by yeasts feasible, there is still the need to shorten the process time to increase volumetric productivity22; in addition, the conditions reported in the literature for preadaptation and cryopreservation of P. pastoris cells are quite variable, which makes the setup of a standard production protocol almost impossible.
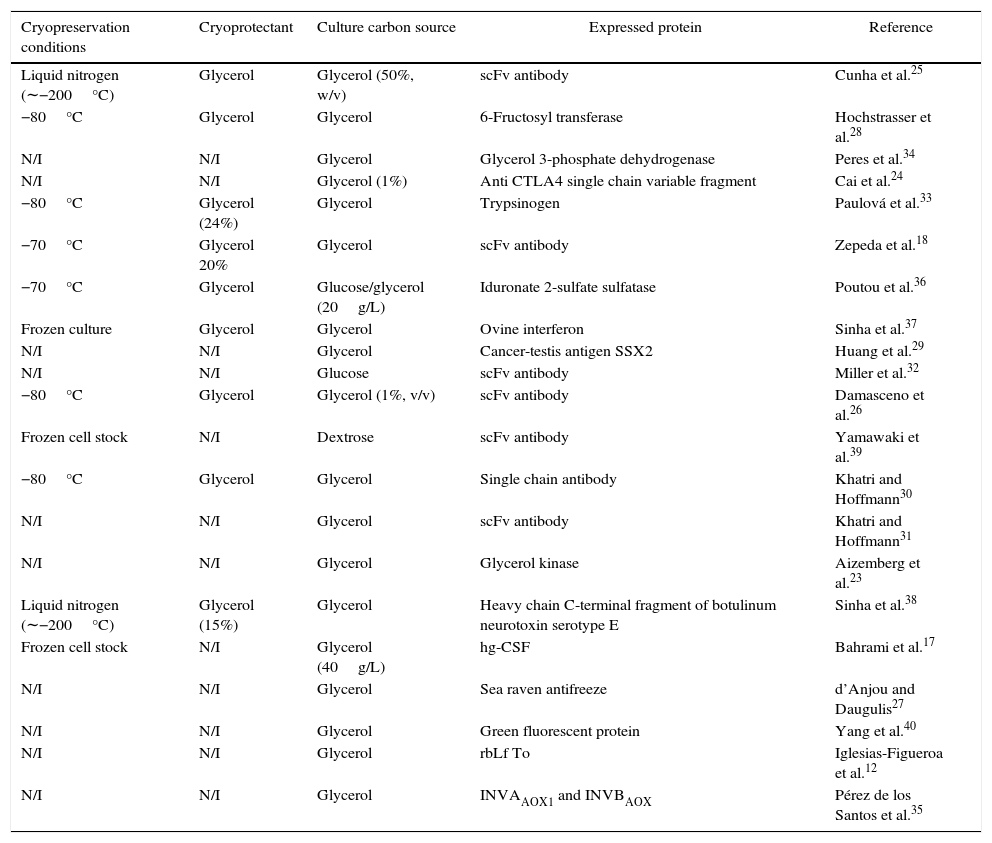
So, we randomly revised the most significant scientific reports on these issues since 1998 from different databases,12,17,18,23–40 with the aim to know (a) how the cryopreservation should be made, (b) what is the best carbon source for preadaptation before cryopreservation, and (c) what is the best carbon source for cultivation.
The main information arising from these studies, which is summarized in Table 1 in terms of cryopreservation conditions, carbon source used in cryopreservation and culture and type of expressed protein, shows that in 5% of selected papers the yeast was cryopreserved at −20°C, in 24% at −70/80°C, in 10% in liquid nitrogen (about −200°C), while in 61% no cryopreservation temperature was indicated. Glycerol was preferred in 43% of cases as carbon source for cryopreservation, whereas no carbon source was specified in the remaining articles. Finally, the preferred carbon source for final culture was glycerol in 86% of cases and glucose in the remaining ones, although the latter choice appears to be contradictory given the known action of glucose as a repressor of heterologous protein expression under the AOX promoter control. Finally, two-thirds of studies where cells were stored at −80°C followed the protocol of the Invitrogen Pichia expression kit for long periods of time.
Survey of the main conditions of cell cryopreservation and culture for heterologous protein expression by recombinant Pichia pastoris.
| Cryopreservation conditions | Cryoprotectant | Culture carbon source | Expressed protein | Reference |
|---|---|---|---|---|
| Liquid nitrogen (∼−200°C) | Glycerol | Glycerol (50%, w/v) | scFv antibody | Cunha et al.25 |
| −80°C | Glycerol | Glycerol | 6-Fructosyl transferase | Hochstrasser et al.28 |
| N/I | N/I | Glycerol | Glycerol 3-phosphate dehydrogenase | Peres et al.34 |
| N/I | N/I | Glycerol (1%) | Anti CTLA4 single chain variable fragment | Cai et al.24 |
| −80°C | Glycerol (24%) | Glycerol | Trypsinogen | Paulová et al.33 |
| −70°C | Glycerol 20% | Glycerol | scFv antibody | Zepeda et al.18 |
| −70°C | Glycerol | Glucose/glycerol (20g/L) | Iduronate 2-sulfate sulfatase | Poutou et al.36 |
| Frozen culture | Glycerol | Glycerol | Ovine interferon | Sinha et al.37 |
| N/I | N/I | Glycerol | Cancer-testis antigen SSX2 | Huang et al.29 |
| N/I | N/I | Glucose | scFv antibody | Miller et al.32 |
| −80°C | Glycerol | Glycerol (1%, v/v) | scFv antibody | Damasceno et al.26 |
| Frozen cell stock | N/I | Dextrose | scFv antibody | Yamawaki et al.39 |
| −80°C | Glycerol | Glycerol | Single chain antibody | Khatri and Hoffmann30 |
| N/I | N/I | Glycerol | scFv antibody | Khatri and Hoffmann31 |
| N/I | N/I | Glycerol | Glycerol kinase | Aizemberg et al.23 |
| Liquid nitrogen (∼−200°C) | Glycerol (15%) | Glycerol | Heavy chain C-terminal fragment of botulinum neurotoxin serotype E | Sinha et al.38 |
| Frozen cell stock | N/I | Glycerol (40g/L) | hg-CSF | Bahrami et al.17 |
| N/I | N/I | Glycerol | Sea raven antifreeze | d’Anjou and Daugulis27 |
| N/I | N/I | Glycerol | Green fluorescent protein | Yang et al.40 |
| N/I | N/I | Glycerol | rbLf To | Iglesias-Figueroa et al.12 |
| N/I | N/I | Glycerol | INVAAOX1 and INVBAOX | Pérez de los Santos et al.35 |
Based on the above literature background, we selected −80°C as the most common cryopreservation temperature and glycerol as both cryoprotectant and culture carbon source, while it is not clear from the literature which preculture carbon source (glycerol or glucose) should be used. The advance of this work compared to previous reports is to provide clear guidelines to perform a typical culture of a recombinant P. pastoris strain carrying the scFv anti LDL (ox) antibody fragment with the aim of developing a new kit to detect LDL (−) in blood for atherosclerosis diagnosis.41 So, to select the best culture conditions, yeast cells were precultured on glucose or glycerol, then cryopreserved in glycerol at −80°C and finally cultured on glycerol either in flasks or bioreactor.
Materials and methodsMicroorganism, preadaptation and cryopreservationThe recombinant P. pastoris SMD 1168 Δpep4::URA3 Δkex::SUC2his4ura3, phenotype His− Mut+ an anti-LDL electronegative His-tagged scFv producing yeast42 was used in this study. It was maintained in Petri dishes on yeast extract–peptone–dextrose (YPD) solid medium containing (per L) 20g dextrose, 10g yeast extract, 20g casein peptone and 20g agar at 30°C for 48h. Two stocks of cells were then prepared to select the best pre-culture conditions. The former was prepared removing a colony from the plate and culturing it in a 500-mL Erlenmeyer containing 200mL of glucose-based YPD medium at 250rpm and 30°C for 32h. To prepare the latter, another colony was grown, under the same conditions, in modified buffered minimal glycerol-complex medium (BMGY) containing (per L) 10g yeast extract, 20g casein peptone, 13.4g yeast nitrogen base with ammonium sulfate but without amino acids, 4×10−4g biotin, 20g glycerol and 20g casamino acids in 100mM potassium phosphate buffer (pH 6.0). For both stocks, in order to get cells in their exponential growth phase and to ensure almost the same concentration of cells pre-adapted in glucose and glycerol (53–78×1012CFU), after about 48h of pre-culture, an aliquot of cell suspension was collected, 1:20 diluted and subjected to optical density (OD) determination at 600nm.
When OD reached 0.36 (corresponding to the above UFC range), 1.0mL of either cell suspension was added to Eppendorf's containing 1.0mL of the cryopreservation medium (BMGY plus 40% glycerol, v/v). Cells (2.0mL) were finally cryopreserved in ultra-freezer at −80°C. All reagents were of analytical grade.
Viability of P. pastoris cellsSamples of P. pastoris cells cryopreserved in 20% (v/v) glycerol at −80°C from both stocks were selected randomly. Before and after cryopreservation, cell counts were performed to determine the percentage of viable cells remaining after this step. To this purpose, cells were subject to serial decimal dilutions with 0.1% (m/v) peptone water, distributed in 20 Petri dishes containing agarized BMGY, and grown at 30°C for 28h.
Inoculum preparationTo prepare the inoculum for flask cultures, 20mL of above cultures were transferred into 500-mL Erlenmeyer flasks containing 180mL of BMGY and reactivated for 28h at 250rpm and 30°C. For cultures in bioreactor, 200mL of cell suspensions were inoculated aseptically into a 3-L bench-scale fermentor (Bioflo 110, New Brunswick Scientific Co., Edison, NJ, USA) containing 1.8L of BMGY under the same conditions. During the growth phase, samples were periodically taken for subsequent analyses of biomass, glycerol and ethanol concentrations.
Expression of the scFv antibody fragmentThe scFv antibody fragment was expressed following the procedure described by Kazuma et al.42 Briefly, 1.0% (v/v) methanol and 1.0mM phenyl methanesulfonyl fluoride (PMSF) were added after 28, 52 and 76h to induce scFv antibody fragment production and to inhibit proteases activity. During this induction phase, samples were periodically withdrawn for subsequent analyses of biomass, glycerol and scFv antibody fragment concentrations. Production of the scFv antibody fragment was performed in triplicate at 20°C either in flasks or in bioreactor. Foaming was controlled by the addition of 0.5mL of dimethylpolysiloxane when necessary. Stirring and specific air flow rate were set at 600rpm and 1.8vvm, respectively.
Analytical methodsCell concentration was determined by a calibration curve correlating OD and dry weight. After the fermentation broth had been centrifuged at 1253×g for 20min at 25°C, cell pellet was washed twice with distilled water, and suspensions were submitted to OD readings at 600nm through a spectrophotometer, model U1800 (Hitachi, Tokyo, Japan). Glycerol and ethanol concentrations were determined by oxidation with acidic periodate43 and dichromate44 methods, respectively. All analyses were performed in triplicate.
scFv antibody fragment purification and quantificationThe scFv antibody fragment was purified, as previously reported,42 by supernatant filtration, dialysis and Ni-Sepharose Fast Flow resin (GE Healthcare, Uppsala, Sweden) chromatography. The eluted protein was finally dialyzed against phosphate buffered saline (PBS). After the addition of loading buffer, the purified protein was heated for 5min at 100°C, and 10μL of this solution were run on a SDS-PAGE (12% polyacrylamide) 1-mm-thick gel in a Bio-Rad Mini-Protein II Electrophoresis System (Bio-Rad, Hercules, CA, USA). Unstained protein standards (cod. 161-0363, Bio-Rad) with molar mass in the range 10–250kDa were used as molecular size markers, Coomassie Brilliant Blue G-250 (cod. 1610406, Bio-Rad) as staining solution, and water for rinsing and destaining. An aliquot (25μl) of the purified anti-body fragment collected from the broth at the end of cultivation was quantified by the modified bicinchoninic acid (BCA) method (Cat. n. 23225, Thermoscientific, Hudson, NH, USA).42
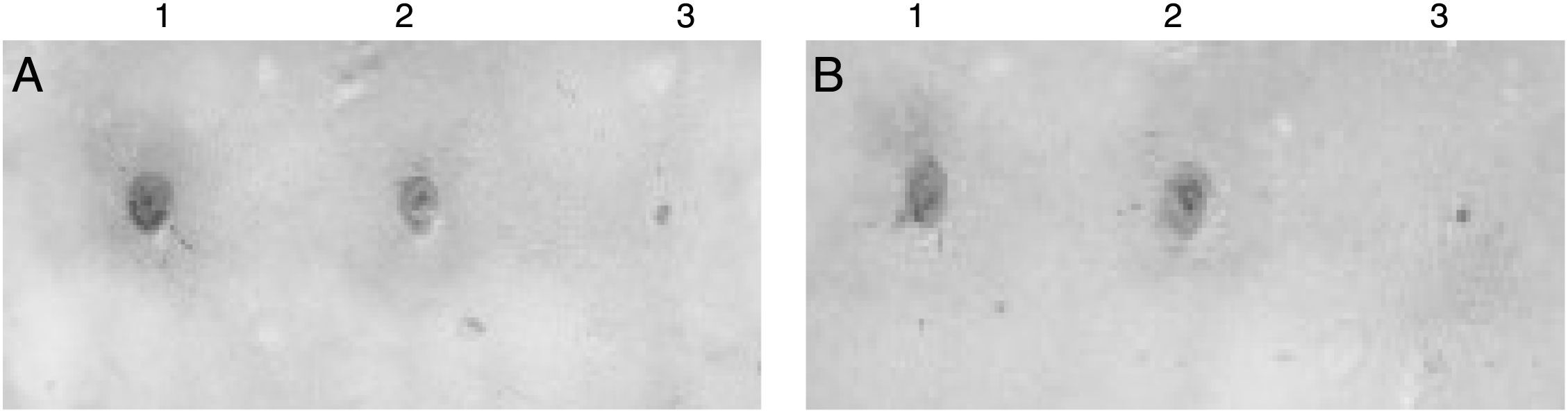
To confirm scFv fragment expression, dot blots were performed. Five microliter-aliquots of the antibody-containing purified fraction, the positive control (E. coli Nha protein labeled with C-terminal 6×HisTag)45 and the negative control (P. pastoris culture without induction) were absorbed onto a polyvinylidene difluoride membrane. To detect the recombinant protein, the membrane was incubated with a blocking solution [5% milk in TBS (10mM Tris–HCl, pH 7.5, plus 150mM NaCl) buffer] for 30–45min at room temperature, washed thrice with TBS buffer for 5min and then incubated with primary antibodies overnight at 4°C. In this case anti-His mouse monoclonal antibodies were used at 1:1000 dilution in a 1% milk solution in TBS buffer. After 24h, the membrane was washed thrice with TBS buffer and incubated for 2h at 4°C under the same conditions with secondary polyclonal anti-mouse antibodies obtained in rabbit and coupled to alkaline phosphatase (A4312 Sigma). After final thrice washing of the membrane with TBS buffer, the chromogenic detection of protein was performed by addition of 5-bromo-4-chloro-3-indolyl-phosphate in conjunction with nitro blue tetrazolium (SigmaFast, Sigma).
Definitions of bioprocess parametersVolumetric productivity of the antibody fragment, P (mgL−1h−1), was defined as:
where Cf and C0 (mg/L) are its concentrations at the end and the beginning of the run, respectively, and tf (h) is the time when the process was stopped.Maximum specific growth rate, μmax (h−1), of the microorganism was determined in the exponential growth phase as follows:
where X is cell concentration (g/L) in a given point of the curve and X0 that at the beginning.The doubling time, td (h), was defined as:
Results and discussionTo investigate the growth behavior of the recombinant P. pastoris strain, two cell stocks with starting cell concentration of the same magnitude order (1010CFUmL−1) were produced using glucose or glycerol as carbon sources. Cell counts revealed that this operation implied a very low cell viability loss either before (7.8 and 6.3% in glucose and glycerol precultures, respectively) or after cryopreservation (18.1 and 15.8% in glucose and glycerol precultures, respectively), with no clear carbon source influence. The results of cultures, performed in flasks and bioreactor, are illustrated in Figs. 1 and 2, respectively.
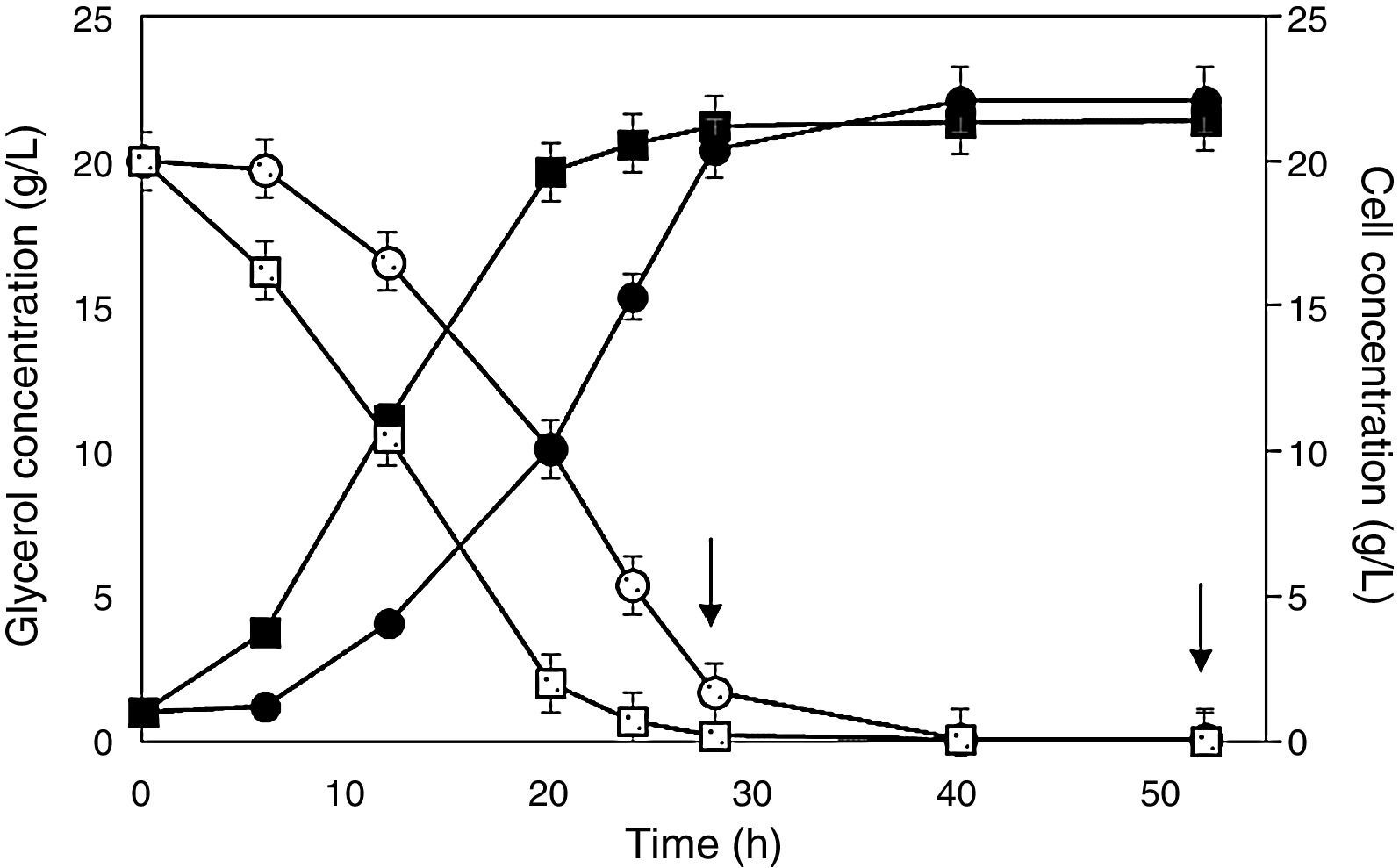
Behavior of cell (full symbols) and glycerol (empty symbols) concentrations during Pichia pastoris SMD 1168 cultures for the expression of scFv in flasks. Cells preadapted to glycerol (squares) or glucose (circles). Arrows point out additions of methanol as an inducer. A further addition of methanol after 76 is not shown because no variation was detected in both glycerol and cell concentrations.
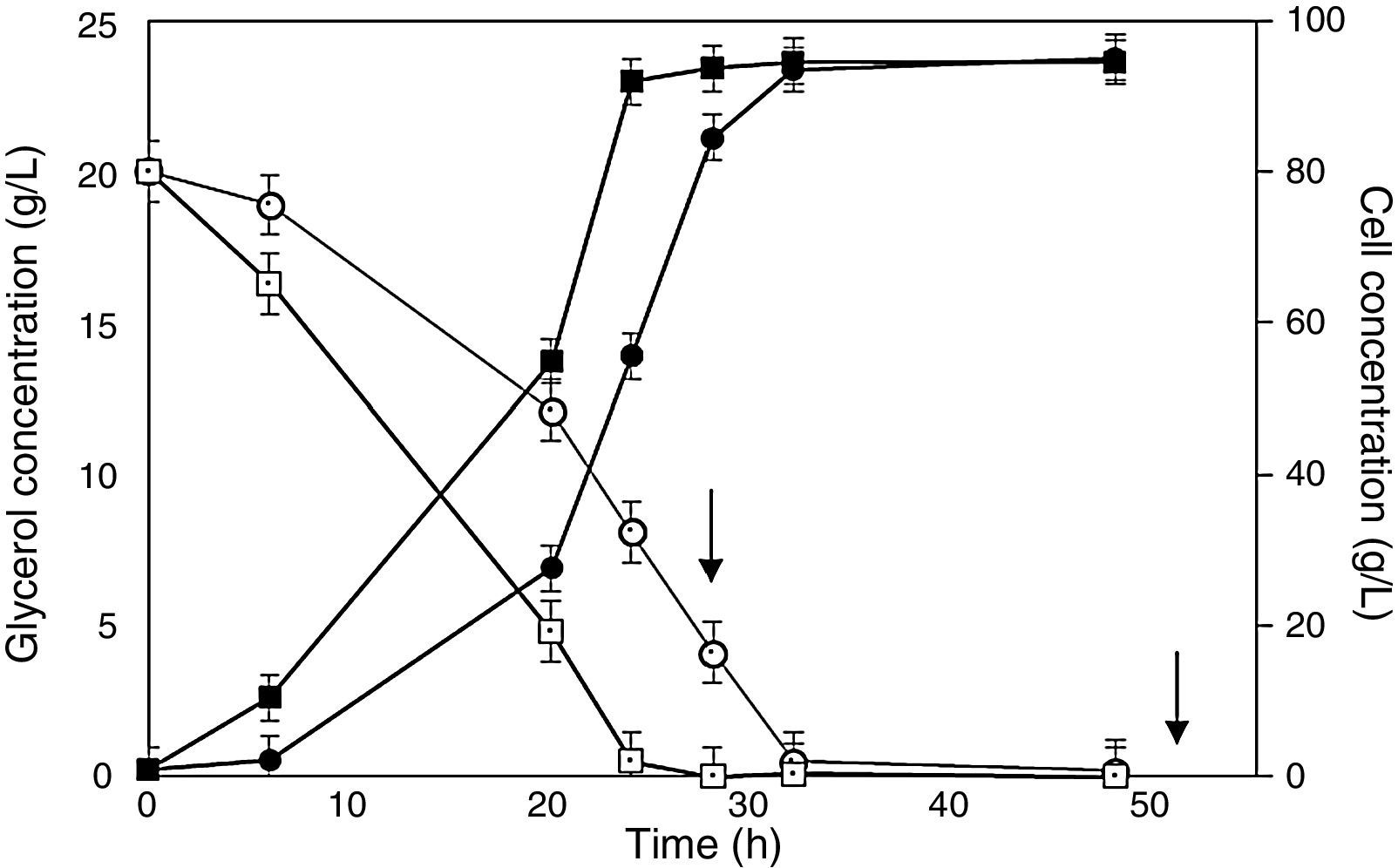
Behavior of cell (full symbols) and glycerol (empty symbols) concentrations during Pichia pastoris SMD 1168 cultures for the expression of scFv in bioreactor. Cells preadapted to glycerol (squares) or glucose (circles). Arrows point out additions of methanol as an inducer. A further addition of methanol after 76 is not shown because no variation was detected in both glycerol and cell concentrations.
In flasks, the final concentration of cells pre-adapted on glycerol (21.4gL−1) and glucose (22.1gL−1) were almost the same, but the latter carbon source required longer time to complete the run, likely because glucose inhibited adenylate cyclase activity, and the consequent low AMPc level prevented the formation of the complex acting on the AOX1 promoter.36 In addition, even though in glycerol-containing medium the residual glucose level from preculturing was likely insignificant to promote any effect, cell adaptation to the new condition may have lasted a time sufficient to delay significantly the growth. As a consequence, after 6h of cultivation, cells precultured on glucose were still at the adaptation stage, and the late log phase was reached 8h later. This behavior was responsible for a remarkable reduction not only of cell productivity, but mainly of antibody fragment productivity (Table 2), which would be a crucial issue in the process scaling up.
Main results of batch cultures of Pichia pastoris SMD 1168 performed in either flasks or bioreactor, using cells preadapted on glycerol or glucose and cryopreserved in glycerol 20% (v/v).
| System | Carbon source | P (mgscFvL−1h−1) | Y (mgscFvgDW−1) | μmax (h−1) | td (h) | Cf (mgscFvL−1) | X (gDWL−1) |
|---|---|---|---|---|---|---|---|
| Flasks | Glycerol | 0.20±0.05 | 0.70±0.03 | 0.14±0.01 | 4.81±0.03 | 14.4±0.01 | 21.4±0.1 |
| Flasks | Glucose | 0.14±0.03 | 0.47±0.03 | 0.16±0.03 | 4.51±1.02 | 10.1±0.01 | 22.1±0.2 |
| Bioreactor | Glycerol | 0.83±0.04 | 0.64±0.02 | 0.17±0.01 | 4.03±0.04 | 60.2±0.01 | 94.7±0.2 |
| Bioreactor | Glucose | 0.77±0.02 | 0.59±0.02 | 0.15±0.01 | 4.47±0.26 | 55.8±0.01 | 95.2±0.2 |
P, volumetric productivity of the antibody fragment; Y, antibody fragment yield; μmax, maximum specific growth rate; td, doubling time; Cf, final antibody fragment concentration; X, final cell concentration.
As is well known, heterologous proteins expression by P. pastoris is subject to induction controlled by the AOX promoter that takes place only in the presence of methanol as the sole carbon source. As soon as the carbon source for growth (glycerol, sorbitol, xylose, ribose, glucose)36,46–48 has been exhausted, the step of induction with methanol begins.12 Some carbon sources, maily glucose, were reported to inhibit the promoter expression,36 while others such as sorbitol and glycerol were described as not inhibitory.49 For this reason, the type of carbon source before the induction is of paramount significance for protein expression.50
Fig. 1 shows that glycerol-preadapted cells consumed glycerol almost entirely within 24h, while those precultured on glucose lasted no less than 32h. Taking into account that methanol was added the first time after 28h, the former cell stock exhibited a high antibody concentration at the end of the process, while the latter did not yet reach the late log phase, and there still was a residual glycerol concentration of 1.7gL−1.
When comparing the results of runs performed in flasks (Table 2), one can see that glycerol-preadapted cells were significantly more effective than those precultured on glucose. Employing the former cell stock, the concentration (Cf=14.4±0.01mgL−1) and overall productivity (P=0.20±0.05mgL−1h−1) of the antibody fragment were in fact 42.9% higher and the maximum specific growth rate (μmax=0.14±0.01h−1) was 12.5% lower, respectively, than employing the latter, and the doubling time (4.81h) was almost 6% longer. The above μmax value is comparable with that reported in the literature for P. pastoris Mut+ expressing lipase from Rhizopus oryzae (0.18h−1).20
Better results were obtained in cultures performed in bioreactor (Fig. 2), in that cell concentration was appreciably higher than in flasks, possibly because the increased dissolved oxygen level in the medium stimulated yeast growth. In bioreactor, cells preadapted to glycerol reached a final concentration almost coincident to that of cells preadapted to glucose, but achieved the late log phase more quickly (24h) and exhibited a final antibody fragment concentration about 8% higher (32h) (Fig. 2). When comparing the related kinetic parameters, the antibody fragment volumetric productivity (Table 2) was in bioreactor 4.2- and 5.5-fold those obtained in flasks with cells preadapted to glycerol and glucose, respectively.
As expected, consistently with the results in flasks, glycerol was almost completely consumed (0.5gL−1) within 24h by cells preadapted to this carbon source, while those precultured on glucose lasted up to 32h to do the same (Fig. 2). A possible reason of this behavior is that glycerol can be assimilated quickly forming glyceraldehyde 3-phosphate and dihydroxy acetone phosphate through a route that requires less energy than the steps involved in the aldohexose uptake.36 Another possible reason may be the different regulation of the genic expression in cells of precultures, mainly in the genes involved in glycerol metabolism.
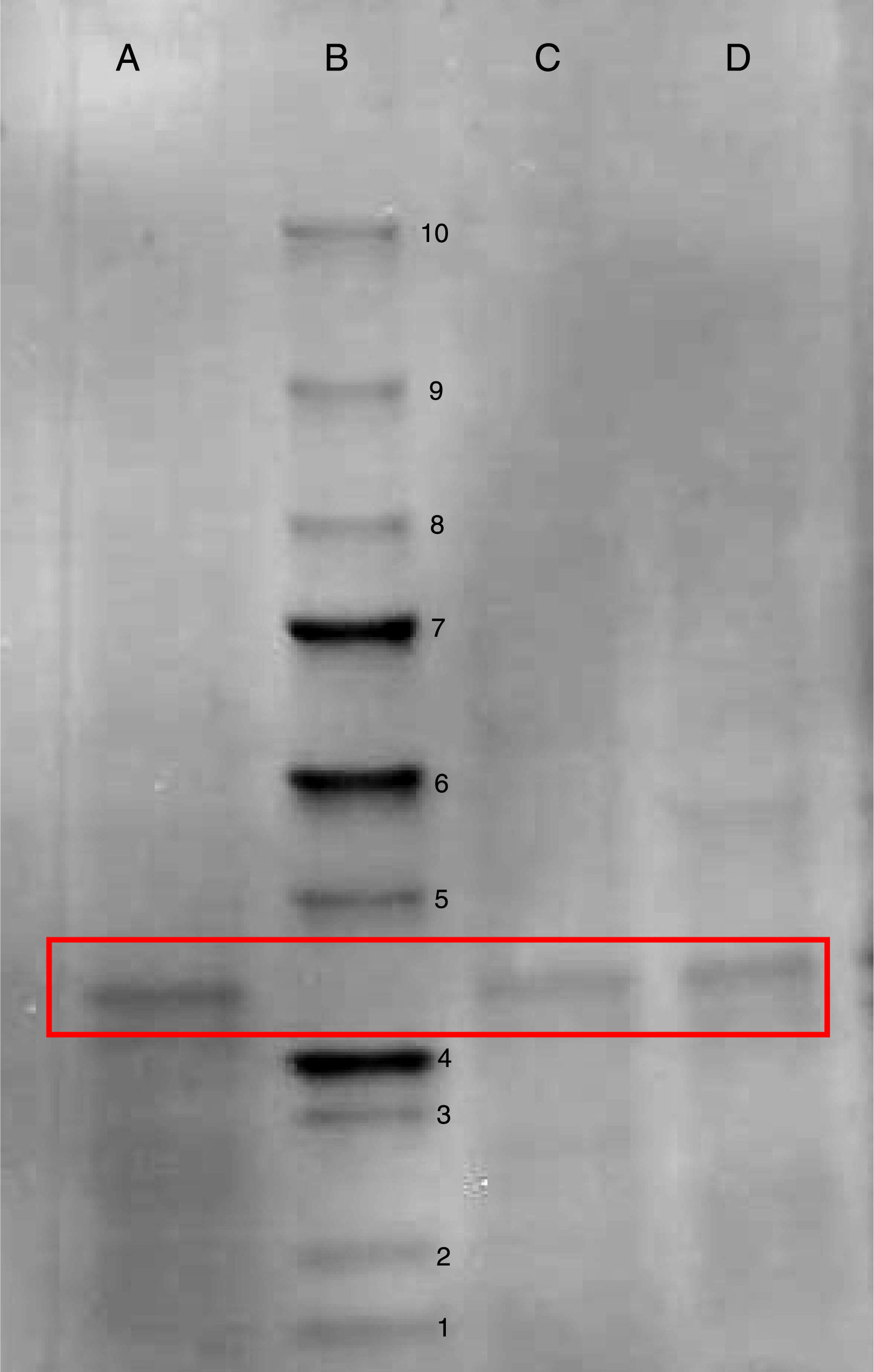
After qualitative confirmation of the antibody fragment activity by dot blot technique (Fig. 3), a bioinformatic study was performed with the aid of Expasy ProtParam program by using its already-obtained aminoacid sequence as an input (unpublished results). These results taken together indicated that the antibody fragment is constituted by 260 aminoacids, molar mass of 27.64kDa and a theoretical isoelectric point of 8.9. To confirm this molar mass, a SDS-PAGE on a sample of purified scFv allowed us to identify a band of about 28kDa (Fig. 4), which is in fair agreement with the above value predicted by the aminoacid sequence.
SDS-PAGE containing 12% polyacrylamide to confirm the production of the antibody fragment scFv anti LDL-ox. Well A: purified fraction from glycerol-based broth taken at the end of culture in bioreactor. Well B: unstained molar mass marker (Bio-Rad): 1=10kDa; 2=15kDa; 3=20kDa; 4=25kDa; 5=37kDa; 6=50kDa; 7=75kDa; 8=100kDa; 9=150kDa; 10=250kDa. Well C: purified fraction from glucose-based broth taken at the end of culture in flask. Well D: purified fraction from glycerol-based broth taken at the end of culture in flask. Boxes enclose the ≈28kDa antibody fragment.
The results of this study demonstrate that recombinant P. pastoris cells cryopreserved in glycerol and preadapted in glycerol-containing BMGY medium provided higher yields of biomass and scFv antibody fragment compared to cells preadapted in glucose. Glycerol was shown to be a better carbon source than glucose to preculture these cells, allowing for high biomass productivity, lower duplication time and greater final concentration and productivity of the antibody fragment. This adaptation shortened the time needed to reach the late log phase, and cells were able to completely consume the carbon source (glycerol) before the induction phase promoted by methanol, hence avoiding any catabolic repression. The results obtained in bioreactor were better than those obtained in flasks and suggest that the use of an appropriate preadaptation protocol to store cells would allow saving up to 8h (more than 8% of the overall production time), thus promising to remarkably increase the volumetric productivity. As a concluding remark, we propose, based on the results of this study, to maintain cells always on glycerol throughout the whole production sequence, i.e. during the steps of preadaption, cryopreservation and cultivation.
Conflicts of interestThe authors declare no conflicts of interest.
This project was supported by Brazilian FAPESP, CNPq and CAPES. Moreover, Prof. Converti thanks CAPES for the Special Visiting Researcher fellowship within the Program Sciences without Borders (project n. 2609/2013).